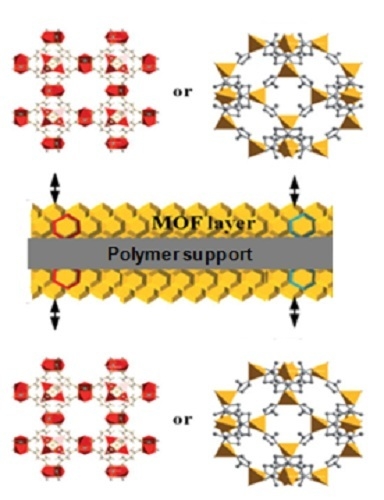
Synthesis and Transport Properties of Novel MOF/PIM-1/MOF Sandwich Membranes for Gas Separation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Membrane Preparation and Properties
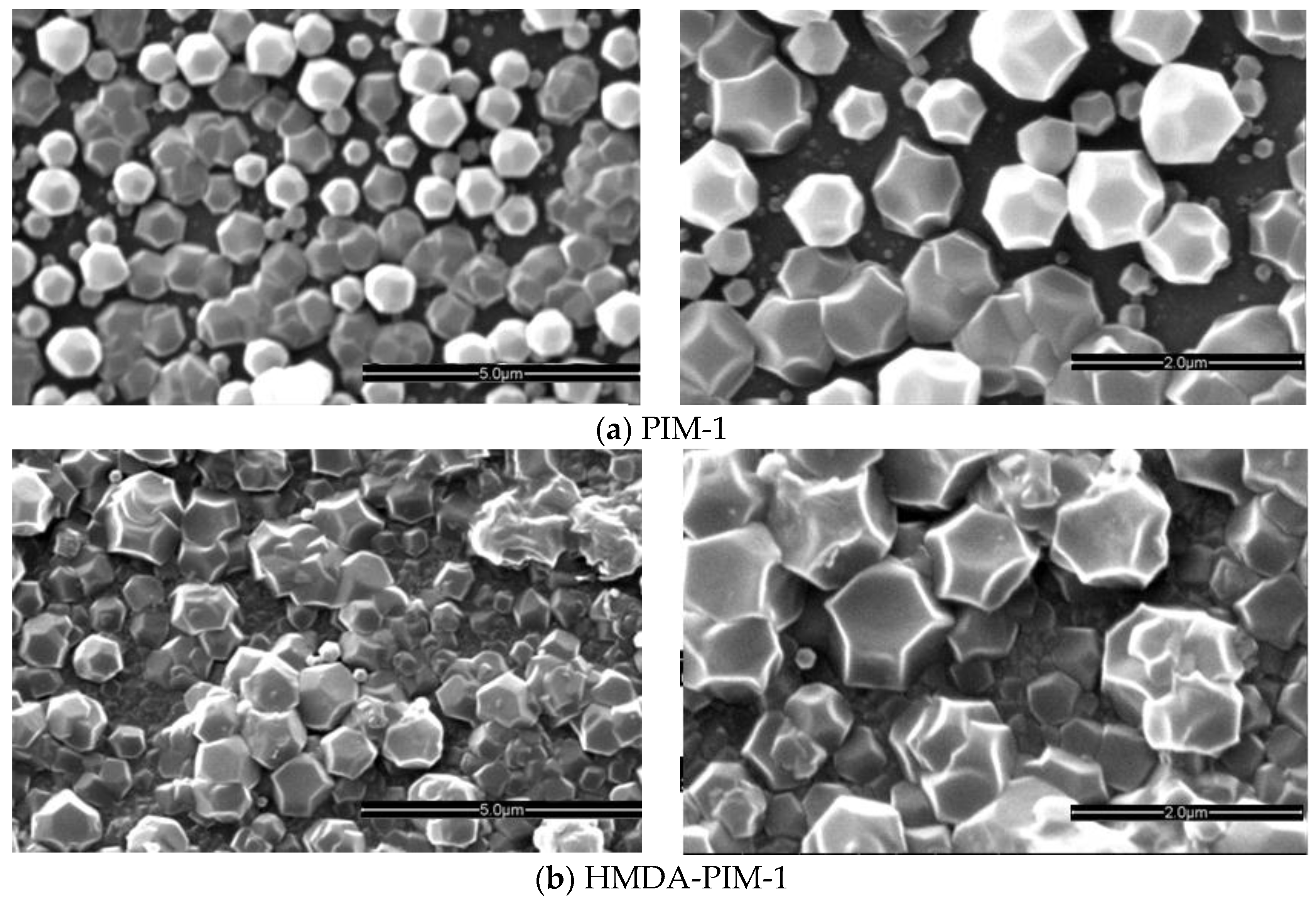
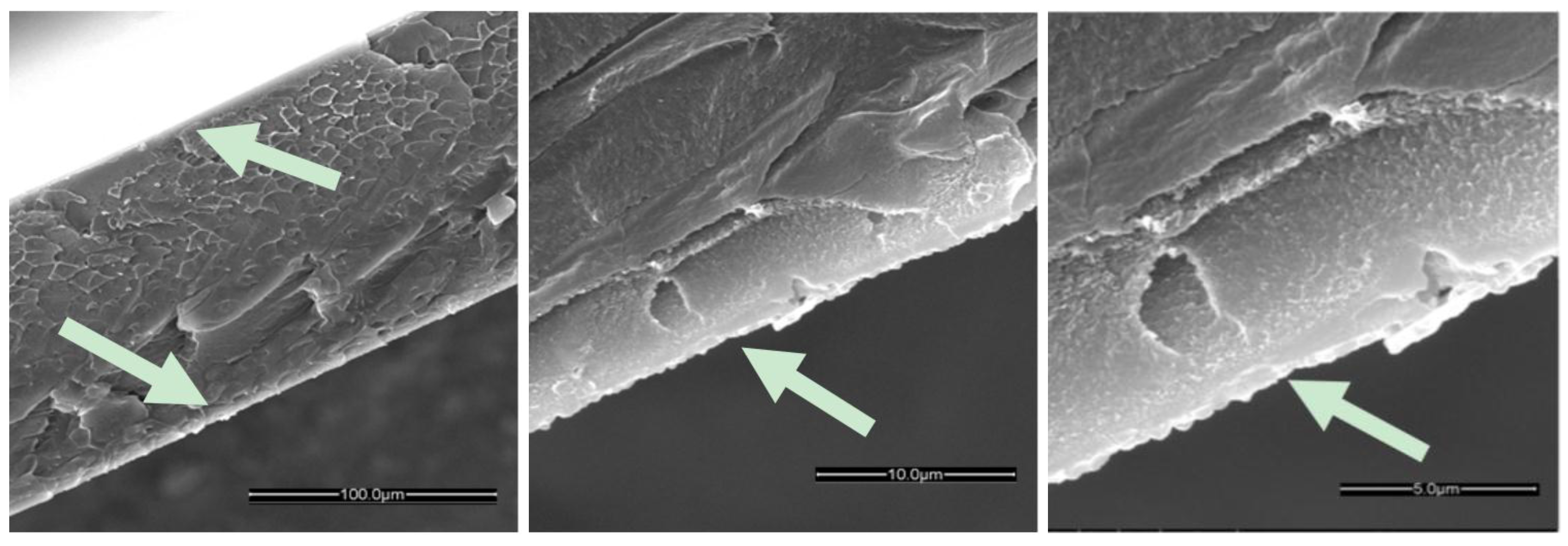
2.1.1. ZIF-8 on PIM-1 and Modified PIM-1 Membranes

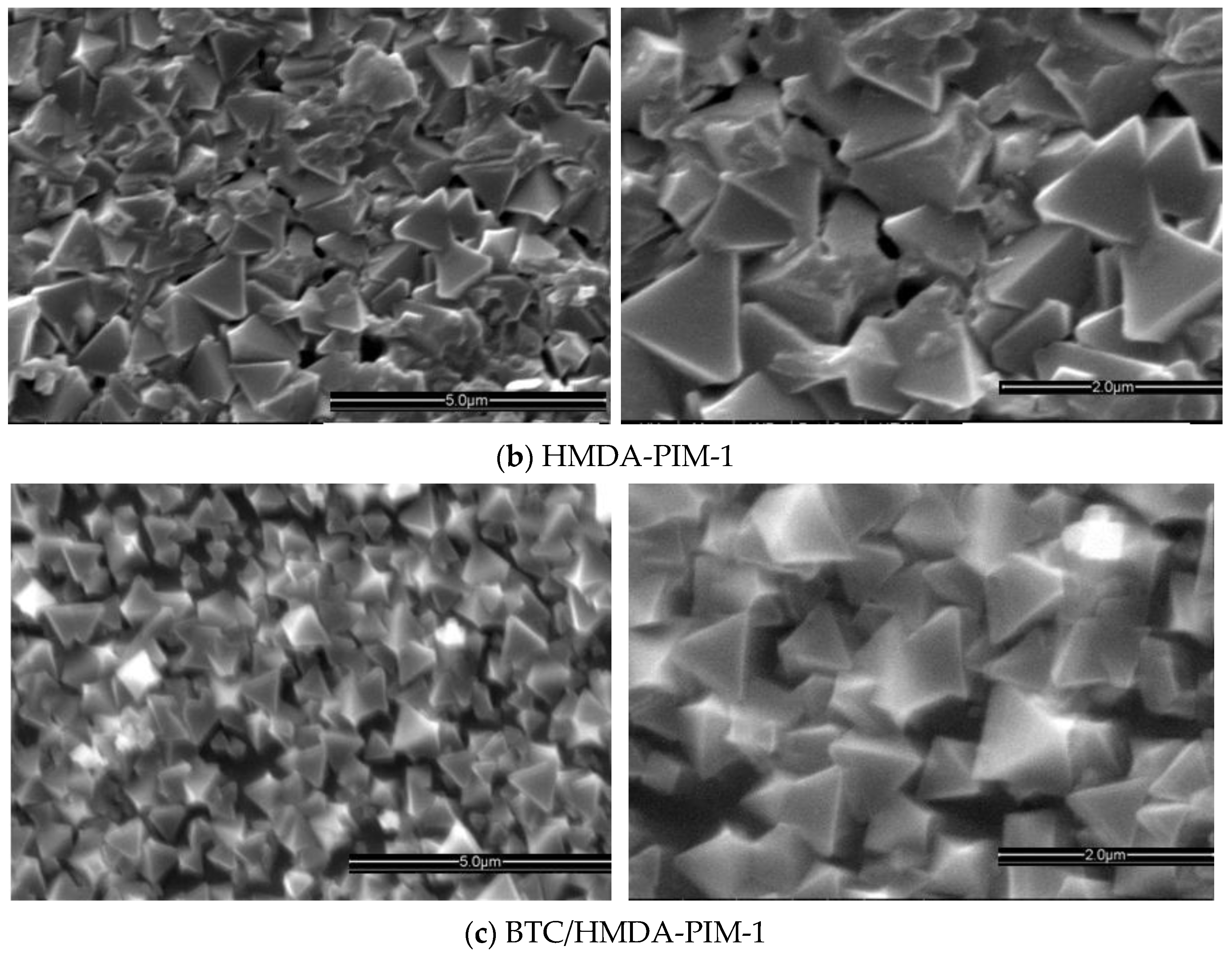
2.1.2. HKUST-1 Supported Surface Modified PIM-1 Membrane
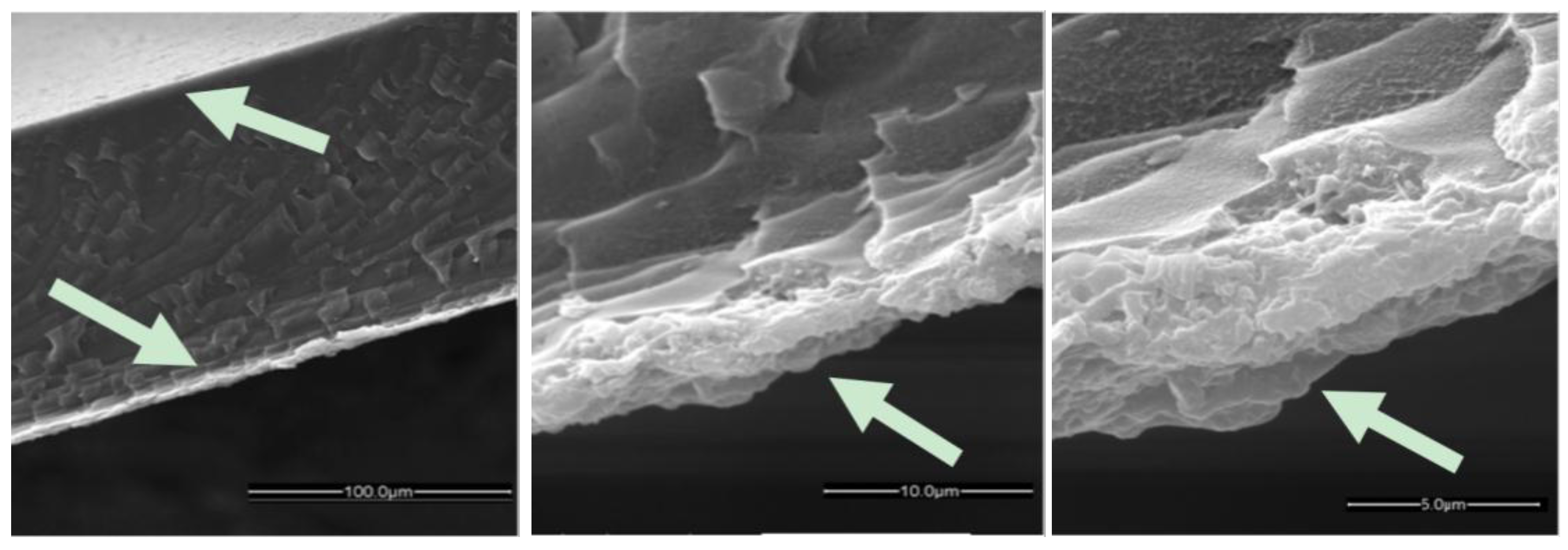
2.2. Gas Transport Properties of MOF/PIM Sandwich Membranes
3. Materials and Methods
3.1. Polymer and Membrane Synthesis
3.1.1. PIM-1 Synthesis
3.1.2. PIM-1 Membrane Preparation
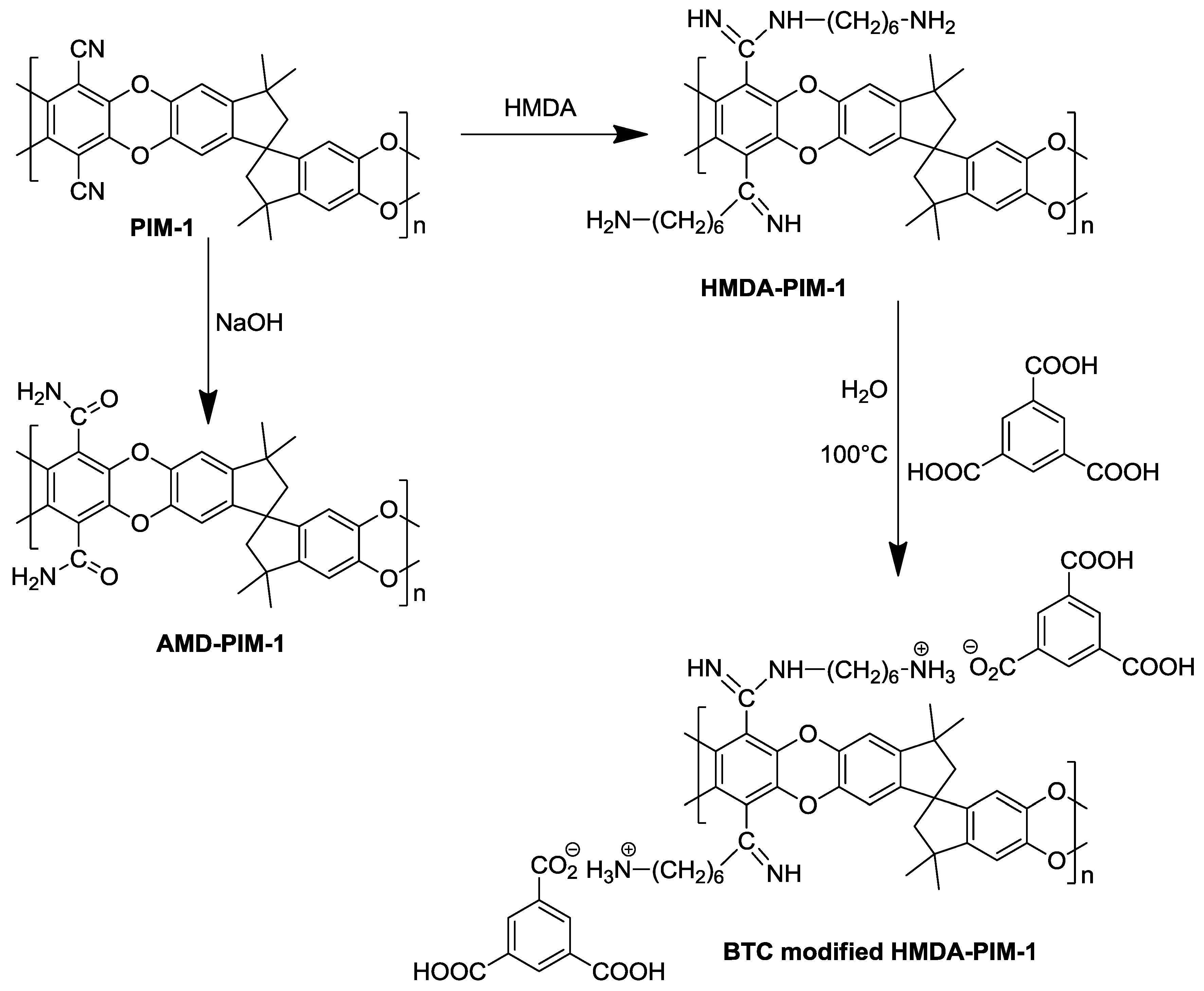
3.1.3. Amide Modification of a PIM-1 Membrane
3.1.4. HMDA Modification of a PIM-1 Membrane
3.1.5. BTC/HMDA Modified PIM-1 Membrane Preparation from HMDA Modified PIM-1 Membrane
3.1.6. Preparation of Membranes of ZIF-8 on PIM-1 and Modified PIM-1
3.1.7. Preparation of Membranes of HKUST-1 on HMDA and BTC/HMDA Modified PIM-1
3.2. Characterisation Techniques
3.2.1. Structural Analysis
3.2.2. Pure Gas Permeation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Bushell, A.F.; Budd, P.M.; Attfield, M.P.; Jones, J.T.A.; Hasell, T.; Cooper, A.I.; Bernardo, P.; Bazzarelli, F.; Clarizia, G.; Jansen, J.C. Nanoporous organic polymer/cage composite membranes. Angew. Chem. Int. Ed. 2013, 52, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- Lanč, M.; Pilnáček, K.; Vopička, O.; Friess, K.; Bernardo, P.; Bazzarelli, F.; Tasselli, F.; Clarizia, G.; Mason, C.R.; Maynard-Atem, L.; et al. Effect of physical aging on the gas transport and sorption in PIM-1 membranes. Polymer 2016. [Google Scholar] [CrossRef]
- Zhang, R.; Ji, S.; Wang, N.; Wang, L.; Zhang, G.; Li, J.R. Coordination-driven in situ self-assembly strategy for the preparation of metal-organic framework hybrid membranes. Angew. Chem. Int. Ed. 2014, 53, 9775–9779. [Google Scholar] [CrossRef] [PubMed]
- Cacho-Bailo, F.; Seoane, B.; Téllez, C.; Coronas, J. ZIF-8 continuous membrane on porous polysulfone for hydrogen separation. J. Membr. Sci. 2014, 464, 119–126. [Google Scholar] [CrossRef]
- Campbell, J.; Davies, R.; Braddock, C.D.; Livingston, A. Improving the permeance of hybrid polymer/metal–organic framework (MOF) membranes for organic solvent nanofiltration (OSN)—Development of MOF thin films via interfacial synthesis. J. Mater. Chem. A 2015, 3, 9668–9674. [Google Scholar] [CrossRef]
- Nagaraju, D.; Bhagat, D.G.; Banerjee, R.; Kharul, U.K. In situ growth of metal-organic frameworks on a porous ultrafiltration membrane for gas separation. J. Mater. Chem. A 2013, 1, 8828–8835. [Google Scholar] [CrossRef]
- Moore, T.T.; Koros, W.J. Non-ideal effects in organic–inorganic materials for gas separation membranes. J. Mol. Struct. 2005, 739, 87–98. [Google Scholar] [CrossRef]
- Mason, C.R.; Buonomenna, M.G.; Golemme, G.; Budd, P.M.; Galiano, F.; Figoli, A.; Friess, K.; Hynek, V. New organophilic mixed matrix membranes derived from a polymer of intrinsic microporosity and silicalite-1. Polymer 2013, 54, 2222–2230. [Google Scholar] [CrossRef]
- Althumayri, K.; Harrison, W.J.; Shin, Y.; Gardiner, J.M.; Casiraghi, C.; Budd, P.M.; Bernardo, P.; Clarizia, G.; Jansen, J.C. The influence of few-layer graphene on the gas permeability of the high-free-volume polymer PIM-1. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Filiz, V.; Bengtson, G.; Rahman, M.M.; Shishatskiy, S.; Abetz, V. Functionalized Carbon Nanotube Mixed Matrix Membranes of Polymers of Intrinsic Microporosity (PIMs) for Gas Separation. Procedia Eng. 2012, 44, 1899–1901. [Google Scholar] [CrossRef]
- Bushell, A.F.; Attfield, M.P.; Mason, C.R.; Budd, P.M.; Yampolskii, Y.; Starannikova, L.; Rebrov, A.; Bazzarelli, F.; Bernardo, P.; Carolus Jansen, J.; et al. Gas permeation parameters of mixed matrix membranes based on the polymer of intrinsic microporosity PIM-1 and the zeolitic imidazolate framework ZIF-8. J. Membr. Sci. 2013, 427, 48–62. [Google Scholar] [CrossRef]
- Khdhayyer, M.R.; Esposito, E.; Fuoco, A.; Monteleone, M.; Giorno, L.; Jansen, J.C.; Attfield, M.P.; Budd, P.M. Mixed matrix membranes based on UiO-66 MOFs in the polymer of intrinsic microporosity PIM-1. Sep. Purif. Technol. 2017, 173, 304–313. [Google Scholar] [CrossRef]
- Alentiev, A.Y.; Bondarenko, G.N.; Kostina, Y.V.; Shantarovich, V.P.; Klyamkin, S.N.; Fedin, V.P.; Kovalenko, K.A.; Yampolskii, Y.P. PIM-1/MIL-101 hybrid composite membrane material: Transport properties and free volume. Pet. Chem. 2014, 54, 477–481. [Google Scholar] [CrossRef]
- Ma, J.; Ying, Y.; Guo, X.; Huang, H.; Liu, D.; Zhong, C. Fabrication of mixed-matrix membrane containing metal–organic framework composite with taskspecific ionic liquid for efficient CO2 separation. J. Mater. Chem. A Mater. Energy Sustain. 2016, 4, 7281–7288. [Google Scholar] [CrossRef]
- Shahid, S.; Nijmeijer, K.; Nehache, S.; Vankelecom, I.; Deratani, A.; Quemener, D. MOF-mixed matrix membranes: Precise dispersion of MOF particles with better compatibility via a particle fusion approach for enhanced gas separation properties. J. Membr. Sci. 2015, 492, 21–31. [Google Scholar] [CrossRef]
- Jin, R.; Bian, Z.; Li, J.; Ding, M.; Gao, L. ZIF-8 crystal coatings on a polyimide substrate and their catalytic behaviours for the Knoevenagel reaction. Dalton Trans. 2013, 42, 3936–3940. [Google Scholar] [CrossRef] [PubMed]
- Ben, T.; Lu, C.; Pei, C.; Xu, S.; Qiu, S. Polymer-supported and free-standing metal-organic framework membrane. Chem. A Eur. J. 2012, 18, 10250–10253. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Meng, Q.; Zhang, C.; Zhang, G. Metal-organic framework/PVDF composite membranes with high H2 permselectivity synthesized by ammoniation. Chem. A Eur. J. 2015, 21, 7224–7230. [Google Scholar] [CrossRef] [PubMed]
- Kida, K.; Fujita, K.; Shimada, T.; Tanaka, S.; Miyake, Y. Layer-by-layer aqueous rapid synthesis of ZIF-8 films on a reactive surface. Dalton Trans. 2013, 42, 11128–11135. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Li, J.; Zhang, M.; Wang, H.; Lan, Y.; Wu, Y.; Li, F.; Li, G. A polydopamine layer as the nucleation center of MOF deposition on “inert” polymer surfaces to fabricate hierarchically structured porous films. Chem. Commun. 2015, 51, 2706–2709. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Xu, Q.; Peng, J.; Ji, Z.; Hu, X. (110)-Oriented ZIF-8 thin films on ITO with controllable thickness. ChemPhysChem 2013, 14, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Liu, Q.; Wang, N.; Caro, J. Highly hydrogen permselective ZIF-8 membranes supported on polydopamine functionalized macroporous stainless-steel-nets. J. Mater. Chem. A 2014, 2, 8246–8251. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Yang, Z.; Zhang, G.; Fan, Z.; Meng, Q.; Shen, C.; Gao, C. Stiff metal–organic framework–polyacrylonitrile hollow fiber composite membranes with high gas permeability. J. Mater. Chem. A 2014, 2, 2110–2118. [Google Scholar] [CrossRef]
- Yao, J.; Wang, H. Zeolitic imidazolate framework composite membranes and thin films: Synthesis and applications. Chem. Soc. Rev. 2014, 43, 4470–4493. [Google Scholar] [CrossRef] [PubMed]
- Munuera, C.; Shekhah, O.; Wang, H.; Wöll, C.; Ocal, C. The controlled growth of oriented metal-organic frameworks on functionalized surfaces as followed by scanning force microscopy. Phys. Chem. Chem. Phys. 2008, 10, 7257–7261. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Ferrand, L.; Li, D.; Lee, D.; Durning, C.J. All-nanoparticle layer-by-layer surface modification of micro- and ultrafiltration membranes. Langmuir 2014, 30, 5545–5556. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; McCarthy, M.C.; Sachdeva, S.; Lee, A.K.; Jeong, H.K. Current status of metal-organic framework membranes for gas separations: Promises and challenges. Ind. Eng. Chem. Res. 2012, 51, 2179–2199. [Google Scholar] [CrossRef]
- Shamsaei, E.; Low, Z.-X.; Lin, X.; Mayahi, A.; Liu, H.; Zhang, X.; Zhe Liu, J.; Wang, H. Rapid synthesis of ultrathin, defect-free ZIF-8 membranes via chemical vapour modification of a polymeric support. Chem. Commun. 2015, 51, 11474–11477. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Sutrisna, P.D.; Zhang, Y.; Chen, V. Formation of Ultrathin, Continuous Metal-Organic Framework Membranes on Flexible Polymer Substrates. Angew. Chem. Int. Ed. 2016, 55, 3947–3951. [Google Scholar] [CrossRef] [PubMed]
- Teplyakov, V.; Meares, P. Correlation aspects of the selective gas permeabilities of polymeric materials and membranes. Gas Sep. Purif. 1990, 4, 66–74. [Google Scholar] [CrossRef]
- Du, N.; Robertson, G.P.; Song, J.; Pinnau, I.; Thomas, S.; Guiver, M.D. Polymers of intrinsic microporosity containing trifluoromethyl and phenylsulfone groups as materials for membrane gas separation. Macromolecules 2008, 41, 9656–9662. [Google Scholar] [CrossRef]
- Budd, P.M.; McKeown, N.B.; Ghanem, B.S.; Msayib, K.J.; Fritsch, D.; Starannikova, L.; Belov, N.; Sanfirova, O.; Yampolskii, Y.; Shantarovich, V. Gas permeation parameters and other physicochemical properties of a polymer of intrinsic microporosity: Polybenzodioxane PIM-1. J. Membr. Sci. 2008, 325, 851–860. [Google Scholar] [CrossRef]
- Satilmis, B.; Budd, P.M. Base-catalysed hydrolysis of PIM-1: Amide versus carboxylate formation. RSC Adv. 2014, 4, 52189–52198. [Google Scholar] [CrossRef]
- Yanaranop, P.; Santoso, B.; Etzion, R.; Jin, J. Facile conversion of nitrile to amide on polymers of intrinsic microporosity (PIM-1). Polymer 2016, 98, 244–251. [Google Scholar] [CrossRef]
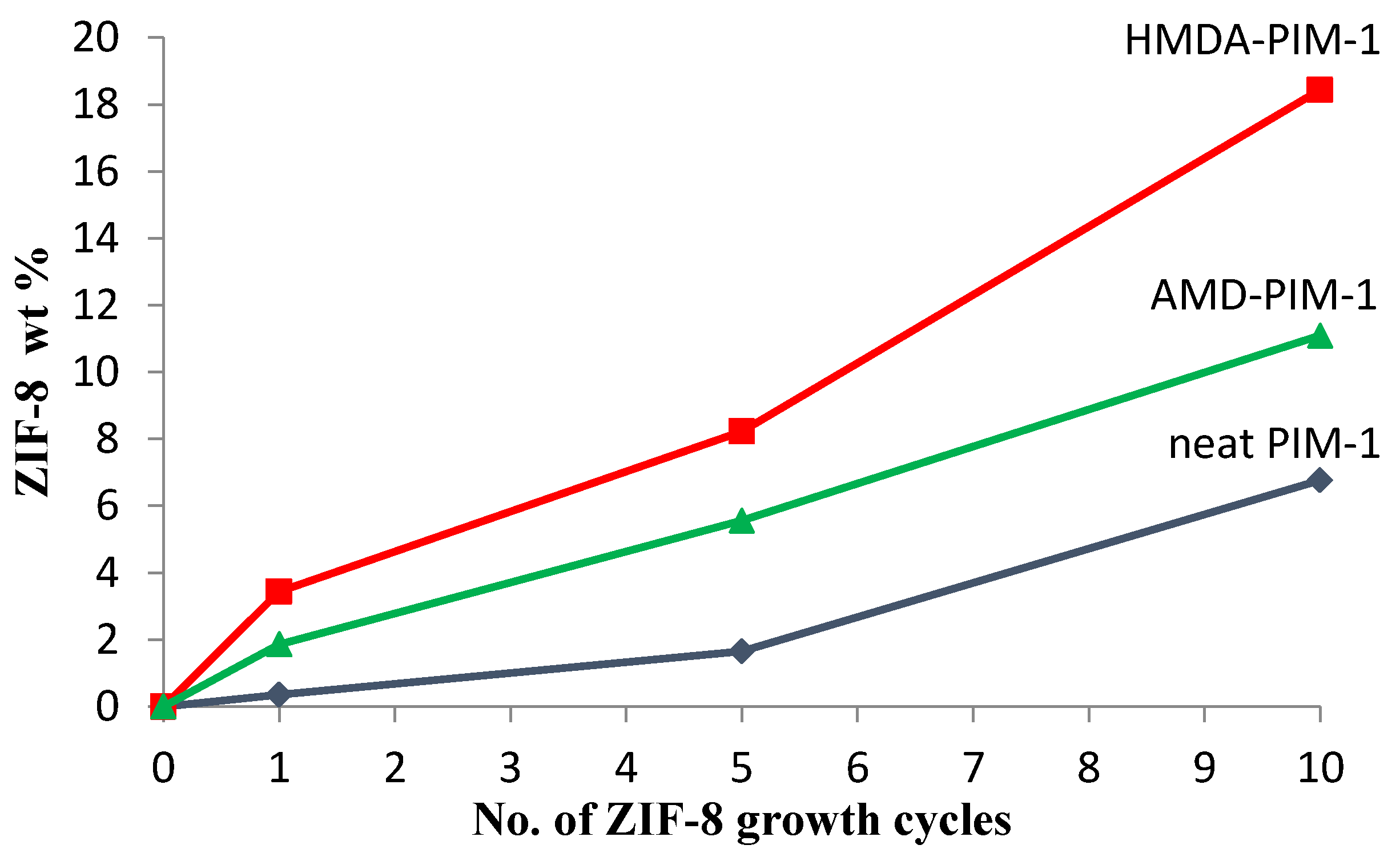

 ) and ZIF-8/PIM-1 after 1(
) and ZIF-8/PIM-1 after 1(  ), 5(
), 5(  ) and 10(
) and 10(  ) growth cycles; Green series: AMD-PIM1 (
) growth cycles; Green series: AMD-PIM1 (  ) and ZIF-8/AMD-PIM-1 after 1(
) and ZIF-8/AMD-PIM-1 after 1(  ), 5(
), 5(  ) and 10(
) and 10(  ) growth cycles; Blue series: HMDA-PIM1 (
) growth cycles; Blue series: HMDA-PIM1 (  ) and ZIF-8/HMDA-PIM-1 after 1(
) and ZIF-8/HMDA-PIM-1 after 1(  ), 5(
), 5(  ) and 10(
) and 10(  ) growth cycles.
) growth cycles.
 ) and ZIF-8/PIM-1 after 1(
) and ZIF-8/PIM-1 after 1(  ), 5(
), 5(  ) and 10(
) and 10(  ) growth cycles; Green series: AMD-PIM1 (
) growth cycles; Green series: AMD-PIM1 (  ) and ZIF-8/AMD-PIM-1 after 1(
) and ZIF-8/AMD-PIM-1 after 1(  ), 5(
), 5(  ) and 10(
) and 10(  ) growth cycles; Blue series: HMDA-PIM1 (
) growth cycles; Blue series: HMDA-PIM1 (  ) and ZIF-8/HMDA-PIM-1 after 1(
) and ZIF-8/HMDA-PIM-1 after 1(  ), 5(
), 5(  ) and 10(
) and 10(  ) growth cycles.
) growth cycles.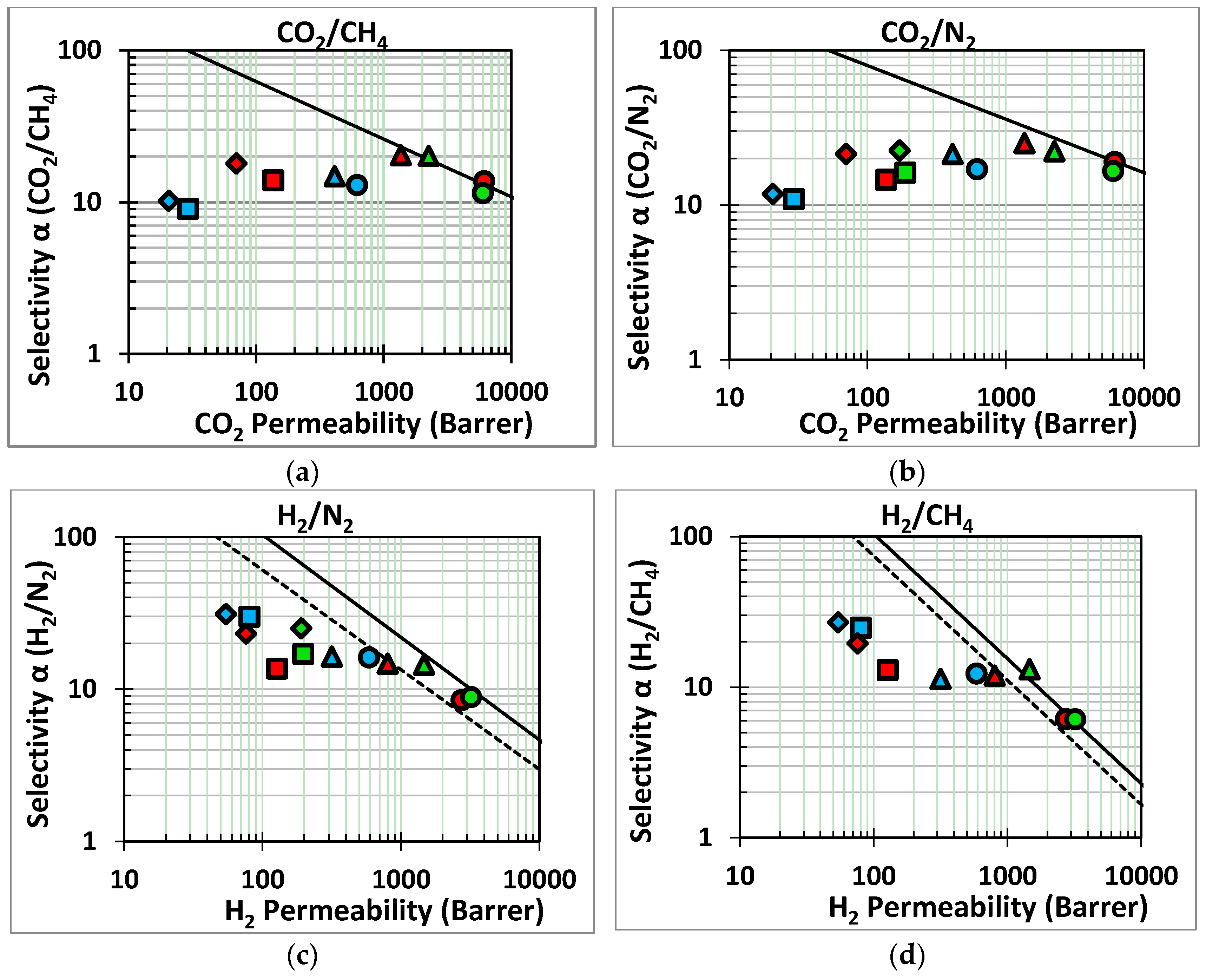
 ) and ZIF-8/PIM-1 after 1(
) and ZIF-8/PIM-1 after 1(  ), 5(
), 5(  ) and 10(
) and 10(  ) growth cycles.
) growth cycles.
 ) and ZIF-8/PIM-1 after 1(
) and ZIF-8/PIM-1 after 1(  ), 5(
), 5(  ) and 10(
) and 10(  ) growth cycles.
) growth cycles.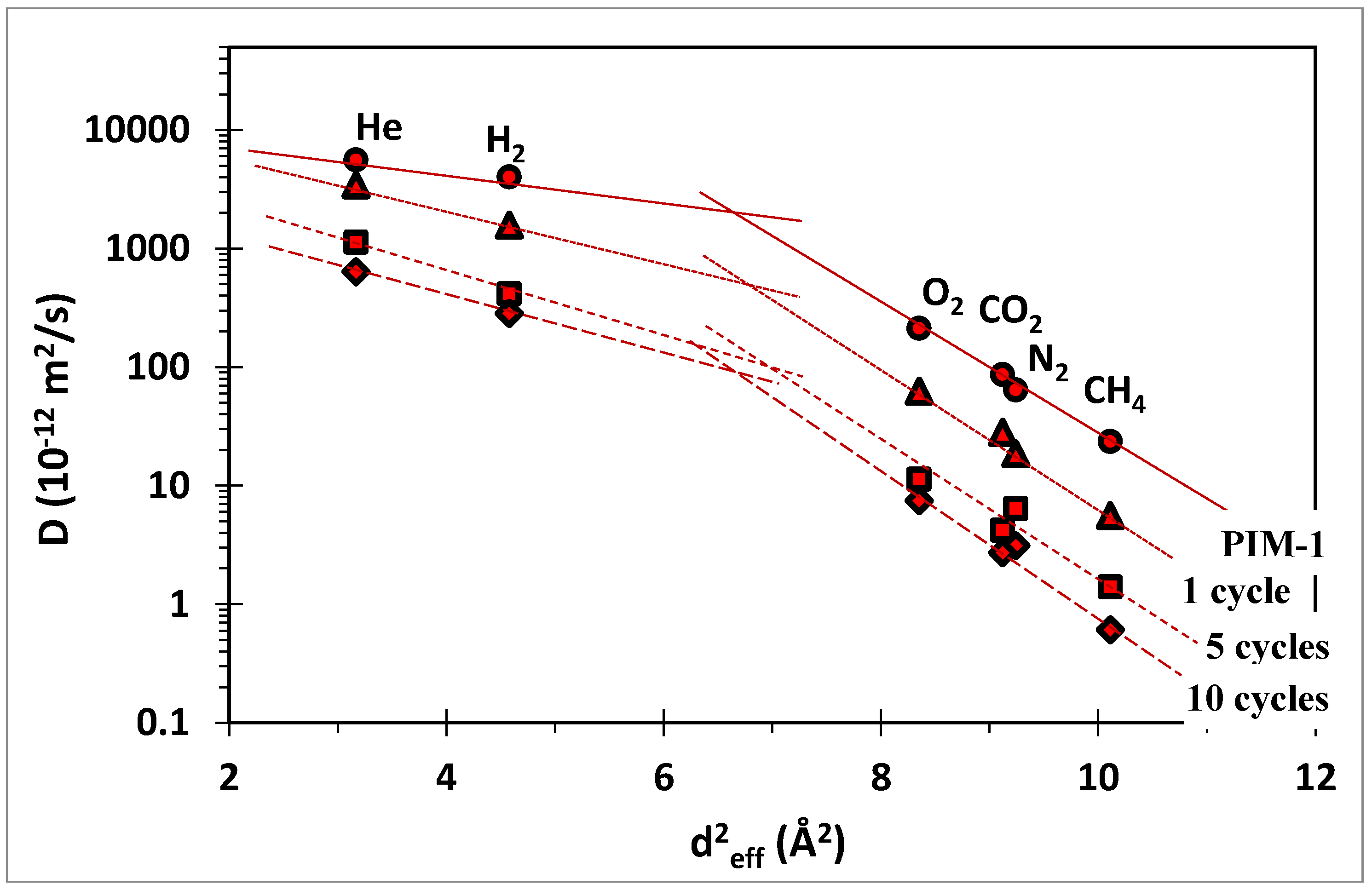
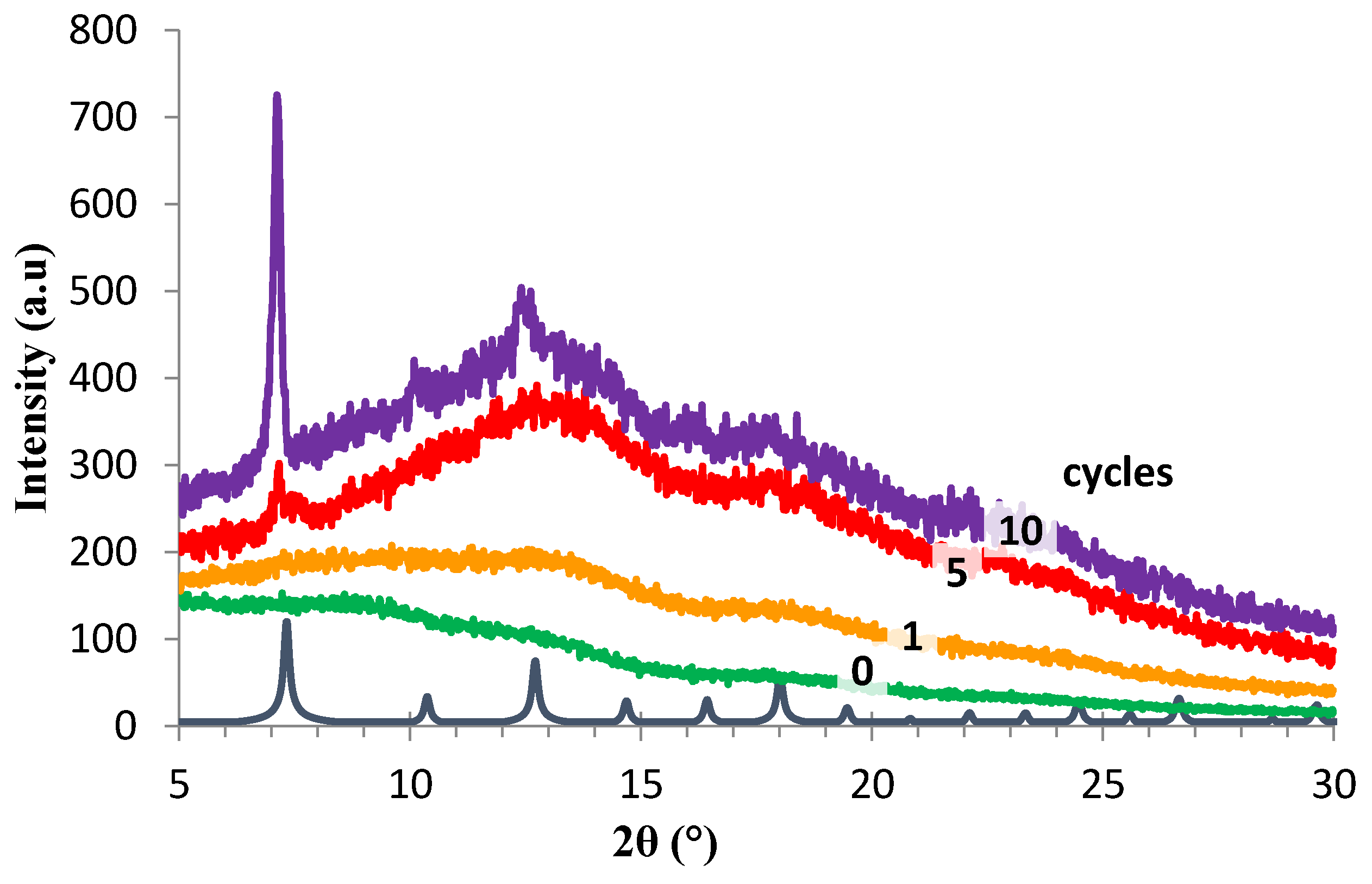
 ); orange series: HKUST-1/BTC/HMDA-PIM1 after 1(
); orange series: HKUST-1/BTC/HMDA-PIM1 after 1(  ), 3(
), 3(  ) and 5(
) and 5(  ) days of HKUST-1 growth; purple series: HKUST-1/HMDA-PIM1 after 3(
) days of HKUST-1 growth; purple series: HKUST-1/HMDA-PIM1 after 3(  ) and 5(
) and 5(  ) days of HKUST-1 growth.
) days of HKUST-1 growth.
 ); orange series: HKUST-1/BTC/HMDA-PIM1 after 1(
); orange series: HKUST-1/BTC/HMDA-PIM1 after 1(  ), 3(
), 3(  ) and 5(
) and 5(  ) days of HKUST-1 growth; purple series: HKUST-1/HMDA-PIM1 after 3(
) days of HKUST-1 growth; purple series: HKUST-1/HMDA-PIM1 after 3(  ) and 5(
) and 5(  ) days of HKUST-1 growth.
) days of HKUST-1 growth.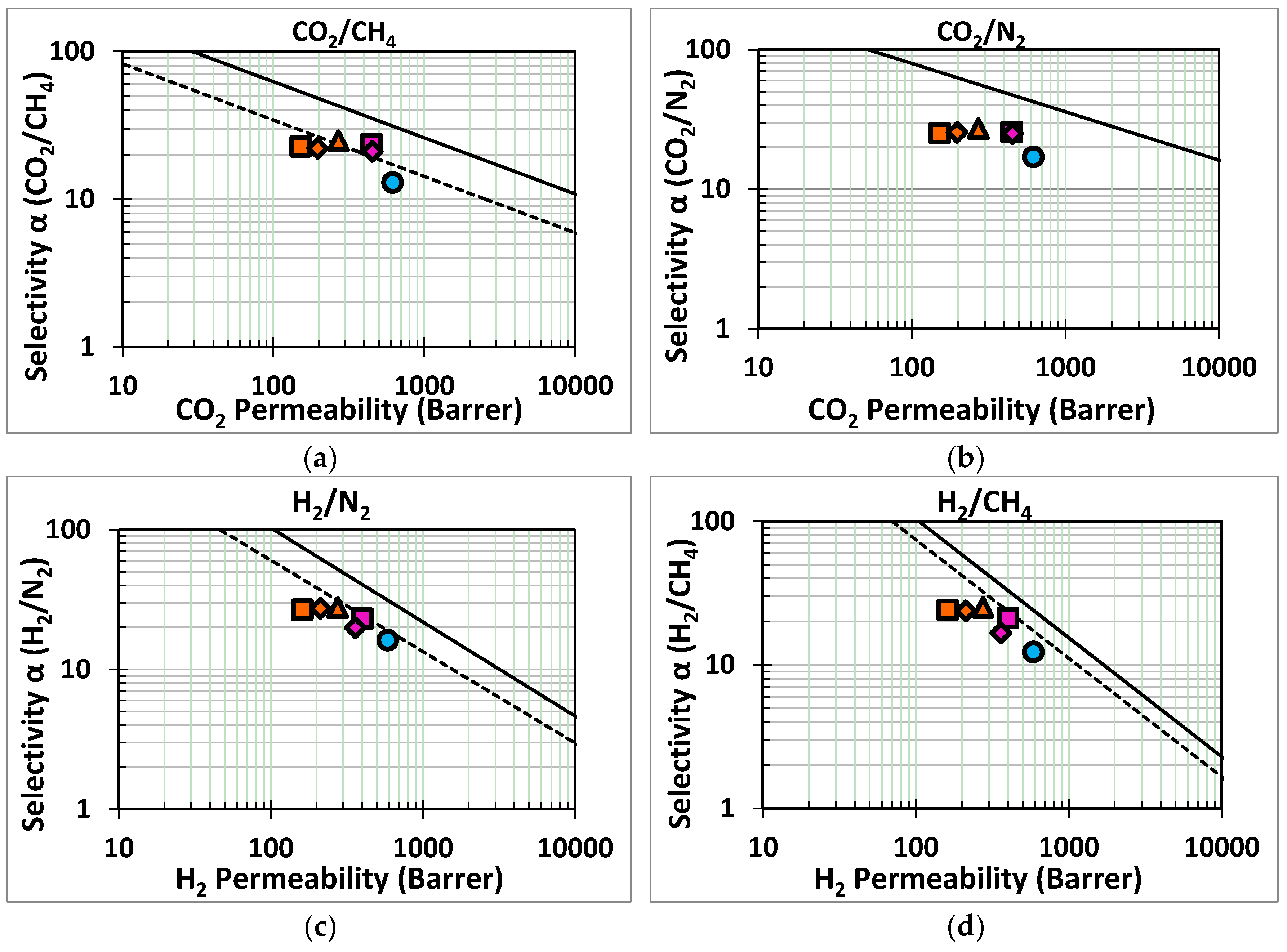
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuoco, A.; Khdhayyer, M.R.; Attfield, M.P.; Esposito, E.; Jansen, J.C.; Budd, P.M. Synthesis and Transport Properties of Novel MOF/PIM-1/MOF Sandwich Membranes for Gas Separation. Membranes 2017, 7, 7. https://doi.org/10.3390/membranes7010007
Fuoco A, Khdhayyer MR, Attfield MP, Esposito E, Jansen JC, Budd PM. Synthesis and Transport Properties of Novel MOF/PIM-1/MOF Sandwich Membranes for Gas Separation. Membranes. 2017; 7(1):7. https://doi.org/10.3390/membranes7010007
Chicago/Turabian StyleFuoco, Alessio, Muhanned R. Khdhayyer, Martin P. Attfield, Elisa Esposito, Johannes C. Jansen, and Peter M. Budd. 2017. "Synthesis and Transport Properties of Novel MOF/PIM-1/MOF Sandwich Membranes for Gas Separation" Membranes 7, no. 1: 7. https://doi.org/10.3390/membranes7010007