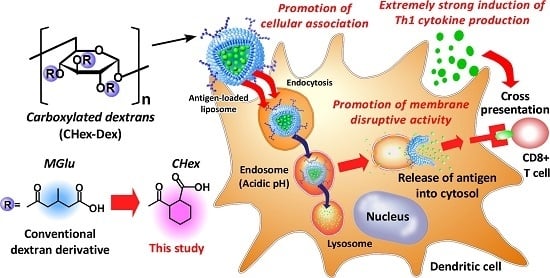
Development of pH-sensitive Dextran Derivatives with Strong Adjuvant Function and Their Application to Antigen Delivery
Abstract
:1. Introduction
2. Results and Discussion
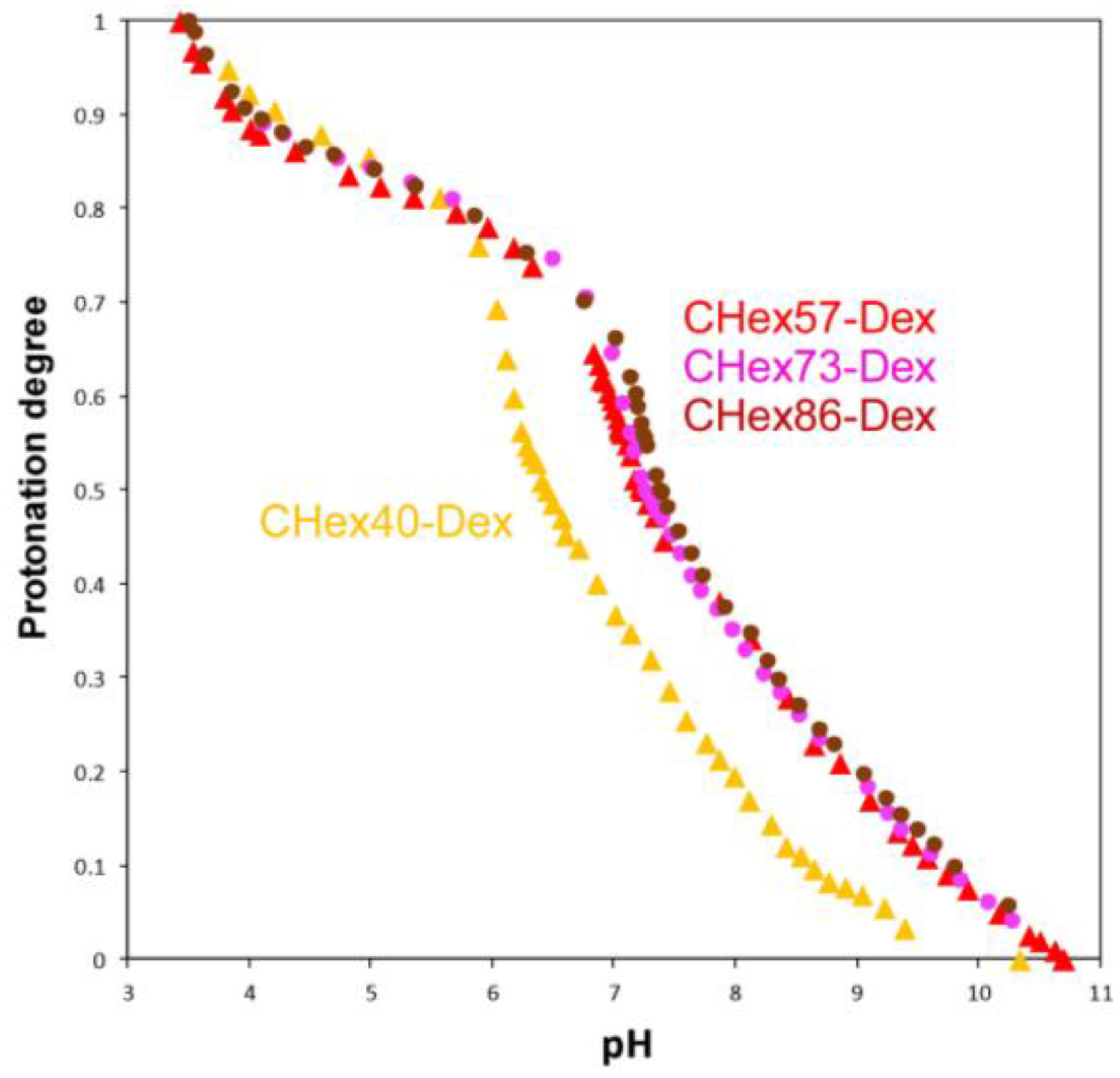
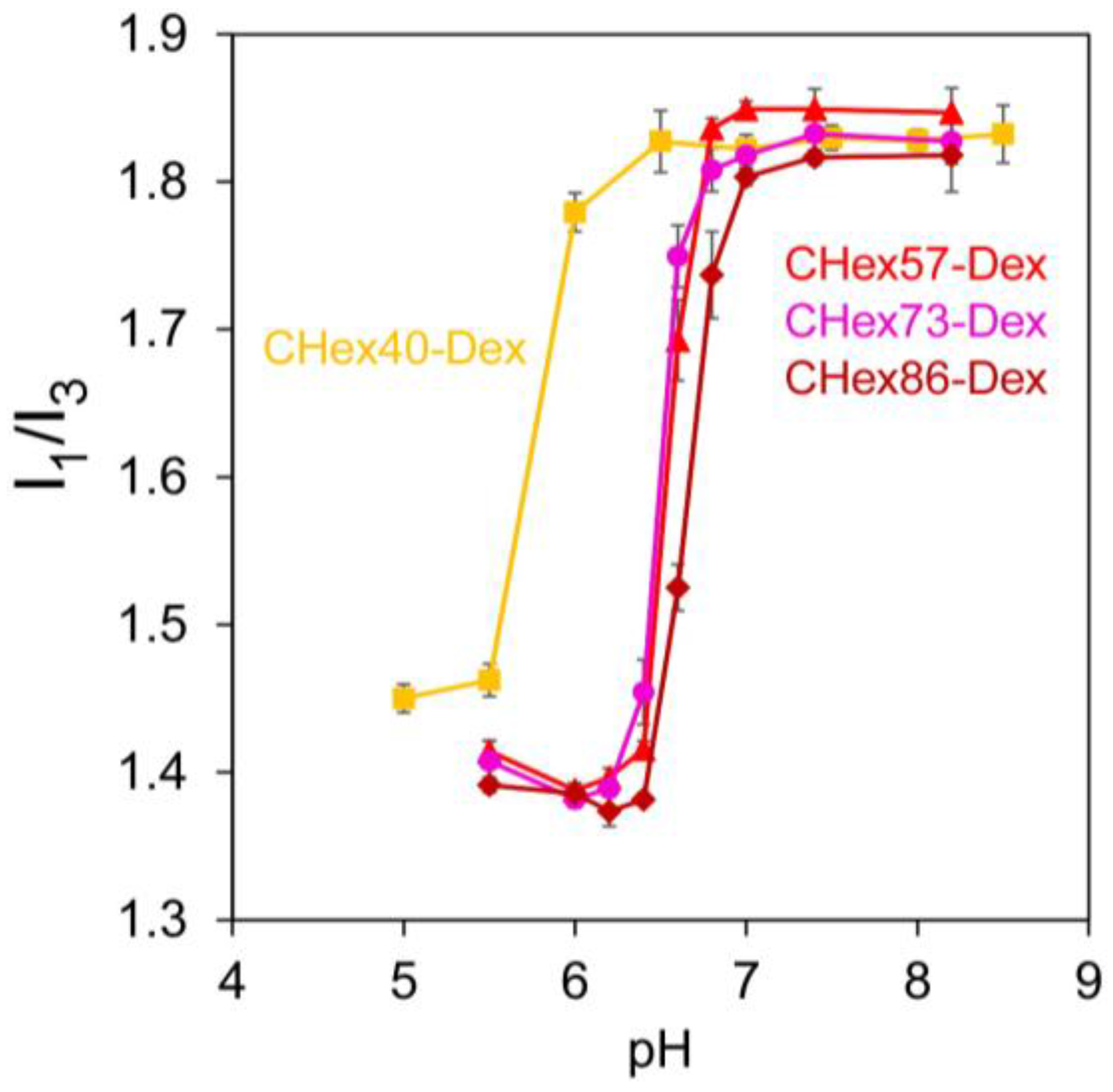
2.1. Characterization of Dextran Derivatives
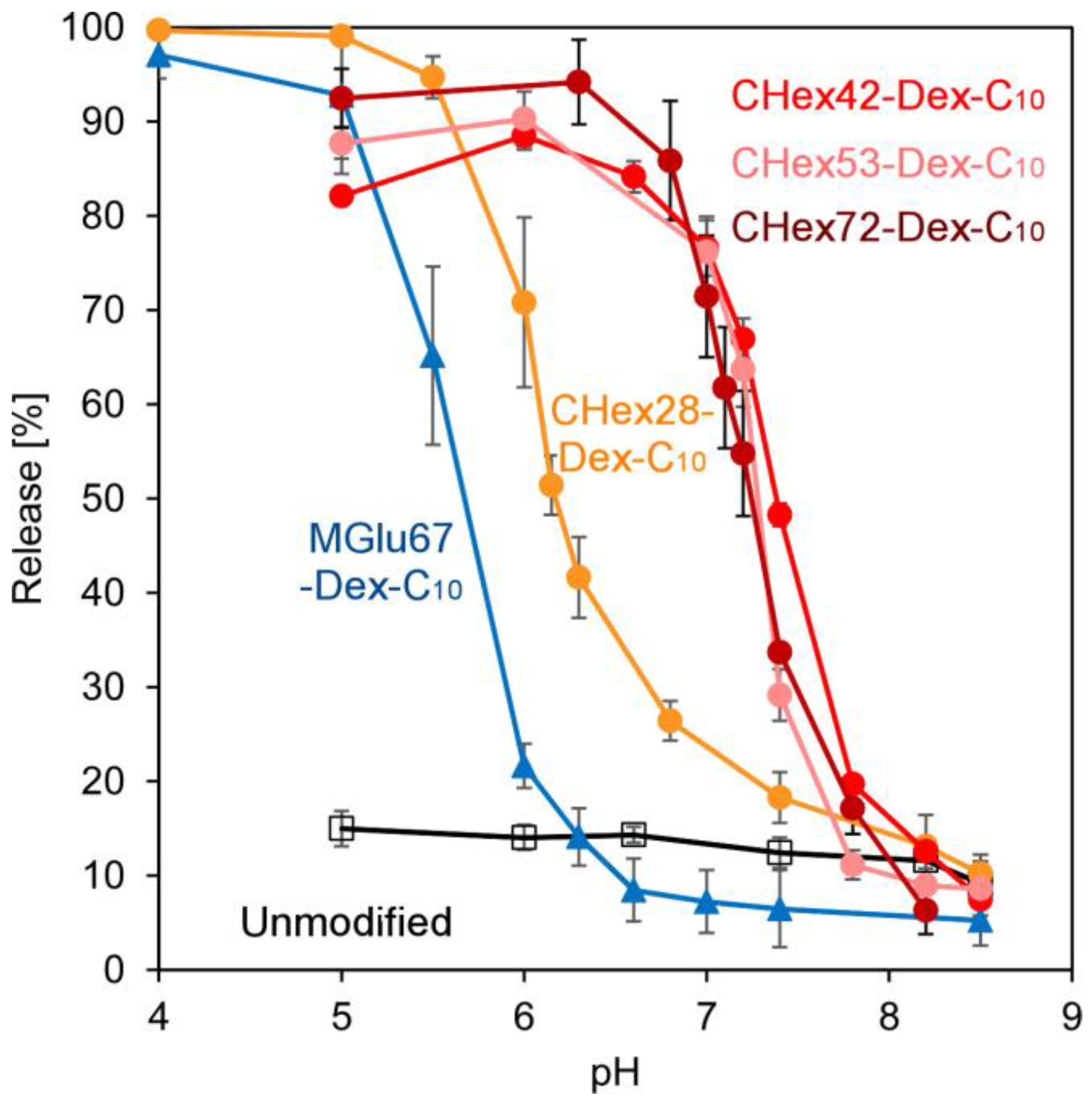
2.2. Preparation of Dextran Derivative-Modified Liposomes
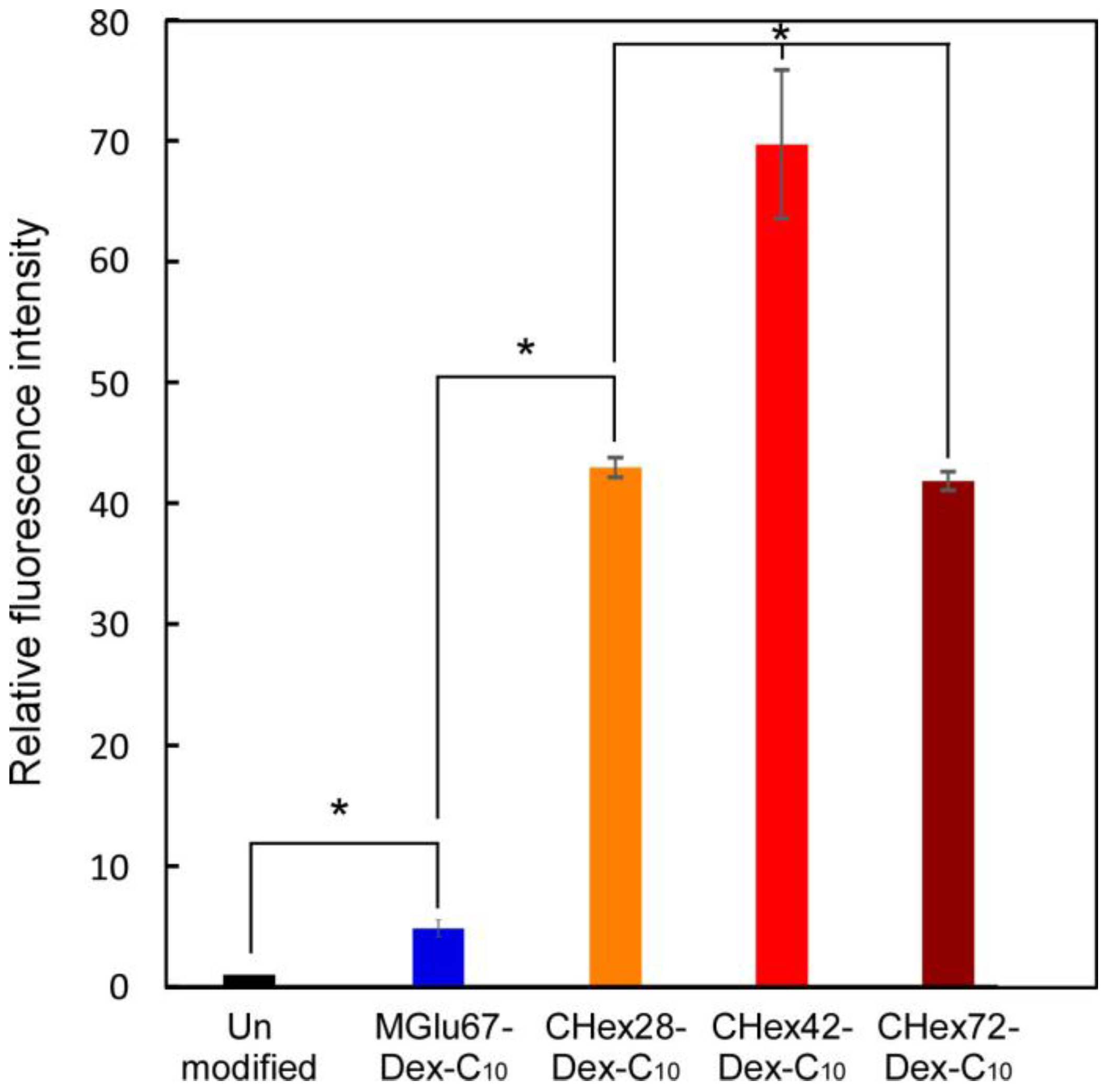
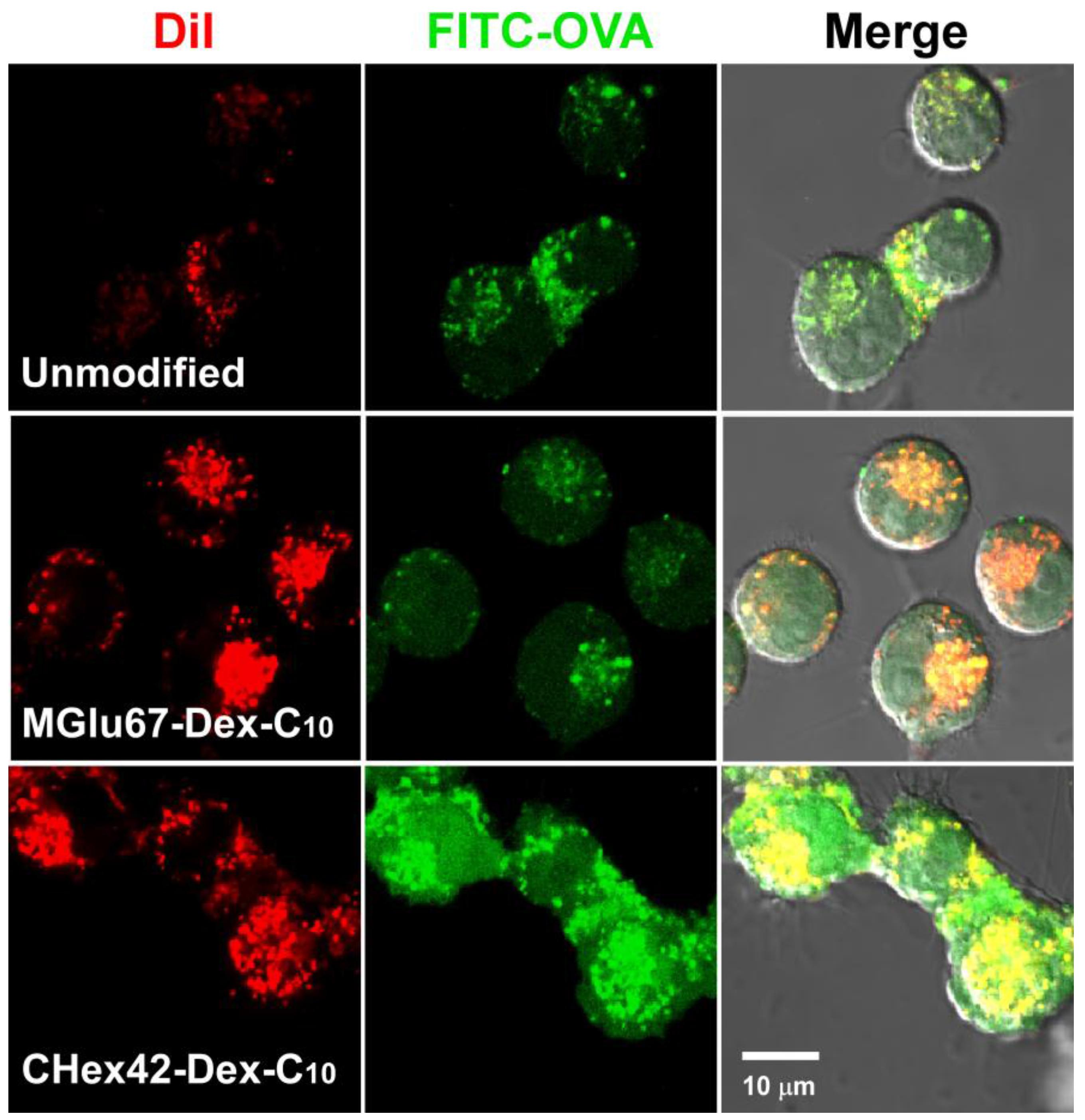
2.3. Interaction of Liposomes with Dendritic Cells
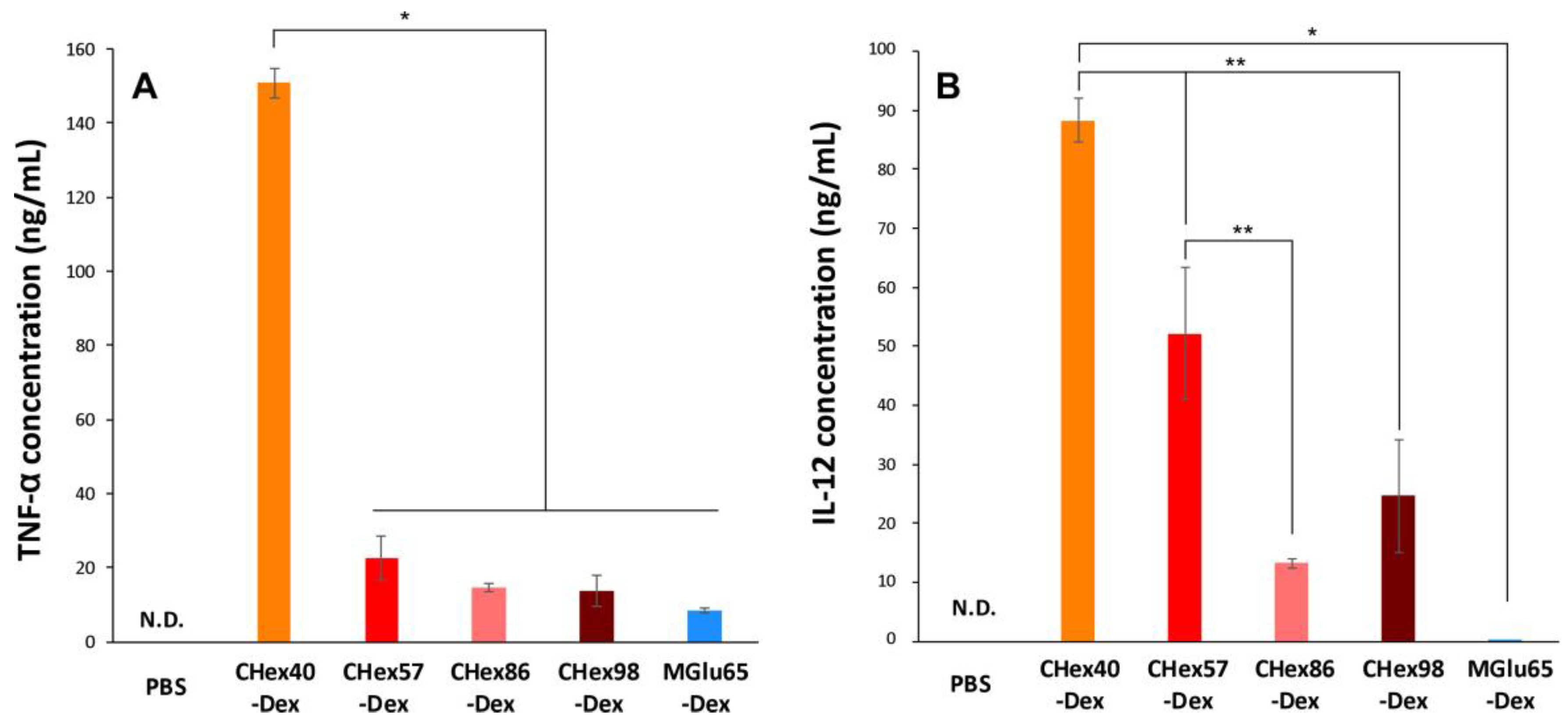
2.4. Activation of Dendritic Cells by Dextran Derivatives
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Dextran Derivatives
3.3. Titration
3.4. Pyrene Fluorescence
3.5. Preparation of Liposomes
3.6. Dynamic Light Scattering and Zeta Potential
3.7. Release of Pyranine from Liposome
3.8. Cell Culture
3.9. Cellular Association of Liposomes
3.10. Intracellular Behavior of Liposomes
3.11. Cytokine Production from Cells Treated with Dextran Derivatives
3.12. Statistical Analysis
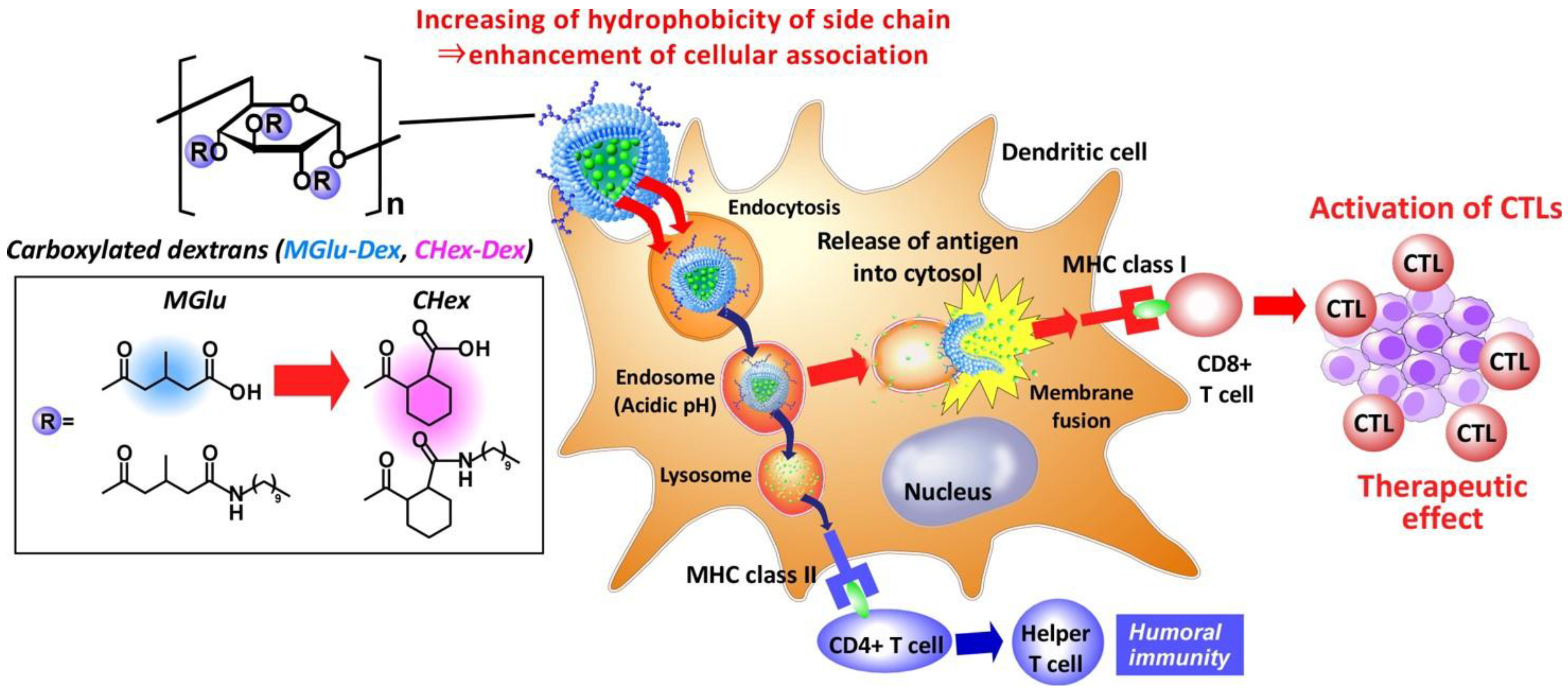
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gedye, C.; van der Westhuizen, A.; John, T. Checkpoint immunotherapy for cancer: Superior survival, unaccustomed toxicities. Intern. Med. J. 2015, 45, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.M.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Mellman, I.; Steinman, R.M. Dendritic cells: Specialized and regulated antigen processing machines. Cell 2001, 106, 255–258. [Google Scholar] [CrossRef]
- Joffre, O.P.; Segura, E.; Savina, A.; Amigorena, S. Cross-presentation by dendritic cells. Nat. Rev. Immunol. 2012, 12, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Panyam, J.; Zhou, W.Z.; Prabha, S.; Sahoo, S.K.; Labhasetwar, V. Rapid endo-lysosomal escape of poly(dl-lactide-co-glycolide) nanoparticles: Implications for drug and gene delivery. FASEB J. 2002, 16, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Bachelder, E.M.; Beaudette, T.T.; Broaders, K.E.; Fréchet, J.M.J.; Albrecht, M.T.; Mateczun, A.J.; Ainslie, K.M.; Pesce, J.T.; Keane-Myers, A.M. In vitro analysis of acetalated dextran microparticles as a potent delivery platform for vaccine adjuvants. Mol. Pharm. 2010, 7, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Boudier, A.; Aubert-Pouëssel, A.; Mebarek, N.; Chavanieu, A.; Quentin, J.; Martire, D.; Boukhaddaoui, H.; Gerardin, C.; Jorgensen, C.; Devoisselle, J.M. Development of tripartite polyion micelles for efficient peptide delivery into dendritic cells without altering their plasticity. J. Control. Release 2011, 154, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Bungener, L.; Serre, K.; Bijl, L.; Leserman, L.; Wilschut, J.; Daemen, T.; Machy, P. Virosome-mediated delivery of protein antigens to dendritic cells. Vaccine 2002, 20, 2287–2295. [Google Scholar] [CrossRef]
- Wang, C.; Li, P.; Liu, L.; Pan, H.; Li, H.; Cai, L.; Ma, Y. Self-adjuvanted nanovaccine for cancer immunotherapy: Role of lysosomal rupture-induced ROS in MHC class I antigen presentation. Biomaterials 2016, 79, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.; Wilson, J.T.; Patilea, G.I.; Kern, H.B.; Convertine, A.J.; Stayton, P.S. Neutral polymer micelle carriers with pH-responsive, endosome-releasing activity modulate antigen trafficking to enhance CD8+ T cell responses. J. Control. Release 2014, 191, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Moriguchi, R.; Kogure, K.; Shastri, N.; Harashima, H. Efficient MHC class I presentation by controlled intracellular trafficking of antigens in octaarginine-modified liposomes. Mol. Ther. 2008, 16, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Yuba, E.; Kojima, C.; Harada, A.; Tana; Watarai, S.; Kono, K. pH-Sensitive fusogenic polymer-modified liposomes as a carrier of antigenic proteins for activation of cellular immunity. Biomaterials 2010, 31, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Yuba, E.; Harada, A.; Sakanishi, Y.; Watarai, S.; Kono, K. A liposome-based antigen delivery system using pH-sensitive fusogenic polymers for cancer immunotherapy. Biomaterials 2013, 34, 3042–3052. [Google Scholar] [CrossRef] [PubMed]
- Yuba, E.; Tajima, N.; Yoshizaki, Y.; Harada, A.; Hayashi, H.; Kono, K. Dextran derivative-based pH-sensitive liposomes for cancer immunotherapy. Biomaterials 2014, 35, 3091–3101. [Google Scholar] [CrossRef] [PubMed]
- Yuba, E.; Yamaguchi, A.; Yoshizaki, Y.; Harada, A.; Kono, K. Bioactive polysaccharide-based pH-sensitive polymers for cytoplasmic delivery of antigen and activation of antigen-specific immunity. Biomaterials 2017, 120, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Murthy, N.; Robichaud, J.R.; Tirrell, D.A.; Stayton, P.S.; Hoffman, A.S. The design and synthesis of polymers for eukaryotic membrane disruption. J. Control. Release 1999, 61, 137–143. [Google Scholar] [CrossRef]
- Harada, A.; Teranishi, R.; Yuba, E.; Kono, K. Effect of the side chain spacer structure on the pH-responsive properties of polycarboxylates. J. Biomater. Sci. Polym. Ed. 2017, 28, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Swift, T.; Swanson, L.; Geoghegan, M.; Rimmer, S. The pH-responsive behaviour of poly(acrylic acid) in aqueous solution is dependent on molar mass. Soft Matter 2016, 12, 2542–2549. [Google Scholar] [CrossRef] [PubMed]
- Yessine, M.; Lafleur, M.; Meier, C.; Petereit, H.; Leroux, J. Characterization of the membrane-distabilizing properties of different pH-sensitive methacrylic acid copolymers. Biochim. Biophys. Acta 2003, 1613, 28–38. [Google Scholar] [CrossRef]
- Sakaguchi, N.; Kojima, C.; Harada, A.; Kono, K. Preparation of pH-sensitive poly(glycidol) derivatives with varying hydrophobicities: Their ability to sensitize stable liposomes to pH. Bioconjug. Chem. 2008, 19, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Yuba, E.; Harada, A.; Sakanishi, Y.; Kono, K. Carboxylated hyperbranched poly(glycidol)s for preparation of pH-sensitive liposomes. J. Control. Release 2011, 149, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Kalyanasundaram, K.; Thomas, J.K. Environmental effects on vibronic band intensities in pyrene monomer fluorescence and their application in studies of micellar systems. J. Am. Chem. Soc. 1977, 99, 2039–2044. [Google Scholar] [CrossRef]
- Thelen, T.; Hao, Y.; Medeiros, A.I.; Curtis, J.L.; Serezani, C.H.; Kobzik, L.; Lisa, L.K.; Aronoff, D.M. The class A scavenger receptor, macrophage receptor with collagenous structure, is the major phagocytic receptor for Clostridium sordellii expressed by human decidual macrophages. J. Immunol. 2010, 185, 4328–4335. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.L.; Pearce, S.F.; Francisco, L.M.; Sauter, B.; Roy, P.; Silverstein, R.L.; Bhardwaj, N. Immature dendritic cells phagocytose apoptotic cells via αvβ5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J. Exp. Med. 1998, 188, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Canton, J.; Neculai, D.; Grinstein, S. Scavenger receptors in homeostasis and immunity. Nat. Rev. Immunol. 2013, 13, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Peiser, L.; Mukhopadhyay, S.; Gordon, S. Scavenger receptors in innate immunity. Curr. Opin. Immunol. 2002, 14, 123–128. [Google Scholar] [CrossRef]
- Fujiwara, M.; Baldeschwieler, J.D.; Grubbs, R.H. Receptor-mediated endocytosis of poly(acrylic acid)-conjugated liposomes by macrophages. Biochim. Biophys. Acta 1996, 1278, 59–67. [Google Scholar] [CrossRef]
- Yuba, E.; Kojima, C.; Sakaguchi, N.; Harada, A.; Koiwai, K.; Kono, K. Gene delivery to dendritic cells mediated by complexes of lipoplexes and pH-sensitive fusogenic polymer-modified liposomes. J. Control. Release 2008, 130, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, X.; Cheng, Y.; Li, J.; Xiao, Y.; Zhang, Q.; Zong, A.; Zhong, C.; Wang, F. Preparation and in vitro immunomodulatory effect of curdlan sulfate. Carbohydr. Polym. 2014, 102, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Muta, T.; Yamazaki, S.; Takeshige, K. Activation of macrophages by linear (1/3)-β-d-glucans: Implications for the recognition of fungi by innate immunity. J. Biol. Chem. 2002, 277, 36825–36831. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, J.; Schmidt, C.; Mescher, M. The roles of IL-12 in providing a third signal for clonal expansion of naive CD8 T cells. J. Immunol. 2002, 169, 6842–6849. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Reznikoff, G.; Dranoff, G.; Rock, K.L. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J. Immunol. 1997, 158, 2723–2730. [Google Scholar] [PubMed]


| Polymer | Dextran (g) | LiCl (g) | DMF (mL) | CHex-Anhydride (g) | Reaction Temperature (°C) | Yield (g) | Yield (%) | CHex (%) 1 |
|---|---|---|---|---|---|---|---|---|
| CHex40-Dex | 0.508 | 0.505 | 18 | 2.507 | 75 | 0.981 | 90 | 40 |
| CHex57-Dex | 0.998 | 0.985 | 12 | 5.016 | 90 | 1.129 | 43 | 57 |
| CHex73-Dex | 1.500 | 1.501 | 29 | 14.999 | 75 | 3.250 | 70 | 73 |
| CHex86-Dex | 1.170 | 0.986 | 16 | 10.290 | 100 | 3.530 | 87 | 86 |
| CHex98-Dex | 0.900 | 0.908 | 16 | 9.007 | 90 | 2.317 | 67 | 98 |
| Polymer | CHex-Dex (g) | n-Decyl Amine (mg) | DMT-MM (mg) | Yield (g) | Yield (%) | CHex (%) 1 | Anchor (%) 1 |
|---|---|---|---|---|---|---|---|
| CHex28-Dex-C10 | 0.507 | 54 | 107 | 0.430 | 90 | 28 | 4 |
| CHex42-Dex-C10 | 0.816 | 86 | 174 | 0.794 | 95 | 51 | 6 |
| CHex53-Dex-C10 | 0.500 | 46 | 81 | 0.426 | 96 | 53 | 4 |
| CHex72-Dex-C10 | 1.214 | 95 | 68 | 0.532 | 43 | 76 | 5 |
| Polymer | pKa | Protonation Degree at pH 7.4 |
|---|---|---|
| CHex40-Dex | 6.42 | 0.27 |
| CHex57-Dex | 7.24 | 0.46 |
| CHex73-Dex | 7.25 | 0.46 |
| CHex86-Dex | 7.34 | 0.49 |
| MGlu13-Dex | 5.30 1 | 0.08 |
| MGlu48-Dex | 5.81 1 | 0.07 |
| MGlu76-Dex | 6.63 1 | 0.22 |
| Liposome | Size (nm) | Zeta Potential (mV) |
|---|---|---|
| Unmodified | 135 ± 3 | −10 ± 5 |
| CHex28-Dex-C10 | 118 ± 4 | −36 ± 0.2 |
| CHex42-Dex-C10 | 120 ± 7 | −31 ± 1 |
| CHex53-Dex-C10 | 112 ± 1 | −27 ± 1 |
| CHex72-Dex-C10 | 109 ± 1 | −37 ± 1 |
| MGlu67-Dex-C10 | 104 ± 1 | −33 ± 1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuba, E.; Uesugi, S.; Miyazaki, M.; Kado, Y.; Harada, A.; Kono, K. Development of pH-sensitive Dextran Derivatives with Strong Adjuvant Function and Their Application to Antigen Delivery. Membranes 2017, 7, 41. https://doi.org/10.3390/membranes7030041
Yuba E, Uesugi S, Miyazaki M, Kado Y, Harada A, Kono K. Development of pH-sensitive Dextran Derivatives with Strong Adjuvant Function and Their Application to Antigen Delivery. Membranes. 2017; 7(3):41. https://doi.org/10.3390/membranes7030041
Chicago/Turabian StyleYuba, Eiji, Shinya Uesugi, Maiko Miyazaki, Yuna Kado, Atsushi Harada, and Kenji Kono. 2017. "Development of pH-sensitive Dextran Derivatives with Strong Adjuvant Function and Their Application to Antigen Delivery" Membranes 7, no. 3: 41. https://doi.org/10.3390/membranes7030041