Fabrication of Defect-Free Cellulose Acetate Hollow Fibers by Optimization of Spinning Parameters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Spinning of CA Fibers
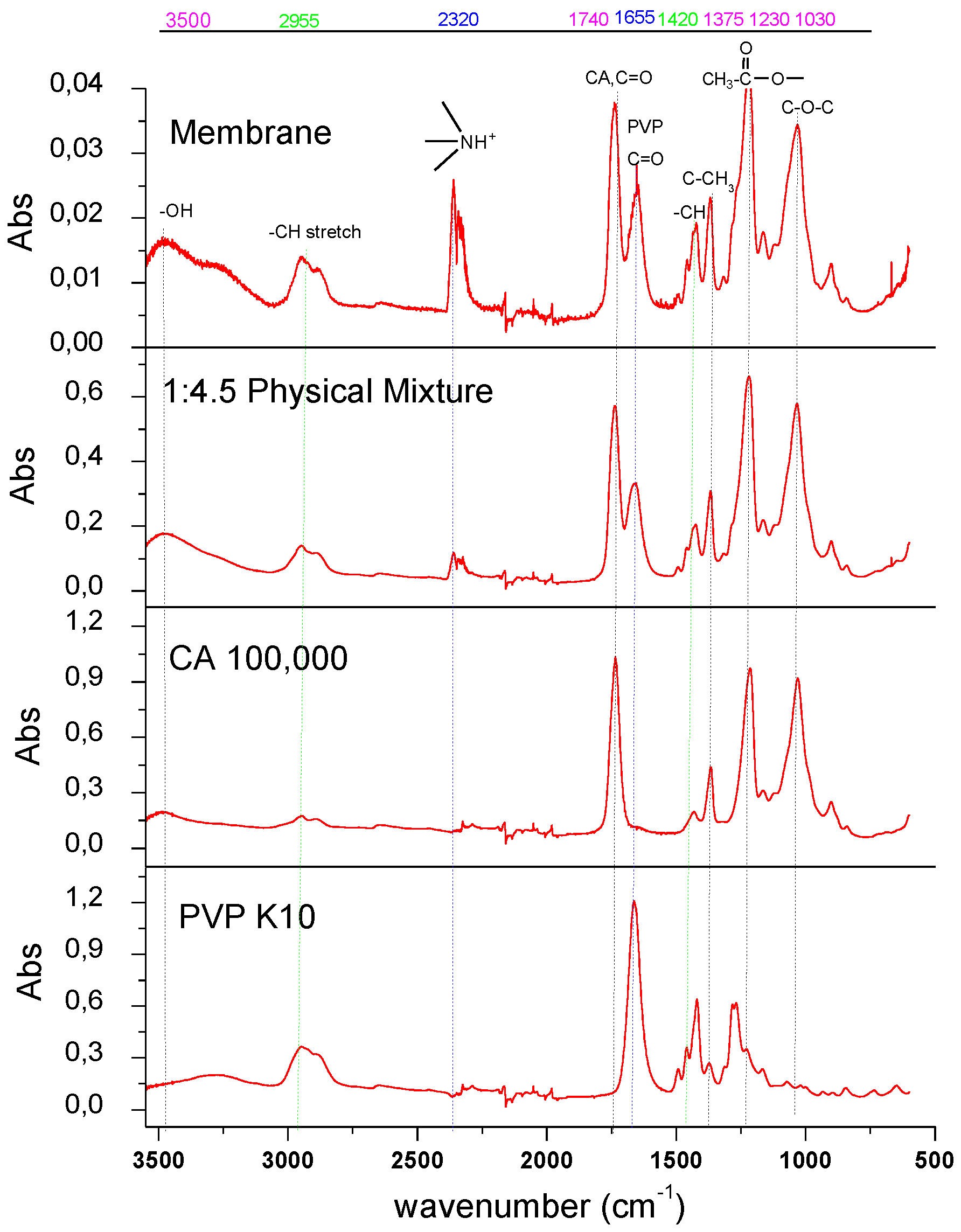
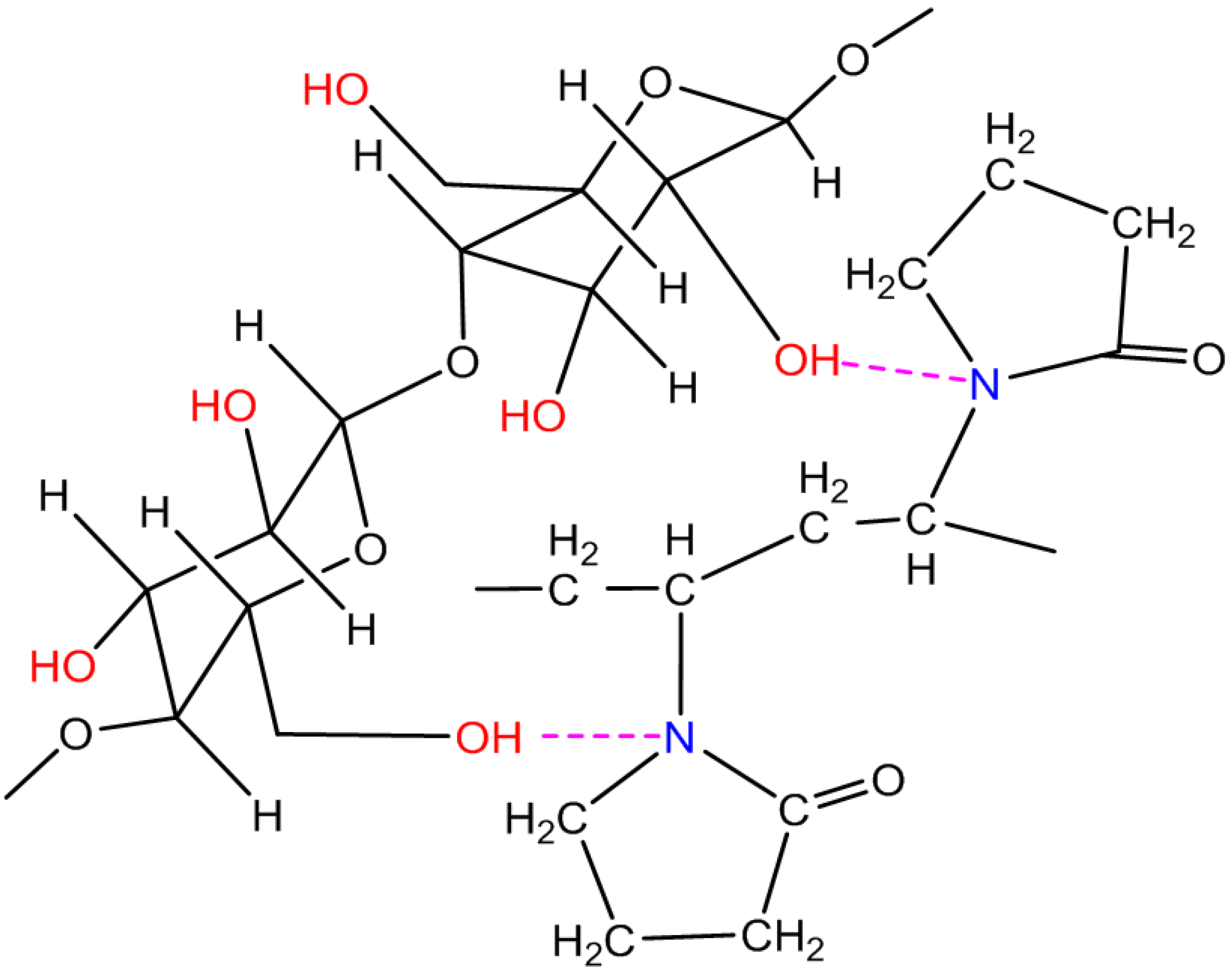
2.3. Measurement and Characterization
3. Results and Discussion
3.1. Experimental Results
3.2. Conjoint Analysis
3.3. Predictions
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Barsema, J.N. Carbon Membranes Precursor, Preparation And Functionalization. Ph.D. Thesis, University of Twente, Enschede, The Netherlands, 2007. [Google Scholar]
- Favvas, E.P.; Kapantaidakis, G.C.; Nolan, J.W.; Mitropoulos, A.C.; Kanellopoulos, N.K. Preparation, characterization and gas permeation properties of carbon hollow fiber membranes based on Matrimid(R) 5218 precursor. J. Mater. Process. Technol. 2007, 186, 102–110. [Google Scholar] [CrossRef]
- Lie, J.A. Synthesis, Performance and Regeneration of Carbon Membranes for Biogas Upgrading—A Future Energy Carrier. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2005. [Google Scholar]
- Saufi, S.M.; Ismail, A.F. Development and characterization of polyacrylonitrile (PAN) based carbon hollow fiber membrane. Songklanakarin J. Sci. Technol. 2002, 24, 843–854. [Google Scholar]
- Vu, D.Q.; Koros, W.J.; Miller, S.J. High Pressure CO2/CH4 Separation Using Carbon Molecular Sieve Hollow Fiber Membranes. Ind. Eng. Chem. Res. 2002, 41, 367–380. [Google Scholar] [CrossRef]
- David, L.I.B.; Ismail, A.F. Influence of the thermastabilization process and soak time during pyrolysis process on the polyacrylonitrile carbon membranes for O2/N2 separation. J. Membr. Sci. 2003, 213, 285–291. [Google Scholar] [CrossRef]
- Soffer, A.; Koresh, J.; Saggy, S. Separation Device. U.S. Patent 4,685,940, 11 August 1987. [Google Scholar]
- Sun, N.; Swatloski, R.P.; Maxim, M.L.; Rahman, M.; Harland, A.G.; Haque, A.; Spear, S.K.; Daly, D.T.; Rogers, R.D. Magnetite-embedded cellulose fibers prepared from ionic liquid. J. Mater. Chem. 2008, 18, 1–9. [Google Scholar] [CrossRef]
- Rosenau, T.; Potthast, A.; Sixta, H.; Kosma, P. The chemistry of side reactions and byproduct formation in the system NMMO/cellulose (Lyocell process). Prog. Polym. Sci. 2001, 26, 1763–1837. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of Cellose with Ionic Liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, J.; Zhang, J.; He, J. 1-Allyl-3-methylimidazolium Chloride Room Temperature Ionic Liquid: A New and Powerful Nonderivatizing Solvent for Cellulose. Macromolecules 2005, 38, 8272–8277. [Google Scholar] [CrossRef]
- Jie, X.; Cao, Y.; Qin, J.-J.; Liu, J.; Yuan, Q. Influence of drying method on morphology and properties of asymmetric cellulose hollow fiber membrane. J. Membr. Sci. 2005, 246, 157–165. [Google Scholar] [CrossRef]
- Qin, J.-J.; Li, Y.; Lee, L.-S.; Lee, H. Cellulose acetate hollow fiber ultrafiltration membranes made from CA/PVP 360 K/NMP/water. J. Membr. Sci. 2003, 218, 173–183. [Google Scholar] [CrossRef]
- Liu, H.; Hsieh, Y.-L. Ultrafine fibrous cellulose membranes from electrospinning of cellulose acetate. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 2119–2129. [Google Scholar] [CrossRef]
- Son, W.K.; Youk, J.H.; Lee, T.S.; Park, W.H. Electrospinning of Ultrafine Cellulose Acetate Fibers: Studies of a New Solvent System and Deacetylation of Ultrafine Cellulose Acetate Fibers. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 5–11. [Google Scholar] [CrossRef]
- Hao, J.-H.; Dai, H.-P.; Yang, P.-C.; Wei, J.-M.; Wang, Z. Cellulose acetate hollow fiber performance for ultra-low pressure reverse osmosis. Desalination 1996, 107, 217–221. [Google Scholar] [CrossRef]
- He, X.; Lie, J.A.; Sheridan, E.; Hägg, M.-B. Preparation and Characterization of Hollow Fiber Carbon Membranes from Cellulose Acetate Precursors. Ind. Eng. Chem. Res. 2011, 50, 2080–2087. [Google Scholar] [CrossRef]
- He, X.; Hägg, M.-B. Structural, kinetic and performance characterization of hollow fiber carbon membranes. J. Membr. Sci. 2012, 390–391, 23–31. [Google Scholar] [CrossRef]
- Chung, T.-S.; Hu, X. Effect of air-gap distance on the morphology and thermal properties of polyethersulfone hollow fibers. J. Appl. Polym. Sci. 1997, 66, 1067–1077. [Google Scholar] [CrossRef]
- Qin, J.-J.; Wong, F.-S.; Li, Y.; Liu, Y.-T. A high flux ultrafiltration membrane spun from PSU/PVP (K90)/DMF/1,2-propanediol. J. Membr. Sci. 2003, 211, 139–147. [Google Scholar] [CrossRef]
- Tang, H.T. Spectroscopic Identification for Organic Compounds; Beijing University Press: Beijing, China, 1992. [Google Scholar]
- SPSS Conjoint™ 17.0. Available online: http://www.sussex.ac.uk/its/pdfs/SPSS_Conjoint_17.0.pdf (accessed on 15 May 2017).
- He, X.; Hagg, M.-B. Optimization of Carbonization Process for Preparation of High Performance Hollow Fiber Carbon Membranes. Ind. Eng. Chem. Res. 2011, 50, 8065–8072. [Google Scholar] [CrossRef]



| Level | Bore Fluid Composition | Air Gap | Flow Rate of Bore Fluid * | Quench Bath Temp. |
|---|---|---|---|---|
| 1 | H2O | 15 mm | 20% | 20 °C |
| 2 | H2O + NMP (85%) | 25 mm | 40% | 50 °C |
| 3 | 35 mm | 60% |
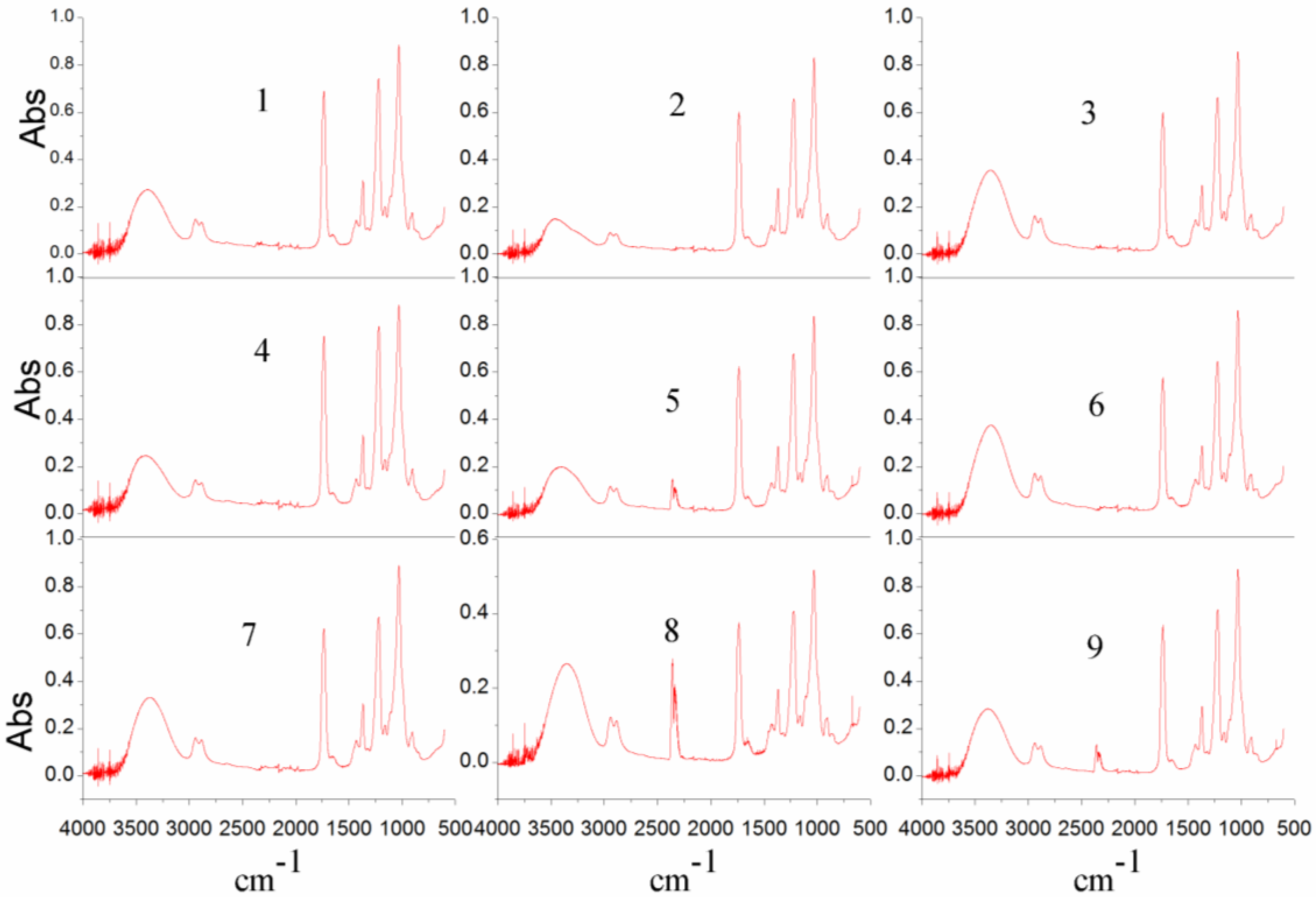
| No. | Bore Fluid | Air Gap (mm) | Flow Rate of Bore Fluid (%) * | Quench Bath Temperature (°C) | Cross-Linking Degree c | PVP Content (%) | Membrane Morphology |
|---|---|---|---|---|---|---|---|
| 1 | water | 15 | 40 | 20 | 5.19 | 9.41 |  |
| 2 | water | 35 | 20 | 50 | 3.14 | 9.01 |  |
| 3 | water + NMP (85%) | 15 | 60 | 50 | 3.68 | 9.11 |  |
| 4 | water | 25 | 60 | 20 | 5.63 | 10.08 |  |
| 5 | water + NMP (85%) | 35 | 40 | 20 | 8.61 | 8.59 |  |
| 6 | water + NMP (85%) | 25 | 20 | 20 | 3.03 | 8.16 |  |
| 7 | water | 25 | 40 | 50 | 4.07 | 9.41 |  |
| 8 | water | 15 | 20 | 20 | 21.47 | 10.83 |  |
| 9 | water | 35 | 60 | 20 | 7.34 | 7.75 |  |
| 10(a) | water | 15 | 60 | 20 | 2.79 | 7.72 |  |
| 11(a) | water + NMP (85%) | 25 | 60 | 20 | 7.17 | 10.37 |  |
| 12(b) | water | 25 | 40 | 20 | |||
| 13(b) | water + NMP (85%) | 25 | 40 | 20 |
| Factors and Levels | Utility | Averaged Importance Score (%) | |
|---|---|---|---|
| Bore fluid composition | water | −0.917 | 28.731 |
| water + NMP(85%) | 0.917 | ||
| Air gap | 15 mm | −1.111 | 29.467 |
| 25 mm | 0.889 | ||
| 35 mm | 0.222 | ||
| Flow rate of bore fluid | 20% | −0.778 | 27.860 |
| 40% | 0.889 | ||
| 60% | −0.111 | ||
| Quench bath Temp. | 20 °C | −0.750 | 13.942 |
| 50 °C | 0.750 | ||
| (Constant) | 5.556 | ||
| Case | Utility | Total Utility | Remarks | ||||
|---|---|---|---|---|---|---|---|
| Bore Fluid Composition | Air Gap | Flow Rate of Bore Fluid | Quench Bath Temp | Constant | |||
| 1 | water (−0.917) | 35 mm (0.222) | 60% (−0.111) | 20 °C (−0.75) | 5.556 | 4 | |
| 2 | water + NMP (85%) (0.917) | 25 mm (0.889) | 40% (0.889) | 50 °C (0.75) | 5.556 | 9.001 | Optimal |
| Card Number | Score | Maximum Utility(a) | BTL | Logit |
|---|---|---|---|---|
| 1 | 5.667 | 33.3% | 43.7% | 26.9% |
| 2 | 7.500 | 66.7% | 56.3% | 73.1% |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X. Fabrication of Defect-Free Cellulose Acetate Hollow Fibers by Optimization of Spinning Parameters. Membranes 2017, 7, 27. https://doi.org/10.3390/membranes7020027
He X. Fabrication of Defect-Free Cellulose Acetate Hollow Fibers by Optimization of Spinning Parameters. Membranes. 2017; 7(2):27. https://doi.org/10.3390/membranes7020027
Chicago/Turabian StyleHe, Xuezhong. 2017. "Fabrication of Defect-Free Cellulose Acetate Hollow Fibers by Optimization of Spinning Parameters" Membranes 7, no. 2: 27. https://doi.org/10.3390/membranes7020027






