1. Introduction
From an environmental standpoint, CO
2 is one of the most significant greenhouse gases and its elimination is necessary, as it is responsible for global climate change [
1,
2].
The most common approach for CO
2 elimination is solvent absorption. High-fixed investment, operating cost, phase distribution, and the mass transfer surface limit are posing impediments to this process [
2,
3].
Recently, one of the techniques considered to be a suitable alternative for CO
2 separation is the execution of the membrane process without the necessity of phase change. This will provide the separation with less energy consumption [
4]. Nowadays, membrane processes are one of the most pioneering technologies for gas separation, specifically in the chemical industry, due to its lower processing and initial cost, lower energy consumption, and less space requirements [
5,
6,
7,
8]. The use of polymeric membranes in capturing CO
2 has drawn the attention of many scientists [
9,
10,
11]. This is due to their high permeability and/or selectivity. Presently, membranologists are trying to develop novel polymer structures to improve the membrane separation properties. However, permeability and selectivity behaviours of the usual polymeric membranes indicate trade-off trends [
10]. These years, studies on the structure of the membrane to increase their efficiency continue. Mixed matrix membranes (MMMs) are polymeric membranes filled with (nano)particles uniformly distributed in the polymer matrix to increase the membrane performance. The possibility of using organic or inorganic composite materials has been examined by researchers to improve the properties of the MMMs [
12,
13,
14,
15].
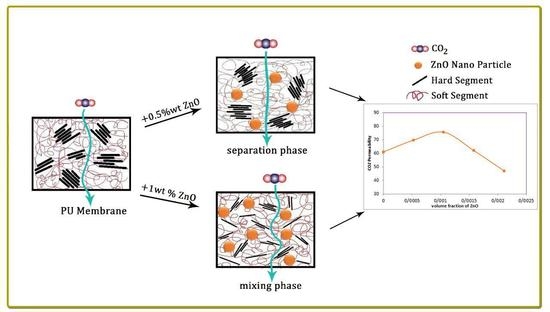
Polyurethane (PU) is a polymeric material with a wide range of applications, including adhesive, fibre, foam, thermoplastic elastomer, and coating. Its specific structural properties are affected by its two-phase structure—the hard urethane and the soft polyester or polyether segments. PU hydrogen bonding competes between the two PU segments. The crystallisability of these segments change the structural features of the PU. Nanoparticles’ interaction with these segments change the balance of the hydrogen bonding between the hard (HS) and soft segments (SS). Then, the mixing or separation phase occurs [
16,
17,
18]. The results of the incorporation of phase mixing or separation affect the physical and chemical properties of the PU.
These special properties of the PU produce good permeation properties for the gas separation system, as well as the good physical, mechanical and thermal properties, fatigue life, and abrasion resistance. Thus, the PU membrane has allocated a lot of the gas separation membrane studies in recent years [
16,
17,
18,
19,
20,
21,
22,
23,
24]. Many organic and inorganic materials have been used as fillers to incorporate into the PU membranes [
25].
Bistricic et al. studied the PU-nanosilica MMM. They reported that the hydrogen bond formation between the nanosilica and the carbonyl groups in the soft segments of PU improved the rheological, thermal, mechanical and adhesive properties of the PU-nanosilica MMM [
16].
In another work, Khosravi et al. examined the role of the silica nanoparticles in the polyether and polyester of the PU MMMs for gas permeation [
26]. They reported that the polyether urethane-silica membranes had higher propane permeability and propane/methane selectivity. The propane permeability and propane/methane selectivity of the polyester urethane decreased due to nanosilica loading [
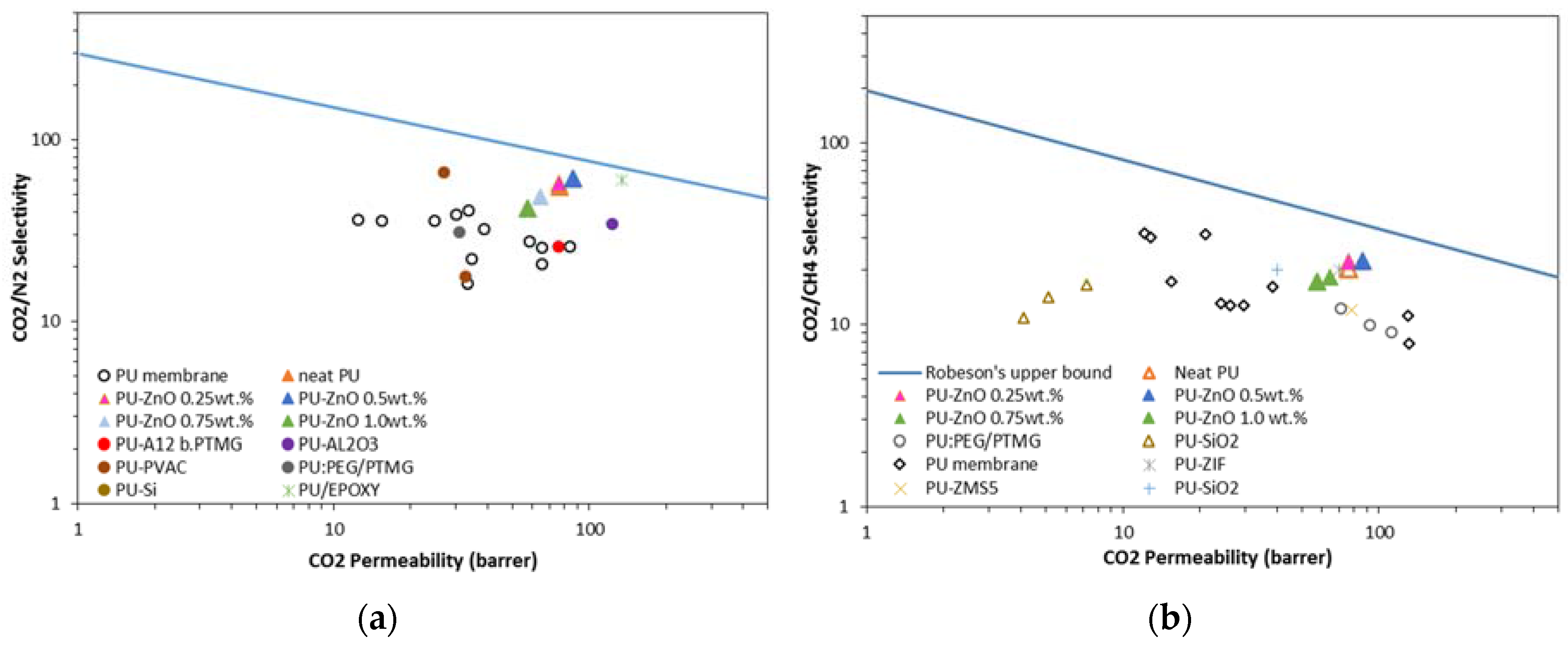
26]. Some of the PU MMMs with different nanoparticles for gas separation are shown in
Table 1.
Formerly, Iron oxide and ZnO catalysts have been used to sweeten sour gas. The use of metal oxide nanoparticles can greatly increase the absorption processes due to its physical and chemical properties [
31]. ZnO could undergo hydrogen bonding with PU. The amines of HS and carbonyls of both HS and SS could be interacted with ZnO and its O–H groups to form hydrogen bonds and affect the PU structure. Therefore, in this study, ZnO nanoparticles were applied to improve the separation performance of PU membranes.
The use of nonporous ZnO nanoparticles as fillers increased the anti-bacterial, mechanical, physical and optical properties of the polymeric composite [
25]. Kim et al. added the ZnO nanoparticles to the polyurethane acrylate nanocomposite film to enhance its optical, physical and mechanical properties, and water transport behaviours. However, the thermal stability of the nanocomposite film was observed to be weak [
32].
Thus, the gas permeation properties of a tiny amount of ZnO on the PU composite membrane were investigated. As it is observed in
Table 1, all the studies on PU MMM have used 5–25% of nanoparticles. In this present study, the effects of the incorporation of a tiny amount of ZnO nanoparticles into the PU MMMs and their gas separation properties were investigated for the first time.
The gas separation properties of PU-ZnO MMMs and the effect of ZnO as a metal oxide on the soft and hard segments of PU were also investigated for the first time. Different amounts of ZnO nanoparticles were added to the PU matrix to study the membranes’ permeability for CO2, CH4, and N2. Moreover, the membrane samples were characterised using precise analysing methods.
2. Experimental
2.1. Materials
Polyester urethane (PU) with the commercial code (Apilon52-A6505) was supplied by API SpA, Neu-Isenburg, Germany. N,N-dimethylformamide (DMF) was purchased from Merck, Darmstadt, Germany. ZnO nanoparticles (purity > 99%; particle size range 20–30 nm) were supplied by Navarrean Nanoproducts Technology (TECNAN, Navarra, Spain). All of the chemicals and materials were used as received.
2.2. Membrane Preparation
The PU and PU-ZnO MMM samples were prepared using solving, casting and solvent evaporating methods.
A sufficient amount of PU was added to the solvent (DMF) and the obtained dope solution (10 wt %) was stirred to get a homogeneous PU solution. The dope solution was then cast on glass plates and the solvents were allowed to evaporate.
In order to allow better evaporation of the solvent from the membranes, the casted films were removed from the glass plates and dried slowly in a vacuum oven. The PU-ZnO membranes were prepared using the same method. The ZnO particles were well dispersed in a mixture of half DMF and 1 wt % of the PU. The target mixture was mixed for about 12 h. Another PU-DMF solution was made and the dope solution, containing ZnO, was added into it. Then, the final mixture was mixed well, sonicated for 20 min, and cast as PU dope.
2.3. Characterization of the Membranes
The IR spectroscopy (Magna IR Spectrometer 550, GMI, Ramsey, MN, USA) was used for monitoring the interaction between the PU and ZnO nanoparticles in the MMMs and confirm the final chemical structures of the membrane samples. The scanning frequency range is 4000–400 cm−1.
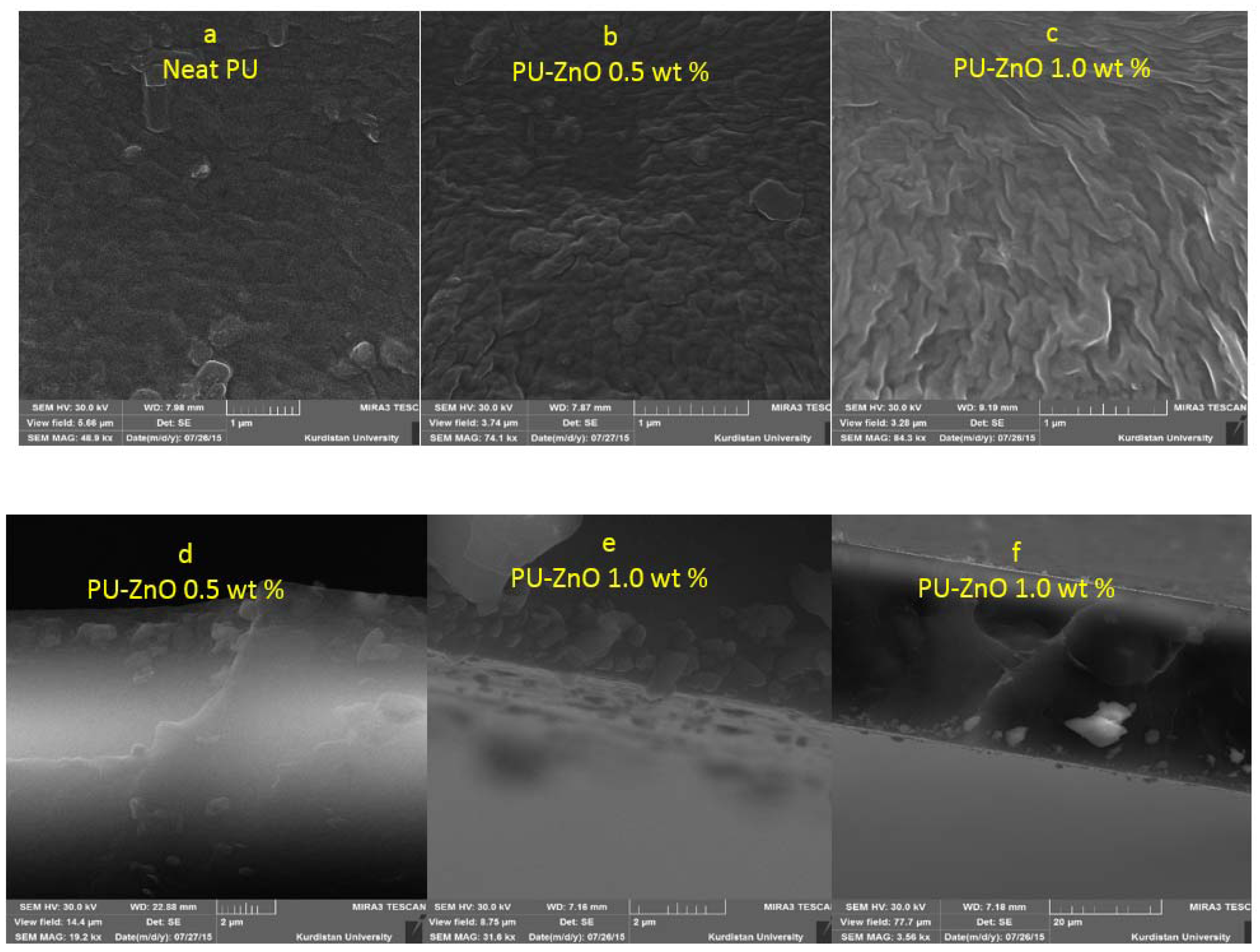
The membranes’ morphology and the distribution of ZnO nanoparticles in the MMMs’ structures were investigated using the scanning electron microscopy (SEM) (TESCAN, Brno, Czech Republic). The MMM samples were well-fractured in liquid nitrogen and coated with gold before the cross-sectional scanning.
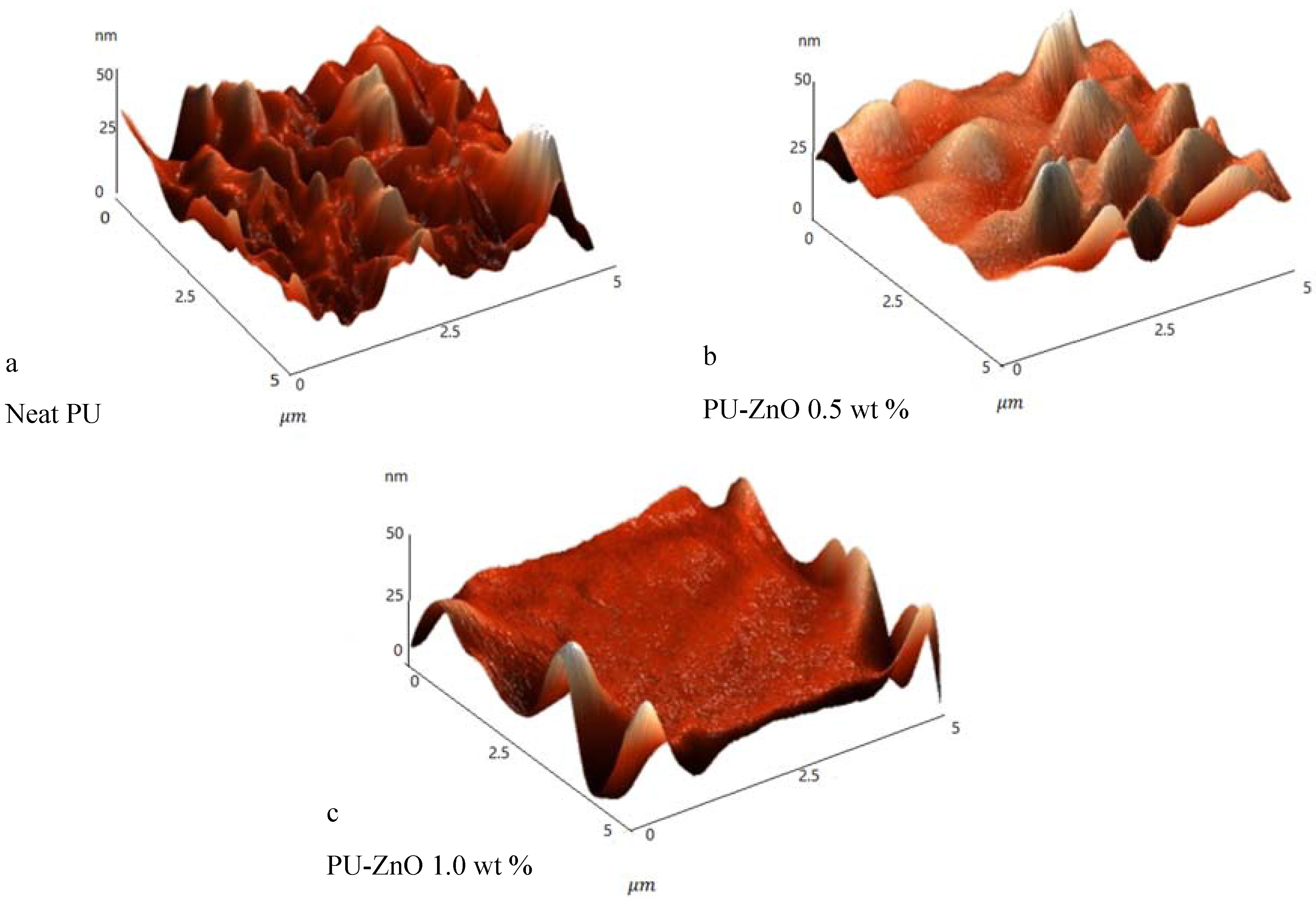
The surface topography of the control sample (neat PU membrane) and the PU-ZnO membranes was studied using the atomic force microscopy (AFM) (SOLVER, NT-MDT, Moscow, Russia) via non-contact mode.
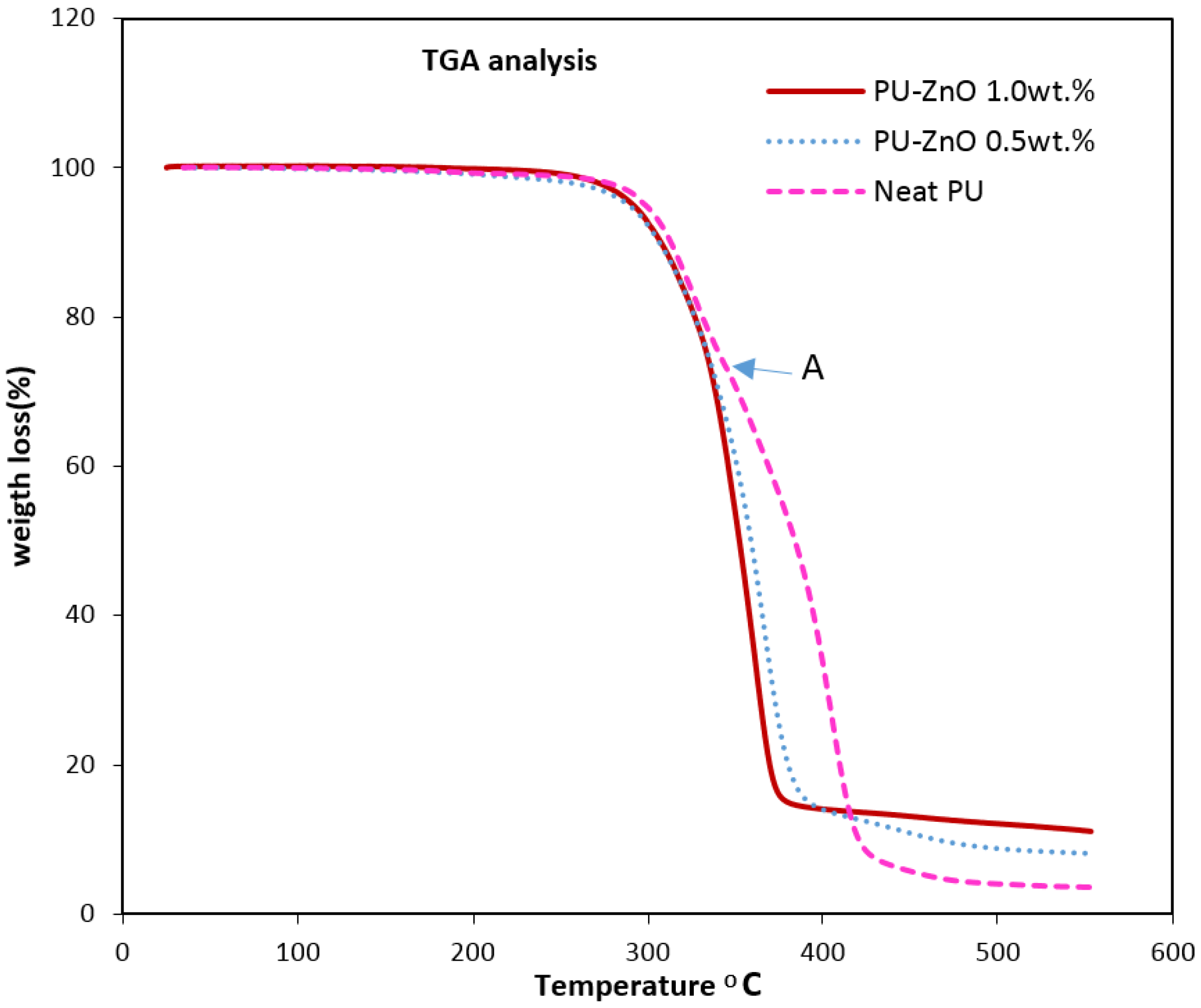
The thermal degradation analysis of the nanocomposite membranes was done through the thermal gravimeter/differential thermal analyser (TG/DTA) (PYRIS DIAMOND, Perkin Elmer, Waltham, MA, USA). The results were obtained from 25 °C to 600 °C at a heating rate of 10 °C/min.
2.4. Permeation Measurements
The gas permeability performance of the membranes was measured using a constant pressure system. The membrane permeability (
P) can be calculated using the Equation (1):
In this equation, the feed pressure (
p) varies from 4 to 14 atm, and the permeate pressure (
p°) is atmospheric. Equation (1) is normalised with the partial pressure difference (
p − p°). The flux (
Q) is measured using a bubble flow meter in a steady state flow. The temperature was maintained at 30 °C and
t is the membrane thickness, which is measured using the SEM images about 35–38 µm. The membrane effective module area in this work was 27.72 cm
2. Moreover, the membrane permeability is expressed in barrers [
1,
4,
18,
19,
20,
29,
33].
4. Conclusions
The effects of a tiny amount of ZnO nanoparticles on the morphological and gas separation performance of the PU-based MMMs were investigated. The IR results of the neat and MMMs indicated that the incorporation of ZnO up to 0.5 wt % in the PU-ZnO MMM led to a phase separation in the membrane, while in the PU-ZnO 1.0 wt % MMM, it led to a phase mixing. The SEM images proved that the ZnO nanoparticles were dispersed well within the polymeric matrix. On the other hand, the AFM observation of the neat PU showed a rougher surface than that of the PU-ZnO MMM. The TGA indicated that the incorporation of ZnO to the PU had no thermal transience in the PU-ZnO MMM. The TGA results also demonstrated that the ZnO nanoparticles dispersed in the SS of the PU.
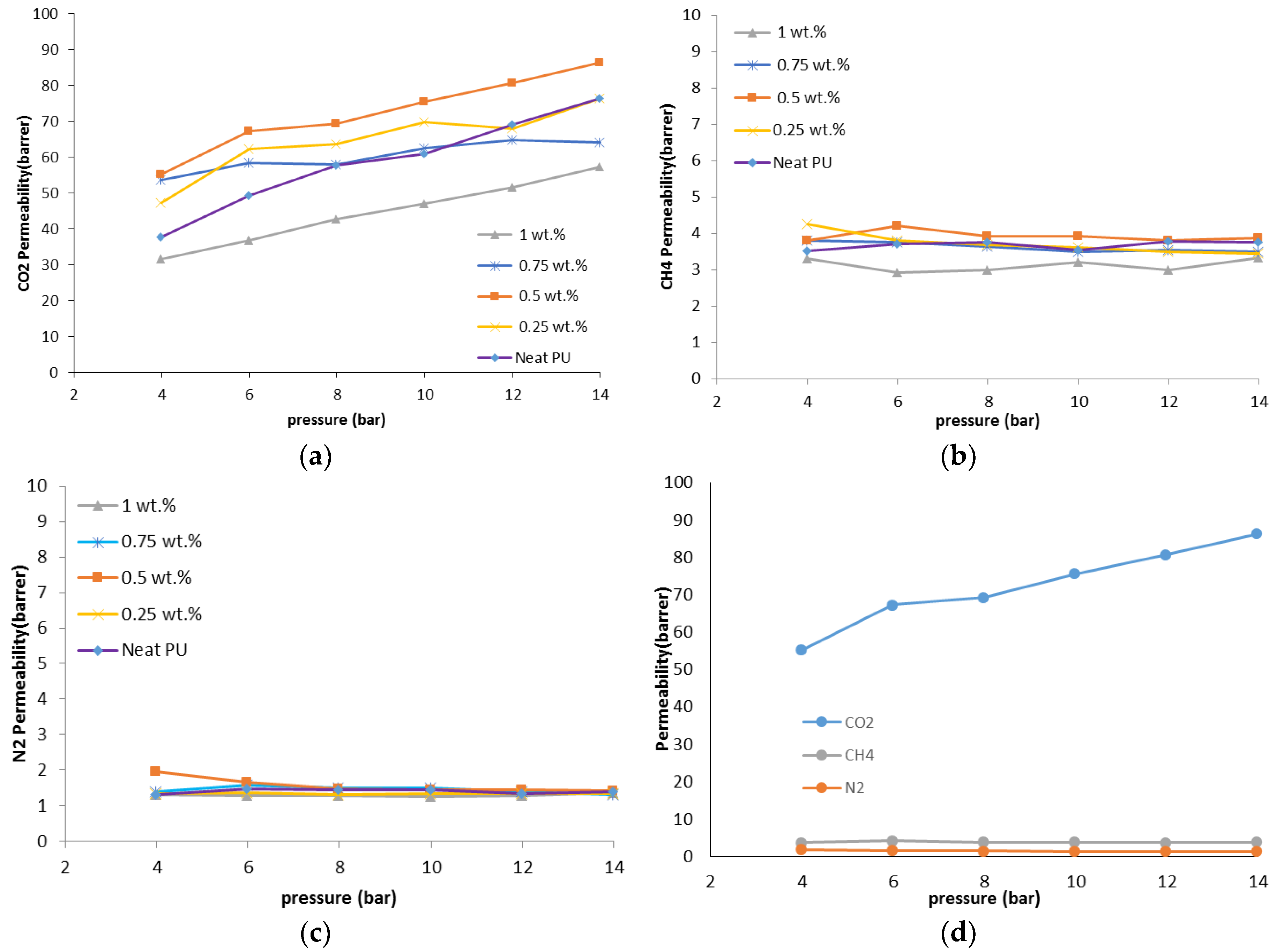
The gas permeation results showed that the permeability of N2, CO2, and CH4 through the PU-ZnO 0.5 wt % MMM was higher than that of the neat PU membrane. The CO2 permeability of PU-ZnO 0.5 wt % MMM was approximately 30% higher than that of the neat PU membrane, while it decreased by about 34% for the membrane containing 1% of ZnO nanoparticles.