Performance of Hybrid Photocatalytic-Ceramic Membrane System for the Treatment of Secondary Effluent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
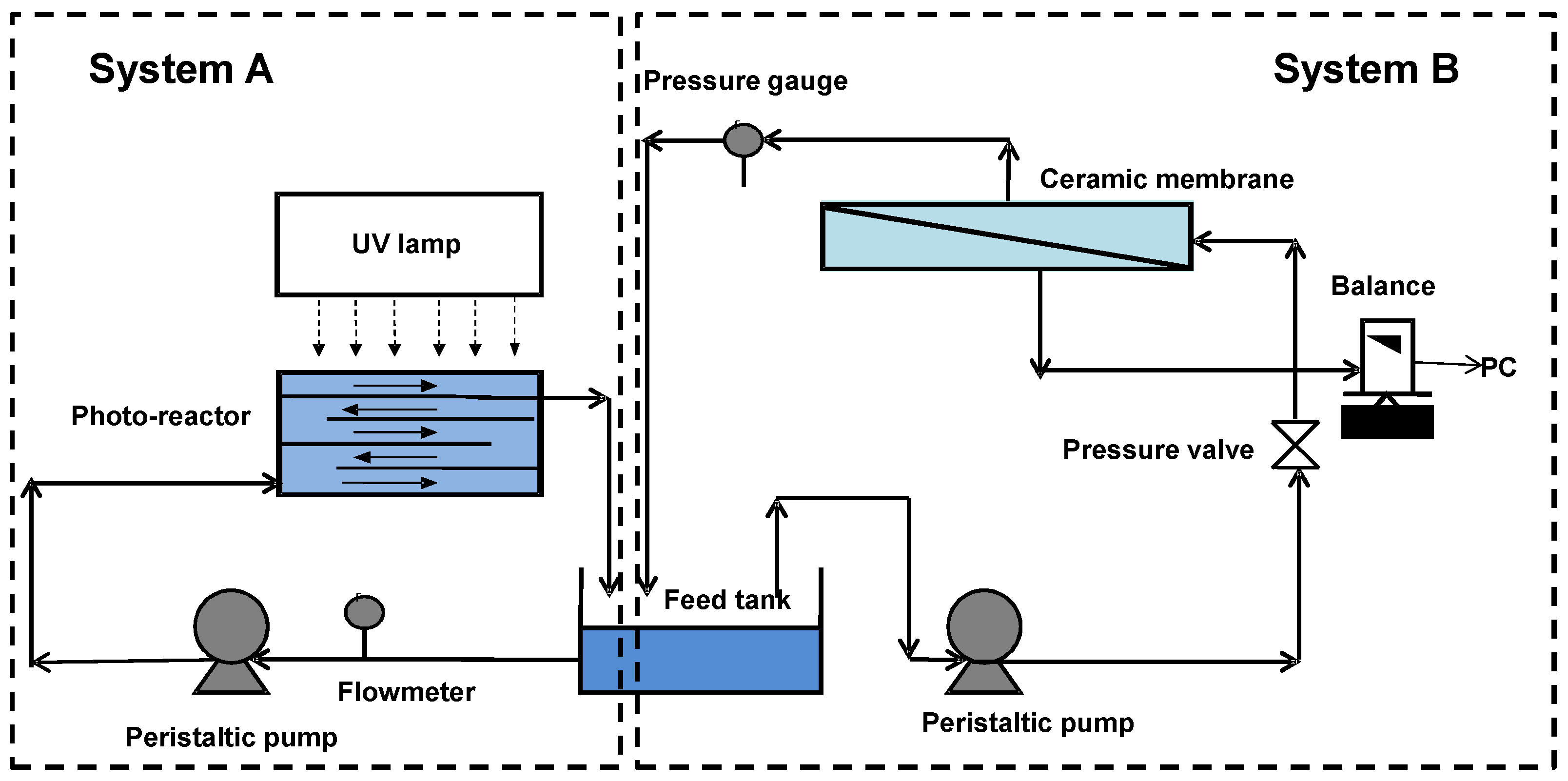
2.2. Apparatus
2.3. Experimental Procedures
2.4. Analyses
2.4.1. DOC and UV254 Measurements
2.4.2. LC-OCD Analysis
3. Results and Discussion
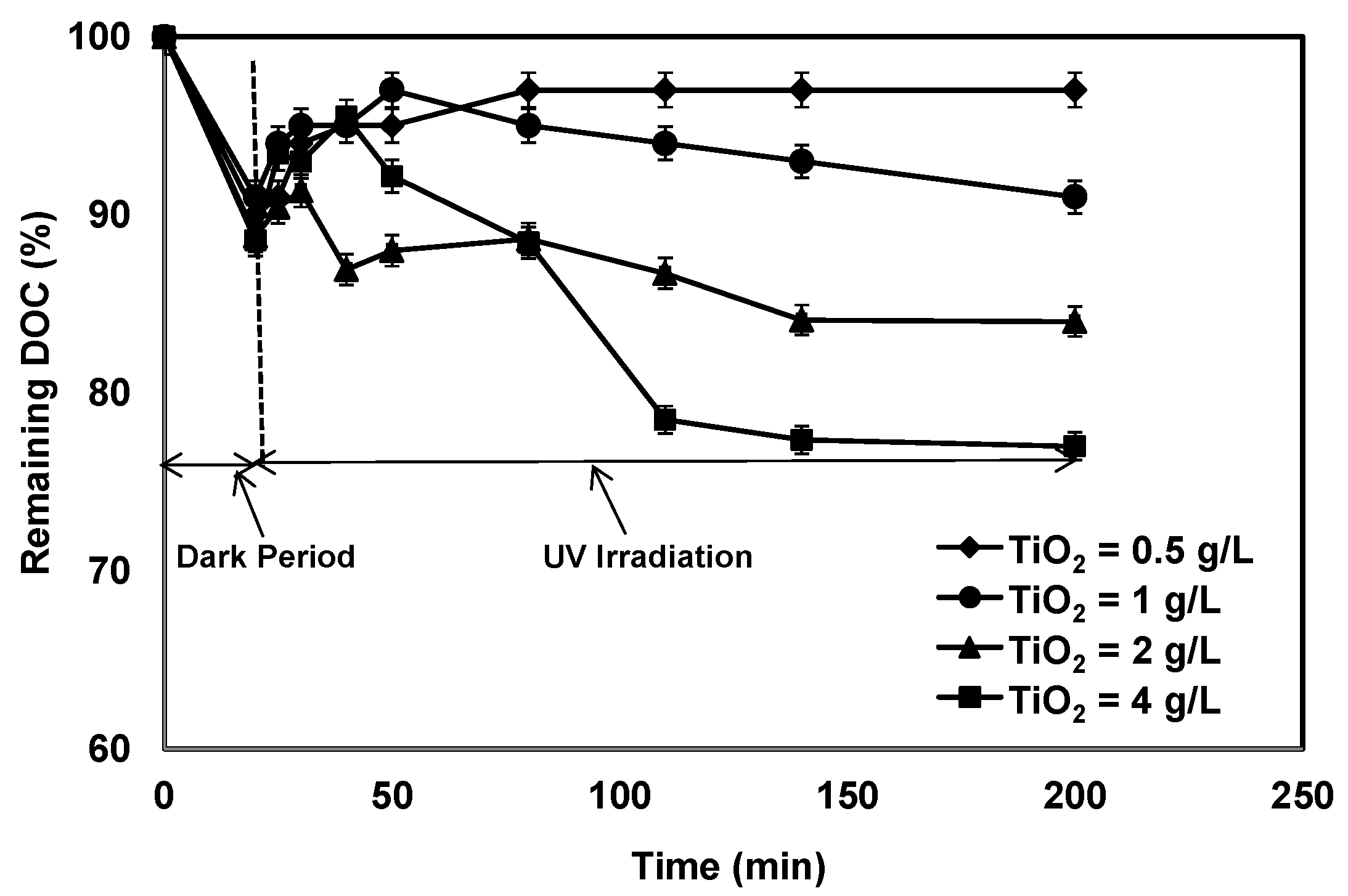
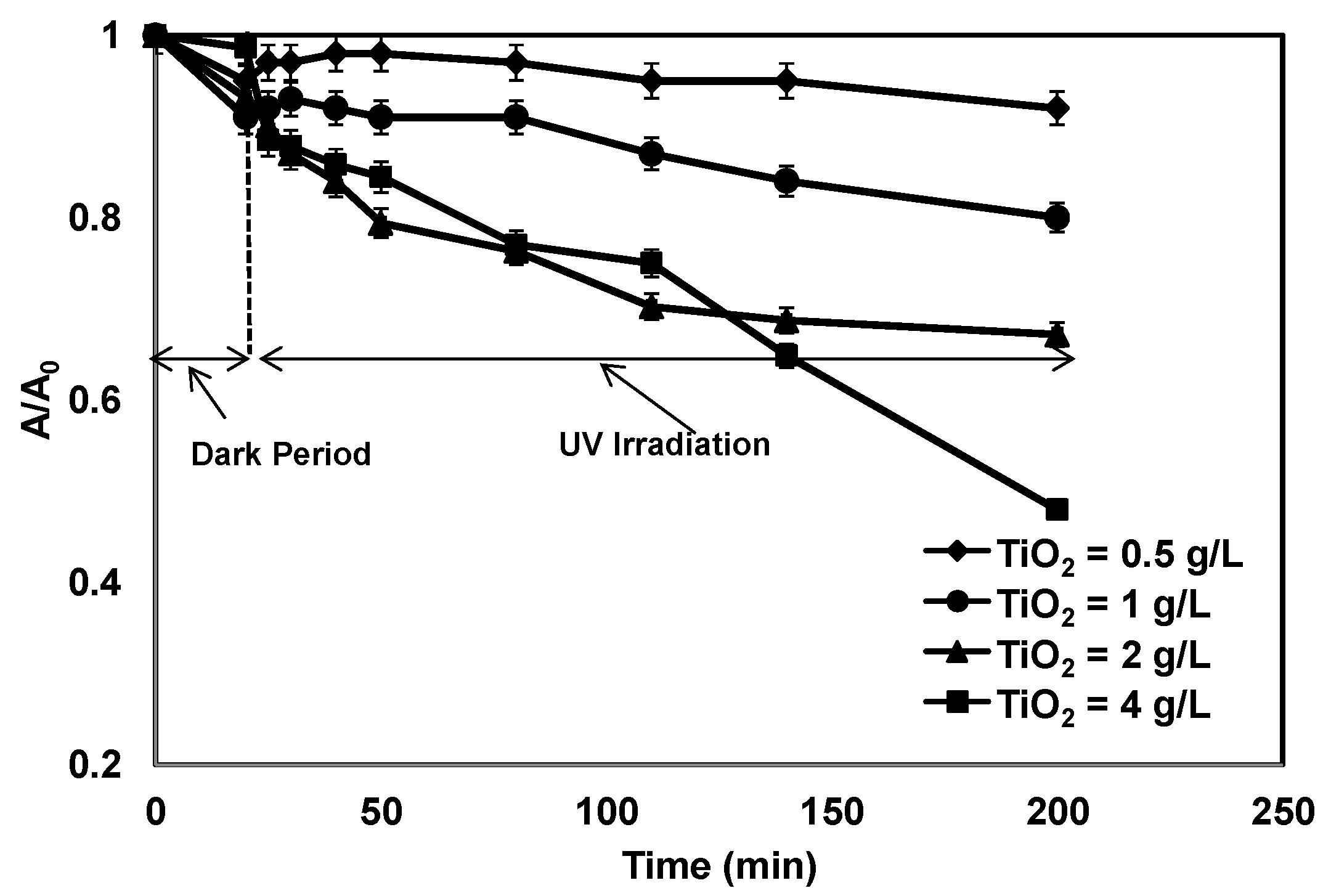
3.1. Effect of TiO2 Concentration on Photocatalytic Treatment
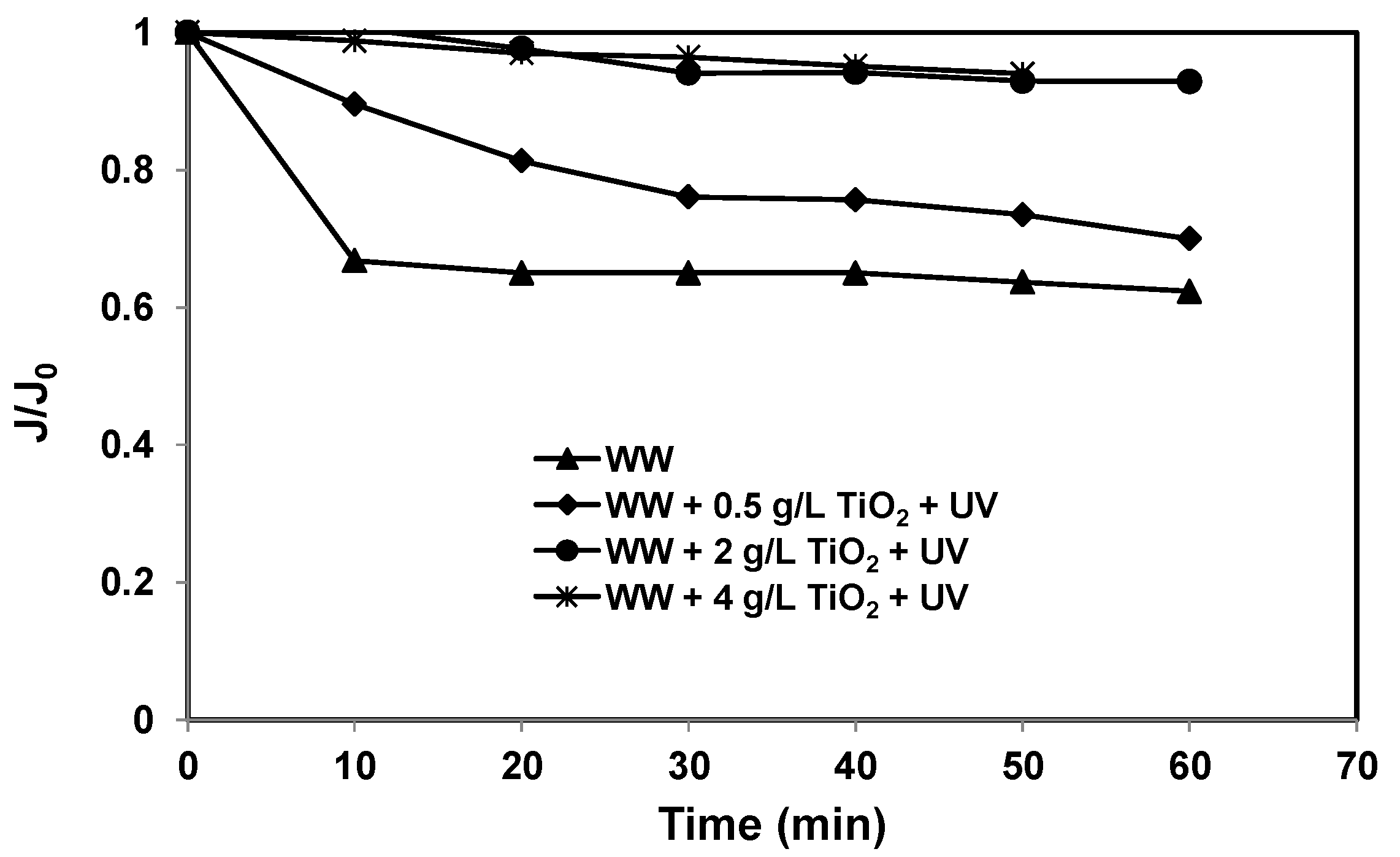
3.2. Effects of TiO2 Slurry Concentration on Membrane Flux and DOC and UV254 Removal
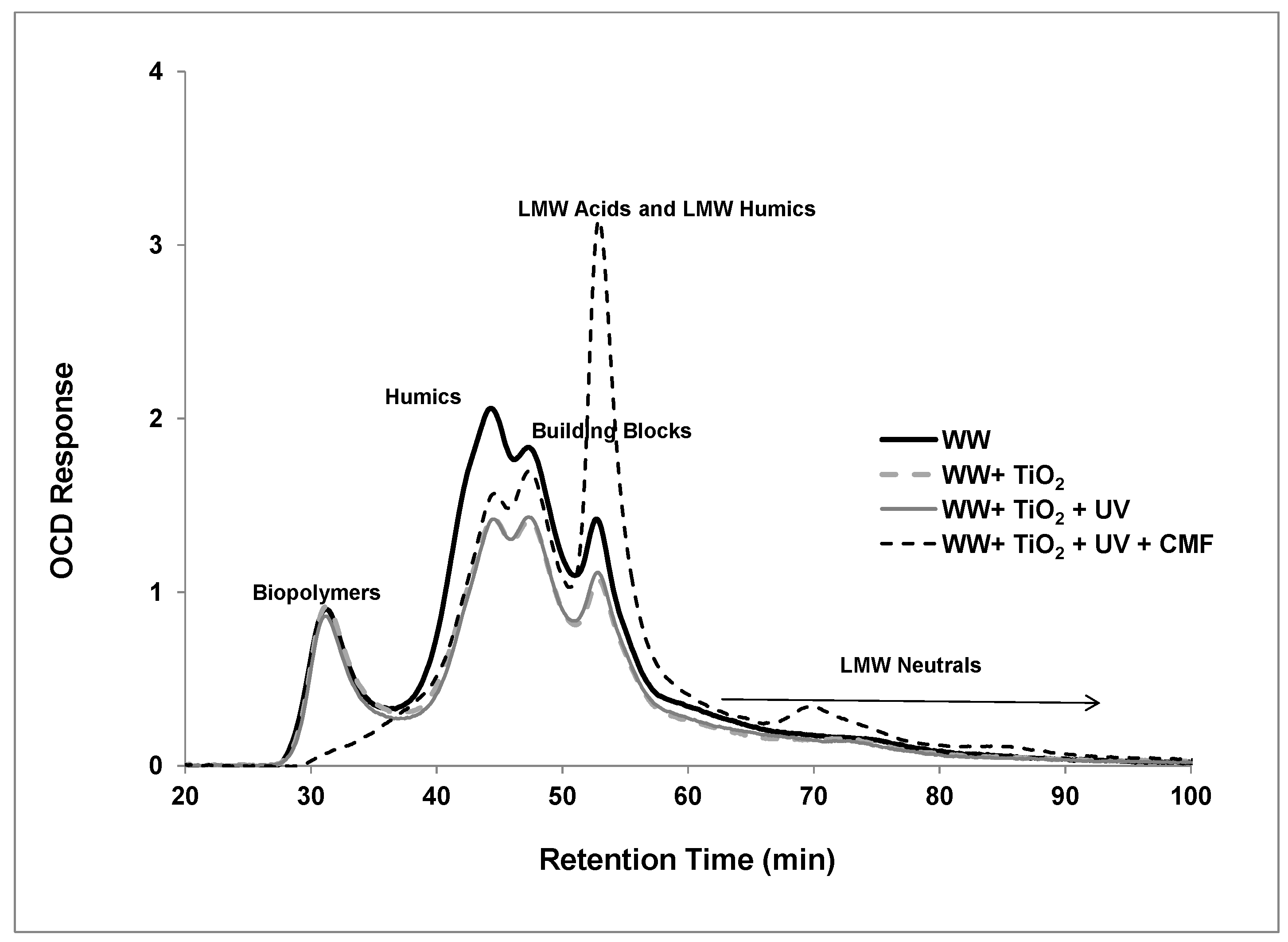
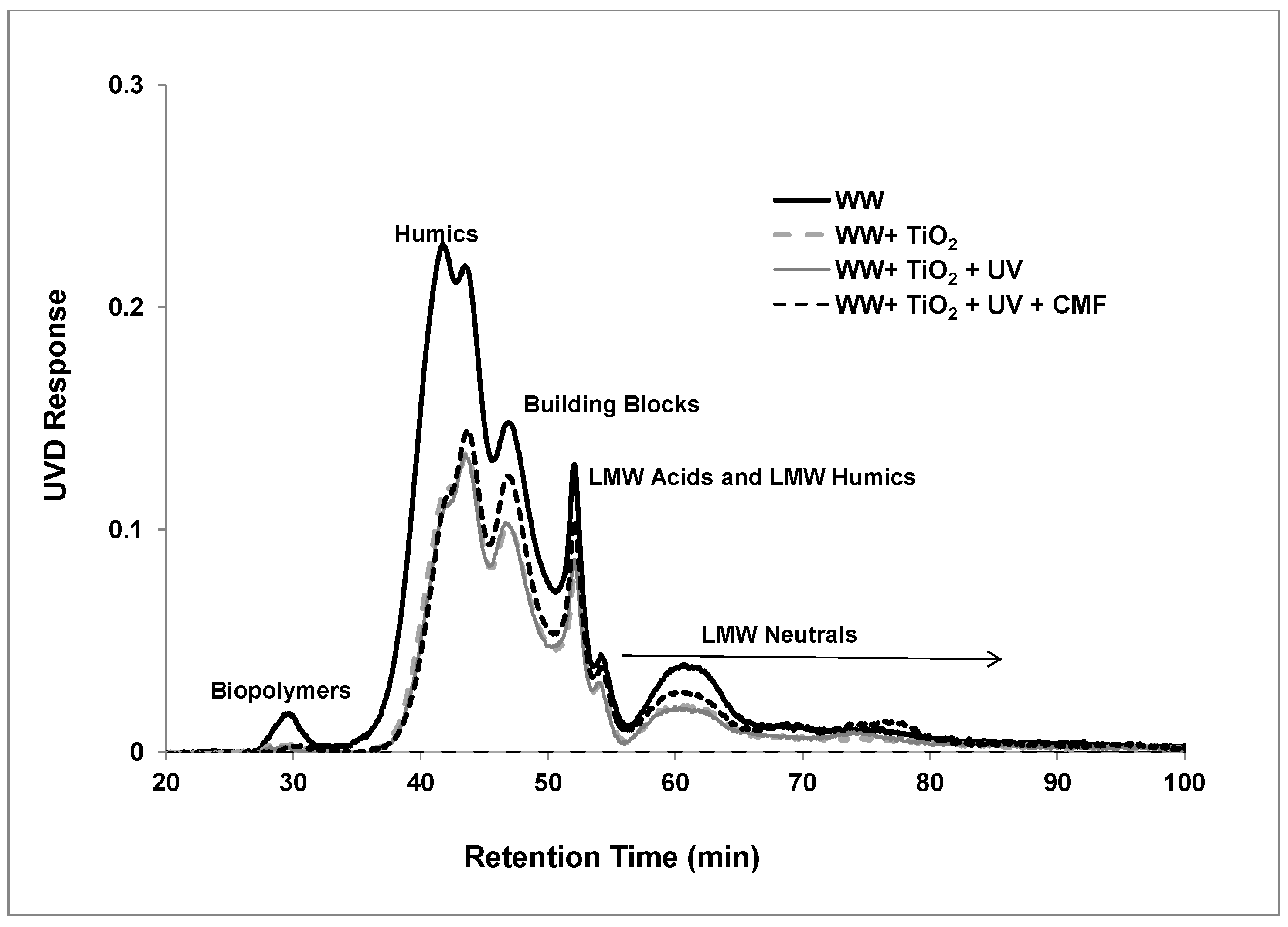
3.3. LC-OCD Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Puspita, P.; Roddick, F.A.; Porter, N.A. Decolourisation of secondary effluent by UV-mediated processes. Chem. Eng. J. 2011, 171, 464–473. [Google Scholar] [CrossRef]
- Mills., A.; Lee., S.-K. A web-based overview of semiconductor photochemistry-based current commercial applications. J. Photochem. Photobiol. A: Chem. 2002, 152, 233–247. [Google Scholar] [CrossRef]
- Patsios, S.I.; Sarasidis, V.C.; Karabelas, A.J. A hybrid photocatalysis–ultrafiltration continuous process for humic acids degradation. Sep. Purif. Technol. 2013, 104, 333–341. [Google Scholar] [CrossRef]
- Shon, H.K.; Vigneswaran, S.; Ngo, H.H.; Kim, J.H. Chemical coupling of photocatalysis with flocculation and adsorption in the removal of organic matter. Water Res. 2005, 39, 2549–2558. [Google Scholar] [CrossRef] [PubMed]
- Shon, H.K.; Phuntsho, S.; Vigneswaran, S. Effect of photocatalysis on the membrane hybrid system for wastewater treatment. Desalination 2008, 225, 235–248. [Google Scholar] [CrossRef]
- Ho, D.P.; Vigneswaran, S.; Ngo, H.H. Photocatalysis-membrane hybrid system for organic removal from biologically treated sewage effluent. Sep. Purif. Technol. 2009, 68, 145–152. [Google Scholar] [CrossRef]
- Mozia, S.; Morawski, A.W. Hybridization of photocatalysis and membrane distillation for purification of wastewater. Catal. Today 2006, 118, 181–188. [Google Scholar] [CrossRef]
- Muthukumaran, S.; Song, L.; Zhu, B.; Myat, D.; Chen, J.-Y.; Gray, S.; Duke, M. UV/TiO2 photocatalytic oxidation of recalcitrant organic matter: Effect of salinity and pH. Water Sci. Technol. 2014, 70, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-A.; Choo, K.-H.; Lee, C.-H.; Lee, H.-I. Use of ultrafiltration membranes for the separation of TiO2 photocatalysts in drinking water treatment. Ind. Eng. Chem. Res. 2001, 40, 1712–1719. [Google Scholar] [CrossRef]
- Lin, C.; Lin, K.S. Photocatalytic oxidation of toxic organohalides with TiO2/UV: The effects of humic substances and organic mixtures. Chemosphere 2007, 66, 1872–1877. [Google Scholar] [CrossRef] [PubMed]
- Le-Clech, P.; Lee, E.K.; Chen, V. Hybrid photocatalysis/membrane treatment for surface waters containing low concentrations of natural organic matters. Water Res. 2006, 40, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Choo, K.-H.; Park, H.-S. Photocatalytic degradation of seawater organic matter using a submerged membrane reactor. J. Photochem. Photobiol. A: Chem. 2010, 216, 215–220. [Google Scholar] [CrossRef]
- Pidou, M.; Parsons, S.A.; Raymond, G.; Jeffrey, P.; Stephenson, T.; Jefferson, B. Fouling control of a membrane coupled photocatalytic process treating greywater. Water Res. 2009, 43, 3932–3939. [Google Scholar] [CrossRef] [PubMed]
- Muthukumaran, S.; Nguyen, D.A.; Baskaran, K. Performance evaluation of different ultrafiltration membranes for the reclamation and reuse of secondary effluent. Desalination 2011, 279, 383–389. [Google Scholar] [CrossRef]
- Lehman, S.G.; Liu, L. Application of ceramic membranes with pre-ozonation for treatment of secondary wastewater effluent. Water Res. 2009, 43, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Benotti, M.J.; Stanford, B.D.; Wert, E.C.; Snyder, S.A. Evaluation of a photocatalytic reactor membrane pilot system for the removal of pharmaceuticals and endocrine disrupting compounds from water. Water Res. 2009, 43, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations. Appl. Catal. B: Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Kormann, C.; Bahnemann, D.W.; Hoffmann, M.R. Photolysis of chloroform and other organic molecules in aqueous suspensions. Environ. Sci. Technol. 1991, 25. [Google Scholar] [CrossRef]
- Butler, E.C.; Davis, A.P. Photocatalytic oxidation in aqueous titanium dioxide suspensions: The influence of dissolved transition metals. J. Photochem. Photobiol. A: Chem. 1993, 70, 273–283. [Google Scholar] [CrossRef]
- Yawalkar, A.A.; Bhatkhande, D.S.; Pangarkar, V.G.; Beenackers, A.A.C.M. Solar assisted photochemical and photocatalytic degradation of phenol. J. Chem.Technol. Biotechnol 2001, 76, 363–370. [Google Scholar] [CrossRef]
- Xi, W.; Geissen, S.U. Separation of titanium dioxide from photo catalytically treated water by cross-flow microfiltration. Water Res. 2001, 35, 1256–1262. [Google Scholar] [CrossRef]
- Abdullah, M.; Low., G.K.-C.; Matthews, R.W. Effects of common inorganic anions on rates of photocatalytic oxidation of organic carbon over illuminated titanium dioxide. J. Phys. Chem. 1990, 94, 6820–6825. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, L.; Wei, M.; Chen, P.; Shan, G. Photolytic reaction mechanism and impacts of coexisting substances on photodegradation of bisphenol a by Bi2WO6 in water. Water Res. 2012, 46, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Zhu, B.; Gray, S.; Duke, M.; Muthukumaran, S. Hybrid processes combining photocatalysis and ceramic membrane filtration for degradation of humic acids in saline water. Membranes 2016, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Leal, M.; Li, Q. Degradation of natural organic matter by TiO2 photocatalytic oxidation and its effect on fouling of low-pressure membranes. Water Res. 2008, 42, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Kaneco, S.; Rahman, M.A.; Suzuki, T.; Katsumata, H.; Ohta, K. Optimization of solar photocatalytic degradation conditions of bisphenol a in water using titanium dioxide. J. Photochem. Photobiol. A: Chem. 2004, 163, 419–424. [Google Scholar] [CrossRef]
- Ates, N.; Kitis, M.; Yetis, U. Formation of chlorination by-products in waters with low SUVA—correlations with SUVA and differential UV spectroscopy. Water Res. 2007, 41, 4139–4148. [Google Scholar] [CrossRef] [PubMed]
- González, O.; Justo, A.; Bacardit, J.; Ferrero, E.; Malfeito, J.J.; Sans, C. Characterization and fate of effluent organic matter treated with UV/H2O2 and ozonation. Chem. Eng. J. 2013, 226, 402–408. [Google Scholar] [CrossRef]
- Liu, S.; Lim, M.; Fabris, R.; Chow, C.W.; Drikas, M.; Korshin, G.; Amal, R. Multi-wavelength spectroscopic and chromatography study on the photocatalytic oxidation of natural organic matter. Water Res. 2010, 44, 2525–2532. [Google Scholar] [CrossRef] [PubMed]
- Laabs, C.; Amy, G.; Jekel, M. Understanding the size and character of fouling-causing substances from effluent organic matter (EfOM) in low-pressure membrane filtration. Environ. Sci. Technol. 2006, 40, 4495–4499. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-H.; Hsieh, Y.-H.; Chen, L.-J. The heterogeneous photocatalytic degradation, intermediates and mineralization for the aqueous solution of cresols and nitrophenols. J. Hazard. Mater. 1998, 59, 251–260. [Google Scholar] [CrossRef]
| Parameters | Disinfected Secondary Effluent |
|---|---|
| pH | 7.5–7.6 |
| Conductivity (µS/cm) | 1700–1800 |
| Dissolved Organic Carbon (DOC) mg/L | 9–10 |
| Total Dissolved Solids (TDS) (mg/L) | 1000–1100 |
| UV 254 (cm−1) | 0.16–0.17 |
| True Colour (Pt-Co) | 25–27 |
| Turbidity (NTU) | 1.9–2.0 |
| Alkalinity (Bicarbonate Alkalinity (mg CaCO3/L)) | 120 * |
| Sulphate (mg/L) | 94 * |
| Calcium (mg/L) | 31 * |
| Magnesium (mg/L) | 26 * |
| Potassium (mg/L) | 27 * |
| Sodium (mg/L) | 271 * |
| Chloride (mg/L) | 420 * |
| Ammonia (mg/L) | 0.2 * |
| Total Nitrogen (mg/) | 11 * |
| Total Phosphorus (mg/L) | 9 * |
| Experimental Conditions | After Photocatalysis | After Hybrid System | ||
|---|---|---|---|---|
| DOC Removal (%) | UV 254 Removal (%) | DOC Removal (%) | UV 254 Removal (%) | |
| 0.5 g/L TiO2 | 3 | 2 | 18 | 10 |
| 2 g/L TiO2 | 16 | 33 | 36 | 52 |
| 4 g/L TiO2 | 23 | 52 | 60 | 54 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, L.; Zhu, B.; Gray, S.; Duke, M.; Muthukumaran, S. Performance of Hybrid Photocatalytic-Ceramic Membrane System for the Treatment of Secondary Effluent. Membranes 2017, 7, 20. https://doi.org/10.3390/membranes7020020
Song L, Zhu B, Gray S, Duke M, Muthukumaran S. Performance of Hybrid Photocatalytic-Ceramic Membrane System for the Treatment of Secondary Effluent. Membranes. 2017; 7(2):20. https://doi.org/10.3390/membranes7020020
Chicago/Turabian StyleSong, Lili, Bo Zhu, Stephen Gray, Mikel Duke, and Shobha Muthukumaran. 2017. "Performance of Hybrid Photocatalytic-Ceramic Membrane System for the Treatment of Secondary Effluent" Membranes 7, no. 2: 20. https://doi.org/10.3390/membranes7020020







