Characterization and Antibiofouling Performance Investigation of Hydrophobic Silver Nanocomposite Membranes: A Comparative Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Surface Modification
2.2. Characterization
2.3. Antibacterial Tests
2.3.1. The Colony Forming Count Methods
2.3.2. Antibacterial Property
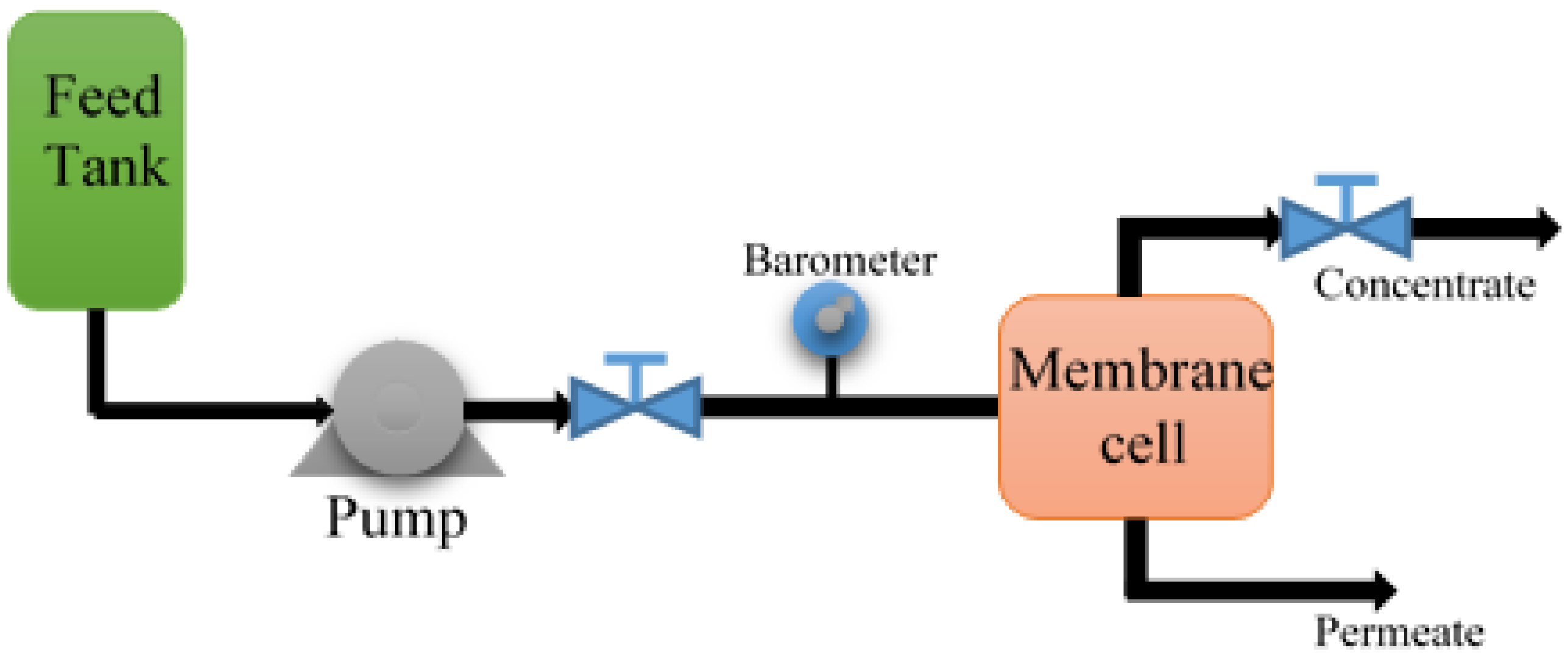
2.4. Microfiltration Test
3. Results and Discussion
3.1. Surface Hydrophilicity
3.2. Silver Loss
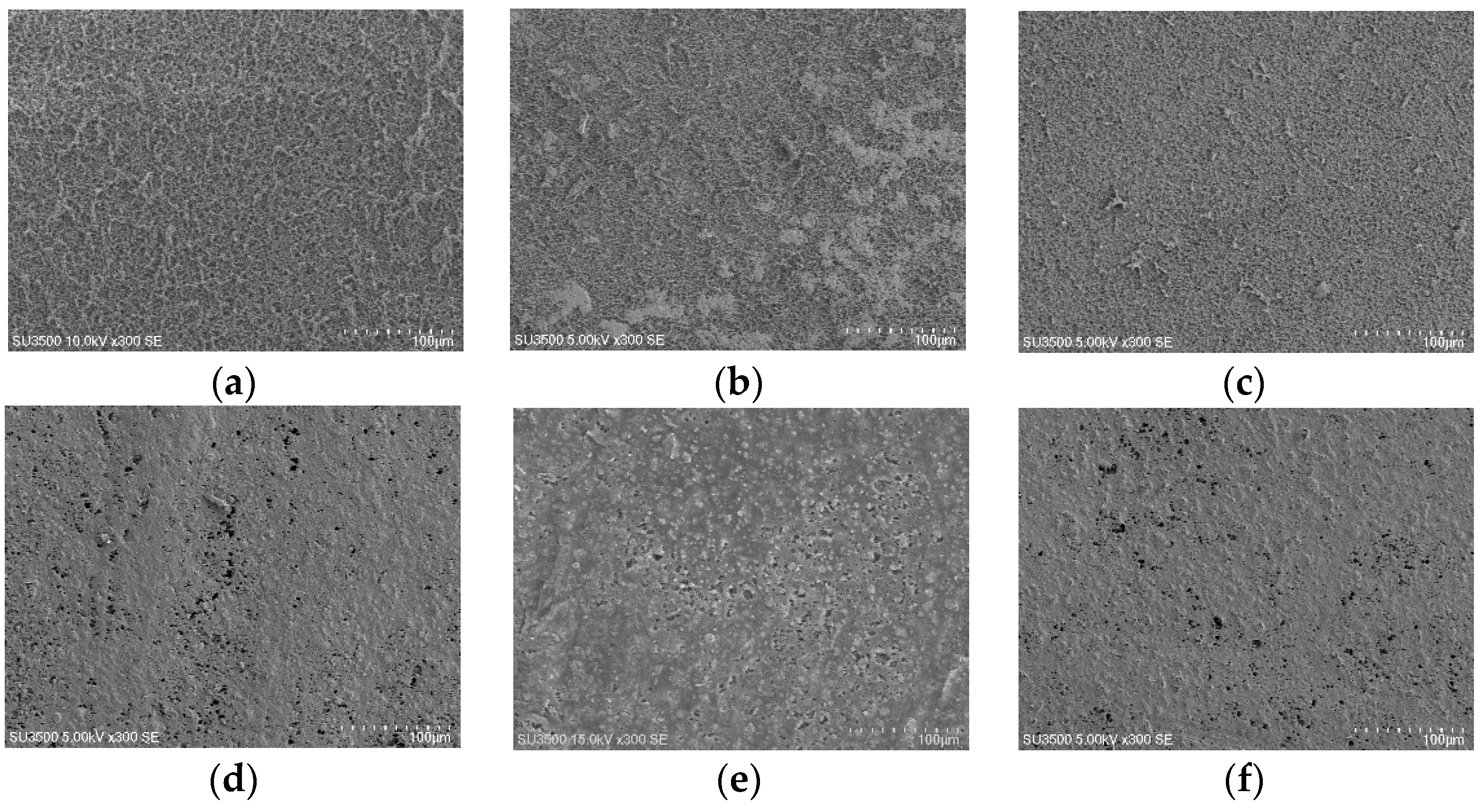
3.3. Membrane Surface Morphology
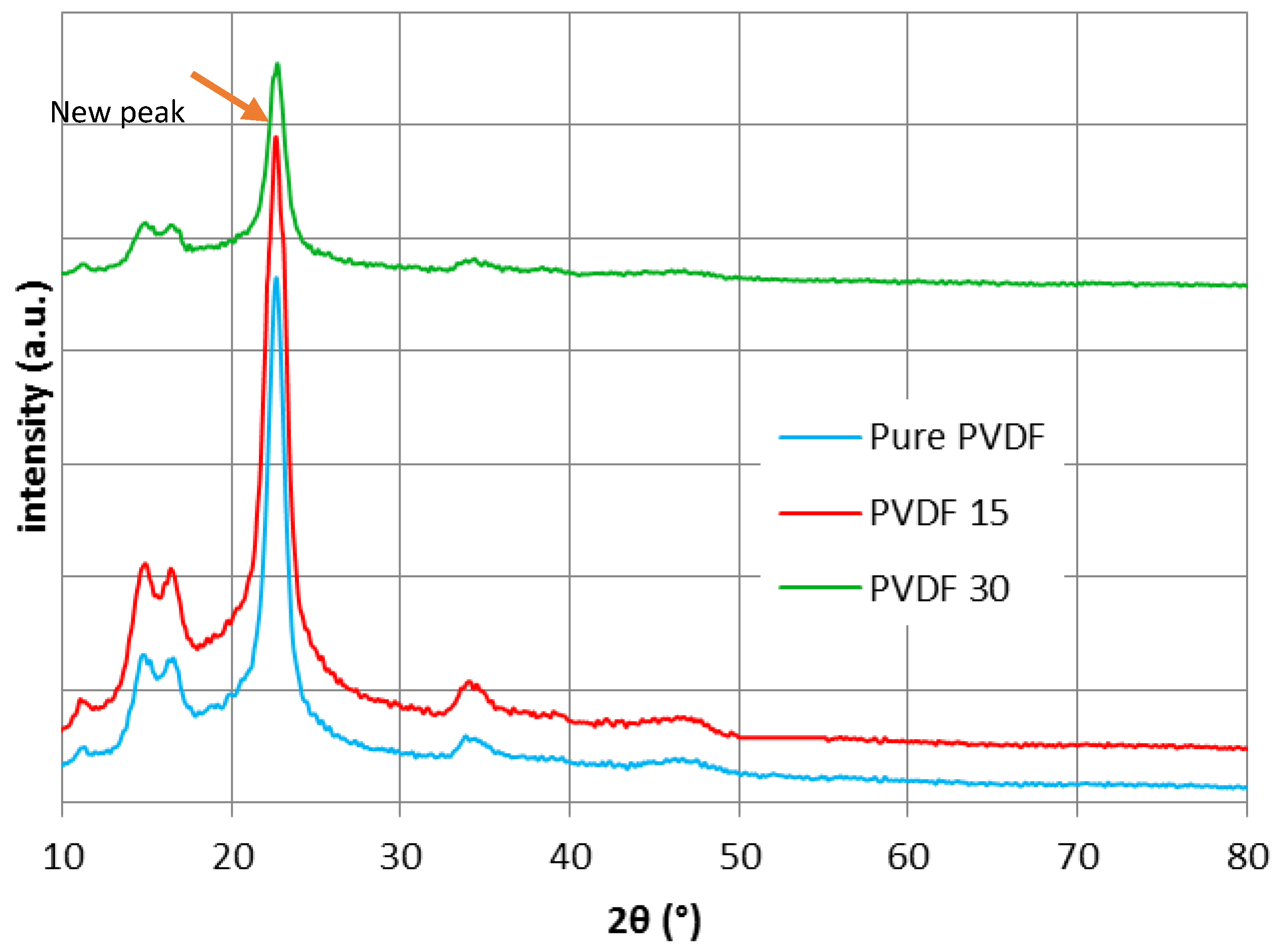
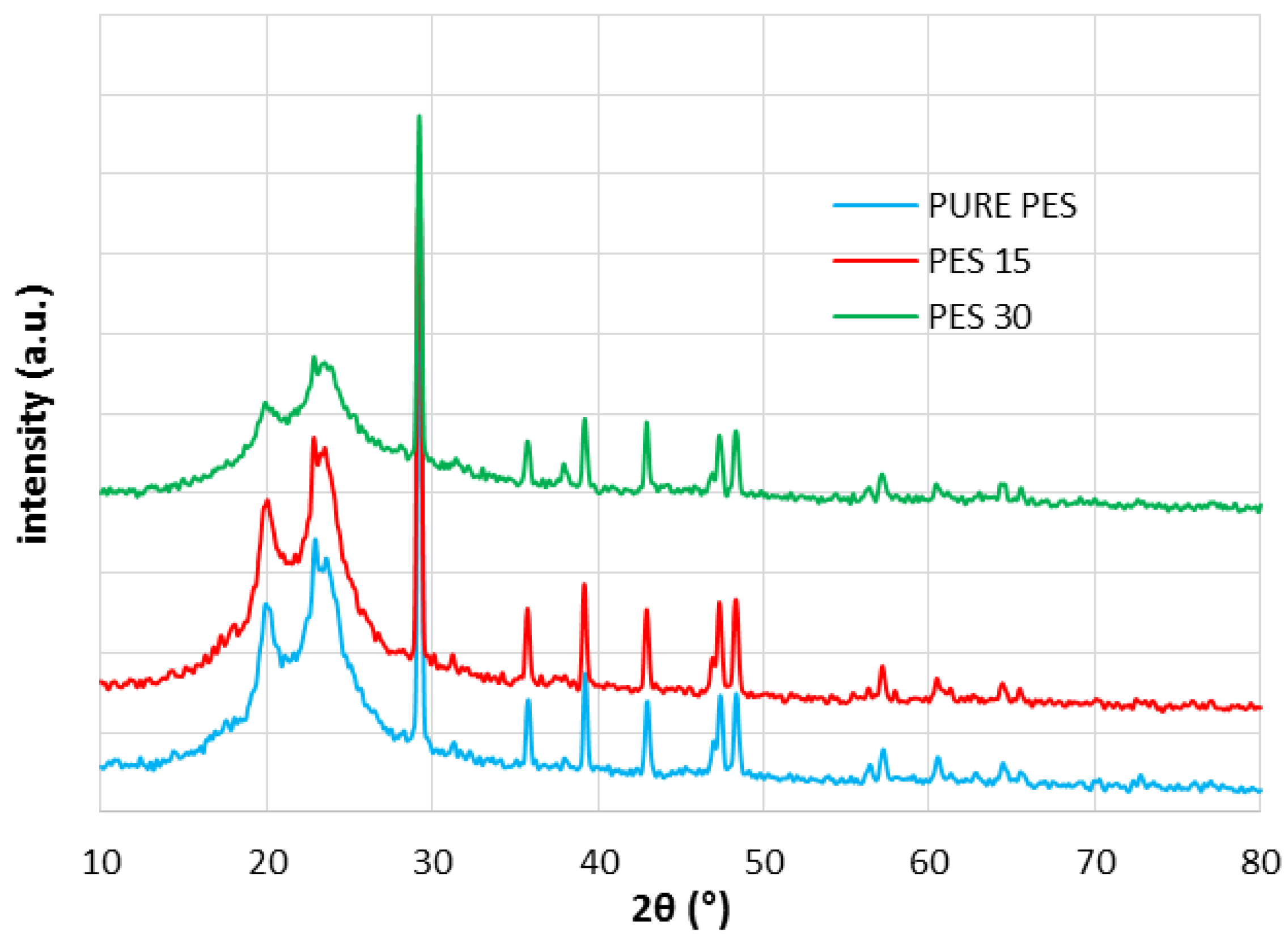
3.4. XRD, ATR-FTIR and AFM Results
3.5. Antibacterial Property
3.5.1. Growth Inhibition
3.5.2. Bactericidal Effect
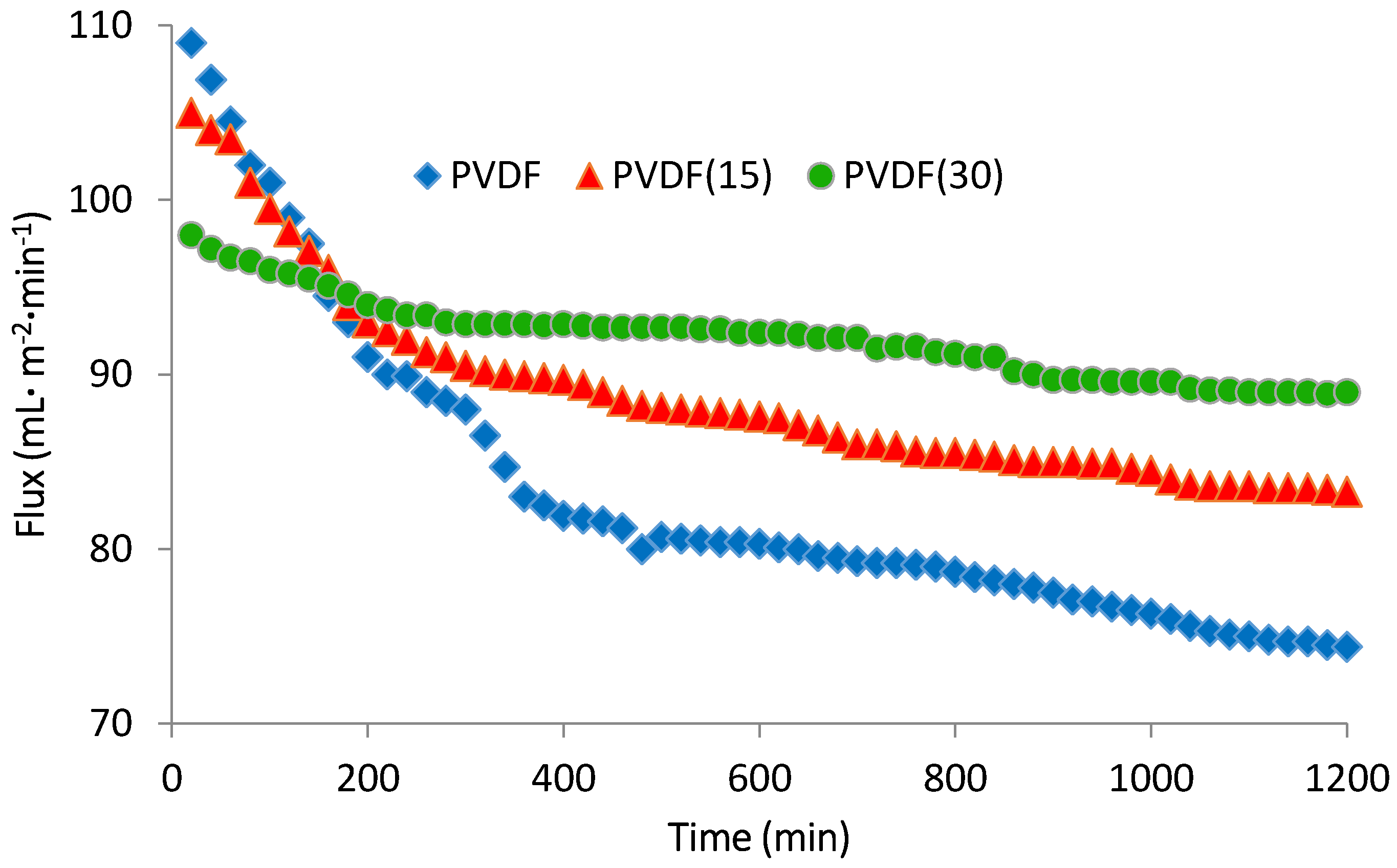
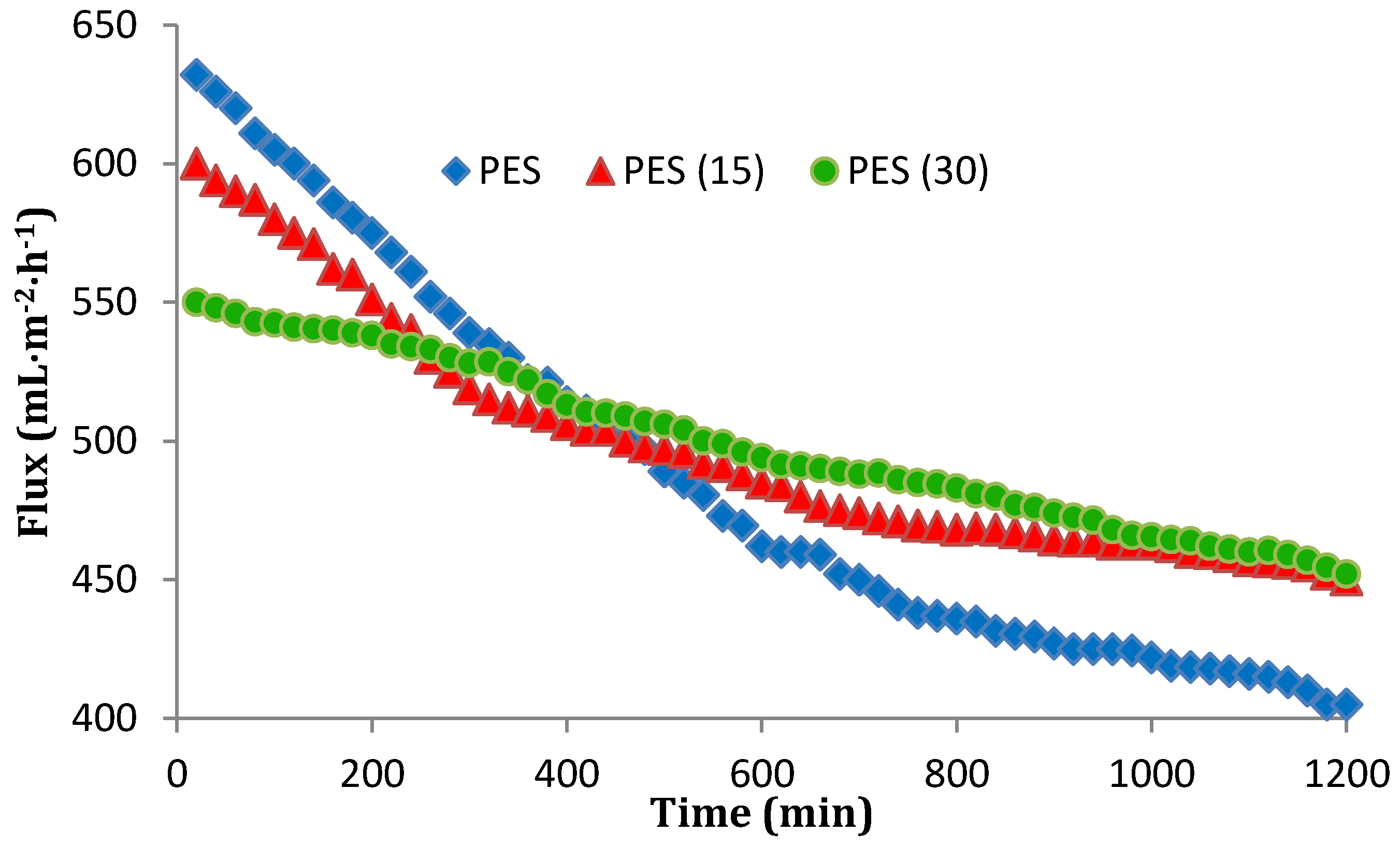
3.6. Antibiofouling Performance
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Peng, Y.; Fan, H.; Ge, J.; Wang, S.; Chen, P.; Jiang, Q. The effects of processing conditions on the surface morphology and hydrophobicity of polyvinylidene fluoride membranes prepared viavapor-induced phase separation. Appl. Surf. Sci. 2012, 263, 737–744. [Google Scholar] [CrossRef]
- Kumar, R.; Ismail, A.F. Fouling control on microfiltration/ultrafiltration membranes: Effects of morphology, hydrophilicity, and charge. J. Appl. Polym. Sci. 2015, 132, 1–20. [Google Scholar] [CrossRef]
- Zhao, Y.-J.; Wu, K.-F.; Wang, Z.-J.; Zhao, L.; Li, S.-S. Fouling and cleaning of membrane—A literature review. Environ. Sci. 2000, 12, 241–251. [Google Scholar]
- Lin, H.; Peng, W.; Zhang, M.; Chen, J.; Hong, H.; Zhang, Y. A review on anaerobic membrane bioreactors: Applications, membrane fouling and future perspectives. Desalination 2013, 314, 169–188. [Google Scholar] [CrossRef]
- Liu, C.X.; Zhang, D.R.; He, Y.; Zhao, X.S.; Bai, R. Modification of membrane surface for anti-biofouling performance: Effect of anti-adhesion and anti-bacteria approaches. J. Membr. Sci. 2010, 346, 121–130. [Google Scholar] [CrossRef]
- Haider, M.S.; Shao, G.N.; Imran, S.M.; Park, S.S.; Abbas, N.; Tahir, M.S.; Hussain, M.; Bae, W.; Kim, H.T. Aminated polyethersulfone-Silver nanoparticles (AgNPs–APES) composite membranes with controlled silver ion release for antibacterial and water treatment applications. Mater. Sci. Eng. C 2016, 62, 732–745. [Google Scholar] [CrossRef] [PubMed]
- Charfi, A.; Amar, N.B.; Harmand, J. Analysis of fouling mechanisms in anaerobic membrane bioreactors. Water Res. 2012, 46, 2637–2650. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zhao, Y.; Chen, L. The construction of a zwitterionic PVDF membrane surface to improve biofouling resistance. Biofouling 2013, 29, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; van der Bruggen, B. The use of nanoparticles in polymeric and ceramic membrane structures:Review of manufacturing procedures and performance improvement for water treatment. Environ. Pollut. 2010, 158, 2335–2349. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.D.; Cao, Y.M. Application and modification of poly(vinylidene fluoride) (PVDF) membranes—A review. J. Membr. Sci. 2014, 463, 145–165. [Google Scholar] [CrossRef]
- Karkhanechi, H.; Takagi, R.; Matsuyama, H. Biofouling resistance of reverse osmosis membrane modified with polydopamine. Desalination 2014, 336, 87–96. [Google Scholar] [CrossRef]
- Bottino, A.; Capannelli, G.; D’asti, V.; Piaggio, P. Preparation and properties of novel organic–inorganic porous membranes. Sep. Purif. Technol. 2001, 22–23, 269–275. [Google Scholar] [CrossRef]
- Faghihi, K.; Hajibeygi, M. Synthesis and properties of polyimide/silver nanocomposite containing dibenzalacetone moiety in the main chain. J. Saudi Chem. Soc. 2013, 17, 419–423. [Google Scholar] [CrossRef]
- Dallas, P.; Sharma, V.K.; Zboril, R. Silver polymeric nanocomposites as advanced antimicrobial agents: Classification, synthetic paths, applications, and perspectives. Adv. Colloid Interface Sci. 2011, 166, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Shao, X.S.; Zhou, Q.; Li, M.Z.; Zhang, Q.Q. The double effects of silver nanoparticles on the PVDF membrane: Surface hydrophilicity and antifouling performance. Appl. Surf. Sci. 2013, 265, 663–670. [Google Scholar] [CrossRef]
- Sonawane, S.H.; Terrien, A.; Figueiredo, A.S.; Clara Goncalves, M.; De Pinho, M.N. The role of silver nanoparticles on mixed matrix Ag/cellulose acetate asymmetric membranes. Polym. Compos. 2017, 38, 32–39. [Google Scholar] [CrossRef]
- Yang, H.L.; Chun-Te Lin, J.; Huang, C. Application of nanosilver surface modification to RO membrane and spacer for mitigating biofouling in seawater desalination. Water Res. 2009, 43, 3777–3786. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Wang, X.; Zhou, Y.; Zhang, L. Preparation and characterization of poly(vinylidene fluoride) composite membranes blended with nano-crystalline cellulose. Prog. Nat. Sci. 2012, 22, 250–257. [Google Scholar] [CrossRef]
- Ji, J.; Liu, F.; Hashim, N.A.; Abed, M.M.; Li, K. Poly(vinylidene fluoride) (PVDF) membranes for fluid separation. React. Funct. Polym. 2015, 86, 134–153. [Google Scholar] [CrossRef]
- Joseph, N.; Singh, S.K.; Sirugudu, R.K.; Murthy, V.R.K.; Ananthakumar, S.; Sebastian, M.T. Effect of silver incorporation into PVDF-barium titanate composites for EMI shielding applications. Mater. Res. Bull. 2013, 48, 1681–1687. [Google Scholar] [CrossRef]
- Gusseme, B.; Hennebel, T.; Christiaens, E.; Saveyn, H.; Verbeken, K.; Fitts, J.P.; Boon, N.; Verstraete, W. Virus disinfection in water by biogenic silver immobilized inpolyvinylidene fluoride membranes. Water Res. 2011, 45, 1856–1864. [Google Scholar] [CrossRef] [PubMed]
- Makdissy, G.; Croué, J.P.; Amy, G.; Buisson, H. Fouling of a polyethersulfone ultrafiltration membrane by natural organic matter. Water Sci. Technol. 2004, 4, 205–212. [Google Scholar]
- Johnson, D.; Hilal, N. Characterisation and quantification of membrane surface properties using atomic force microscopy: A comprehensive review. Desalination 2015, 356, 149–164. [Google Scholar] [CrossRef]
- Riedl, K.; Girard, B.; Lencki, R.W. Influence of membrane structure on fouling layer morphology during apple juice clarification. J. Membr. Sci. 1998, 139, 155–166. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Zinadini, S.; Vatanpou, V. Preparation of superhydrophobic nanofiltration membrane by embedding multi walled carbon nanotube and polydimethylsiloxane in pores of microfiltration membrane. Sep. Purif. Technol. 2013, 111, 98–107. [Google Scholar] [CrossRef]
- Hu, N.; Xiao, T.; Cai, X.; Ding, L.; Fu, Y.; Yang, X. Preparation and Characterization of Hydrophilically Modified PVDF Membranes by a Novel Nonsolvent Thermally Induced Phase Separation Method. Membranes 2016, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Mahendra, S.; Lyon, D.Y.; Brunet, L.; Liga, M.V.; Li, D.; Alvarez, P.J. Antimicrobial nanomaterials for water disinfection and microbial control: Potential applications and implications. Water Res. 2008, 42, 4591–4602. [Google Scholar] [CrossRef] [PubMed]
- Razmjou, A.; Arifin, E.; Dong, G.; Mansouri, J.; Chen, V. Superhydrophobic modification of TiO2 nanocomposite PVDF membranes for applications in membrane distillation. J. Membr. Sci. 2012, 415–416, 850–863. [Google Scholar] [CrossRef]
- Kujawa, J.; Kujawski, W. Functionalization of ceramic metal oxide powders and ceramic membranes by perfluoroalkylsilanes and alkylsilanes possessing different reactive groups: Physicochemical and tribological properties. ACS Appl. Mater. Interfaces 2016, 8, 7509–7521. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Xue, J.; Ran, F.; Sun, S. Modification of polyethersulfone membranes-A review of methods. Prog. Mater. Sci. 2013, 58, 76–150. [Google Scholar] [CrossRef]
- Liu, F.; Hashim, N.A.; Liu, Y.; Moghareh Abed, M.R.; Li, K. Progress in the production and modification of PVDF membranes. J. Membr. Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Satapathy, S.; Gupta, P.K.; Pawar, S.; Varma, K.B.R. Crystallization of β-Phase Poly(vinylidene fluoride) Films Using Dimethyl Sulfoxide (DMSO) Solvent and at Suitable Annealing Condition. Available online: https://arxiv.org/abs/0808.0419 (accessed on 4 August 2008).
- Cardoso, V.F.; Martins, P.; Botelho, G.; Rebouta, L.; Lanceros-Méndez, S.; Minas, G. Degradation studies of transparent conductive electrodes on electroactive poly(vinylidene fluoride) for uric acid measurements. Sci. Technol. Adv. Mater. 2010, 11, 045006. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Lee, J.Y.; Porter, R.S. The crystalline phase transformation of poly(viny1idene fluoride)/poly(vinyl fluoride) blend films. Polym. Eng. Sci. 1998, 38, 1359–1365. [Google Scholar] [CrossRef]
- Abdullah, I.Y.; Yahaya, M.; Jumali, M.H.H.; Shanshool, H.M. Enhancement piezoelectricity in poly(vinylidene fluoride) by filler piezoceramics lead-free potassium sodium niobate (KNN). Opt. Quant. Electron. 2016, 48, 1–9. [Google Scholar] [CrossRef]
- Dillon, D.R.; Tenneti, K.K.; Li, C.Y.; Ko, F.K.; Sics, I.; Hsiao, B.S. On the structure and morphology of polyvinylidene fluoride–nanoclaynanocomposites. Polymer 2006, 47, 1678–1688. [Google Scholar] [CrossRef]
- Buonomenna, M.G.; Macchi, P.; Davoli, M.; Drioli, E. Poly(vinylidene fluoride) membranes by phase inversion: The role the casting and coagulation conditions play in their morphology, crystalline structure and properties. Eur. Polym. J. 2007, 43, 1557–1572. [Google Scholar] [CrossRef]
- Liu, X.; Qi, S.; Li, Y.; Yang, L.; Cao, B.; Tang, C. Synthesis and characterization of novel antibacterial silver nanocomposite nanofiltration and forward osmosis membranes based on layer-by-layer assembly. Water Res. 2013, 47, 3081–3092. [Google Scholar] [CrossRef] [PubMed]
- Tsonos, C.; Pandis, C.; Soin, N.; Sakellari, D.; Myrovali, E.; Kripotou, S.; Kanapitsas, A.; Siores, E. Multifunctional nanocomposites of poly(vinylidene fluoride)reinforced by carbon nanotubes and magnetitenanoparticles. Express Polym. Lett. 2015, 9, 1104–1118. [Google Scholar] [CrossRef]
- Toroghi, M.; Raisi, A.; Aroujalian, A. Preparation and characterization of polyethersulfone/silver nanocomposite ultrafiltration membrane for antibacterial applications. Polym. Adv. Technol. 2014, 25, 711–722. [Google Scholar] [CrossRef]
- Salimi, A.; Yousefi, A.A. FTIR studies of β-phase crystal formation in stretched PVDF films. Polym. Test. 2003, 22, 699–704. [Google Scholar] [CrossRef]
- Luo, B.; Wang, X.; Wang, Y.; Li, L. Fabrication, characterization, properties and theoretical analysis of ceramic/PVDF composite flexible films with high dielectric constant and low dielectric loss. J. Mater. Chem. A 2014, 2, 510–519. [Google Scholar] [CrossRef]
- Li, J.-H.; Yan, B.-F.; Shao, X.-S.; Wang, S.-S.; Tian, H.-Y.; Zhang, Q.-Q. Influence of Ag/TiO2 nanoparticle on the surface hydrophilicity and visible-light response activity of polyvinylidene fluoride membrane. Appl. Surf. Sci. 2015, 324, 82–89. [Google Scholar] [CrossRef]
- Prince, J.A.; Bhuvana, S.; Boodhoo, K.V.K.; Anbharasi, V.; Singh, G. Synthesis and characterization of PEG–Ag immobilized PES hollow fiber ultrafiltration membranes with long lasting antifouling properties. J. Membr. Sci. 2014, 454, 538–548. [Google Scholar] [CrossRef]
- Chang, Y.; Shih, Y.J.; Ruaan, R.C.; Higuchi, A.; Chen, W.Y.; Lai, J.Y. Preparation of poly(vinylidene fluoride) microfiltration membrane with uniform surface-copolymerized poly (ethylene glycol) methacrylate and improvement of blood compatibility. J. Membr. Sci. 2008, 309, 165–174. [Google Scholar] [CrossRef]
- Mollahosseini, A.; Rahimpour, A.; Jahamshahi, M.; Peyravi, M.; Khavarpour, M. The effect of silver nanoparticle size on performance and antibacteriality of polysulfone ultrafiltration membrane. Desalination 2012, 306, 41–50. [Google Scholar] [CrossRef]
- Dong, C.; He, G.; Li, H.; Zhao, R.; Han, Y.; Deng, Y. Application of Mg(OH)2 nanoplatelets as pore former to prepare PVDF ultrafiltration membranes. J. Environ. Chem. Eng. 2017, 5, 877–883. [Google Scholar] [CrossRef]
- Basri, H.; Ismail, A.F.; Aziz, M. Polyethersulfone (PES)–silver composite UF membrane: Effect of silver loading and PVP molecular weight on membrane morphology and antibacterial activity. Desalination 2011, 273, 72–80. [Google Scholar] [CrossRef]
- EPA 833-R-04-002A Local Limits Development Guidance; U.S. Environmental Protection Agency: Washington, DC, USA, 2004.
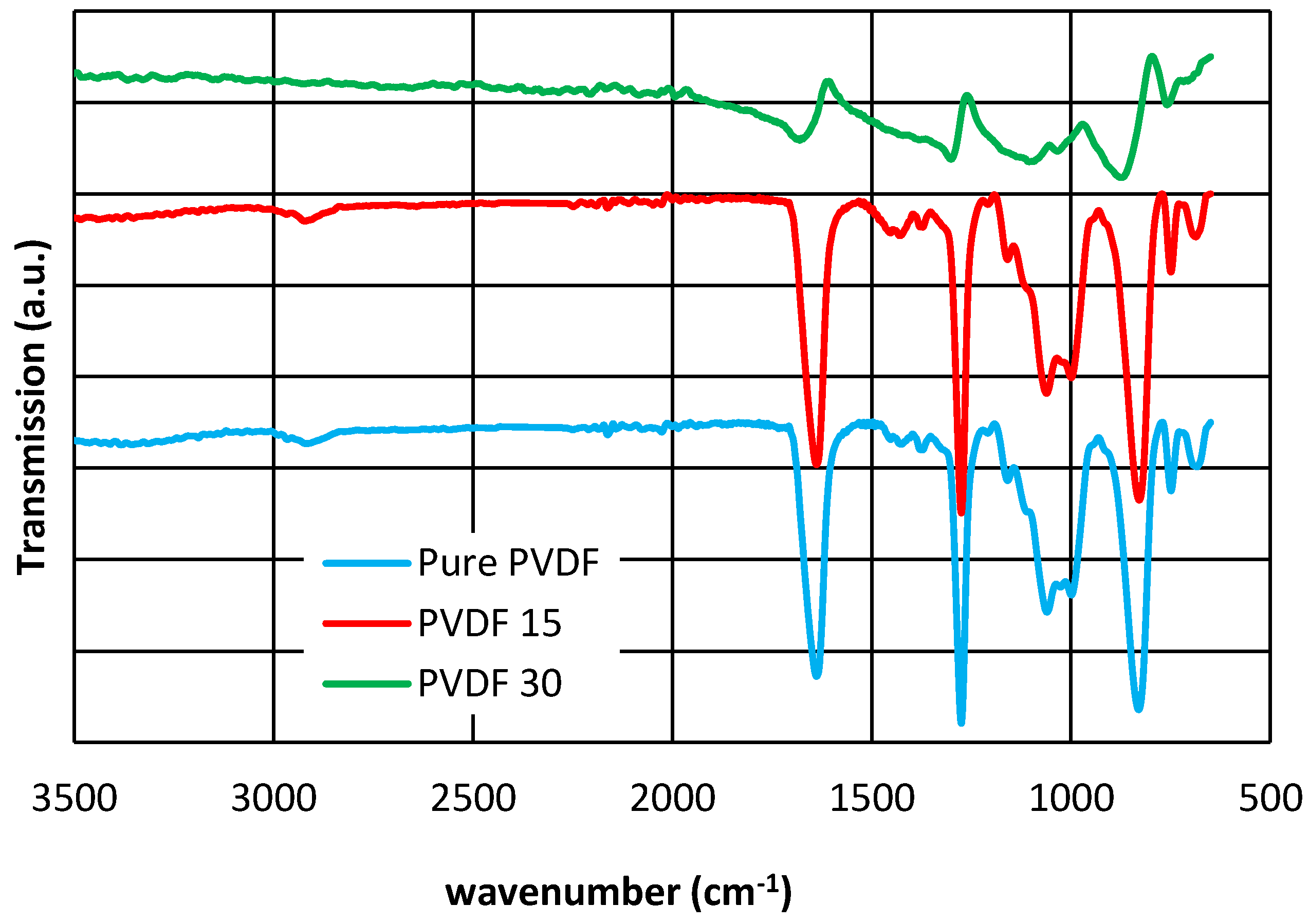
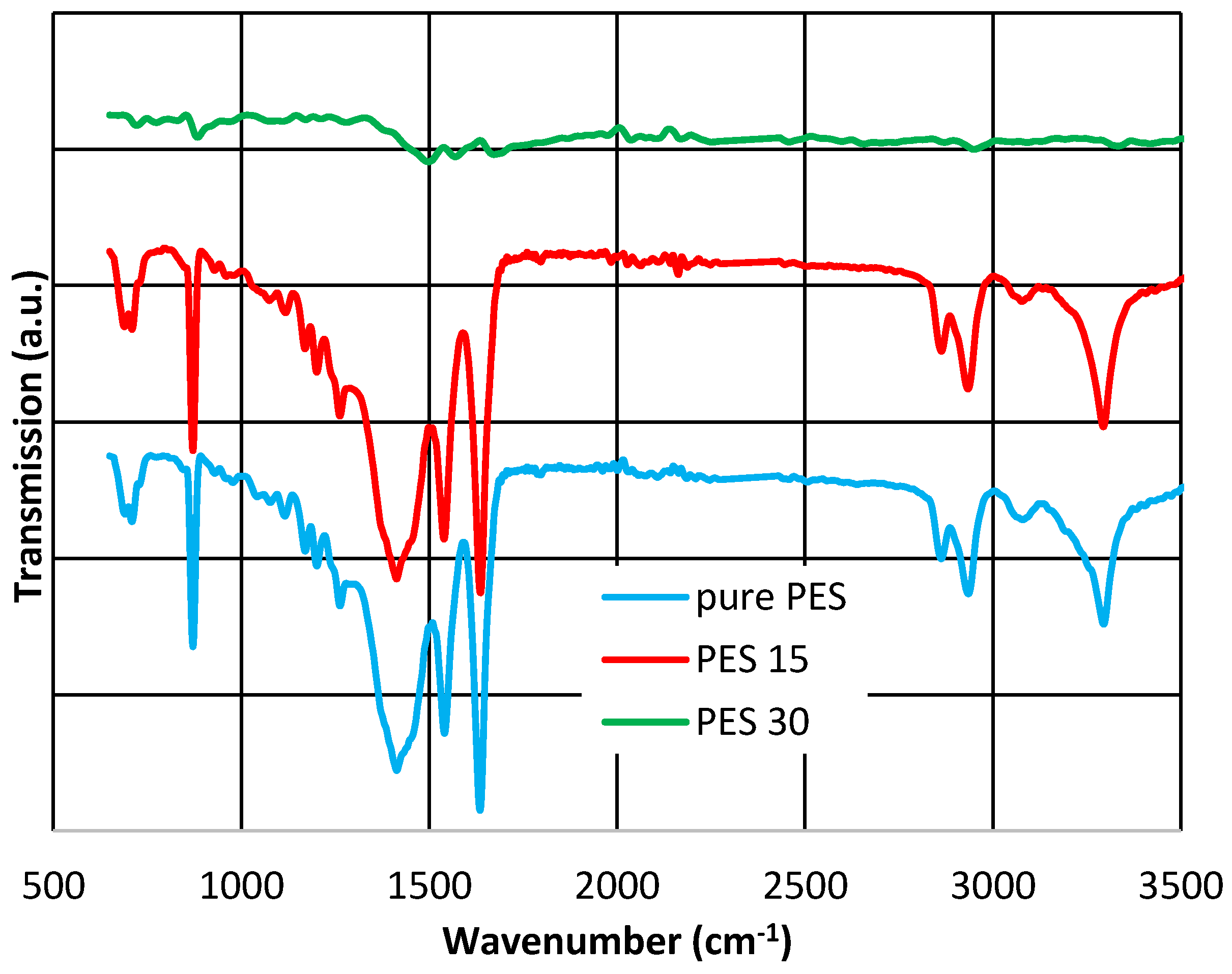


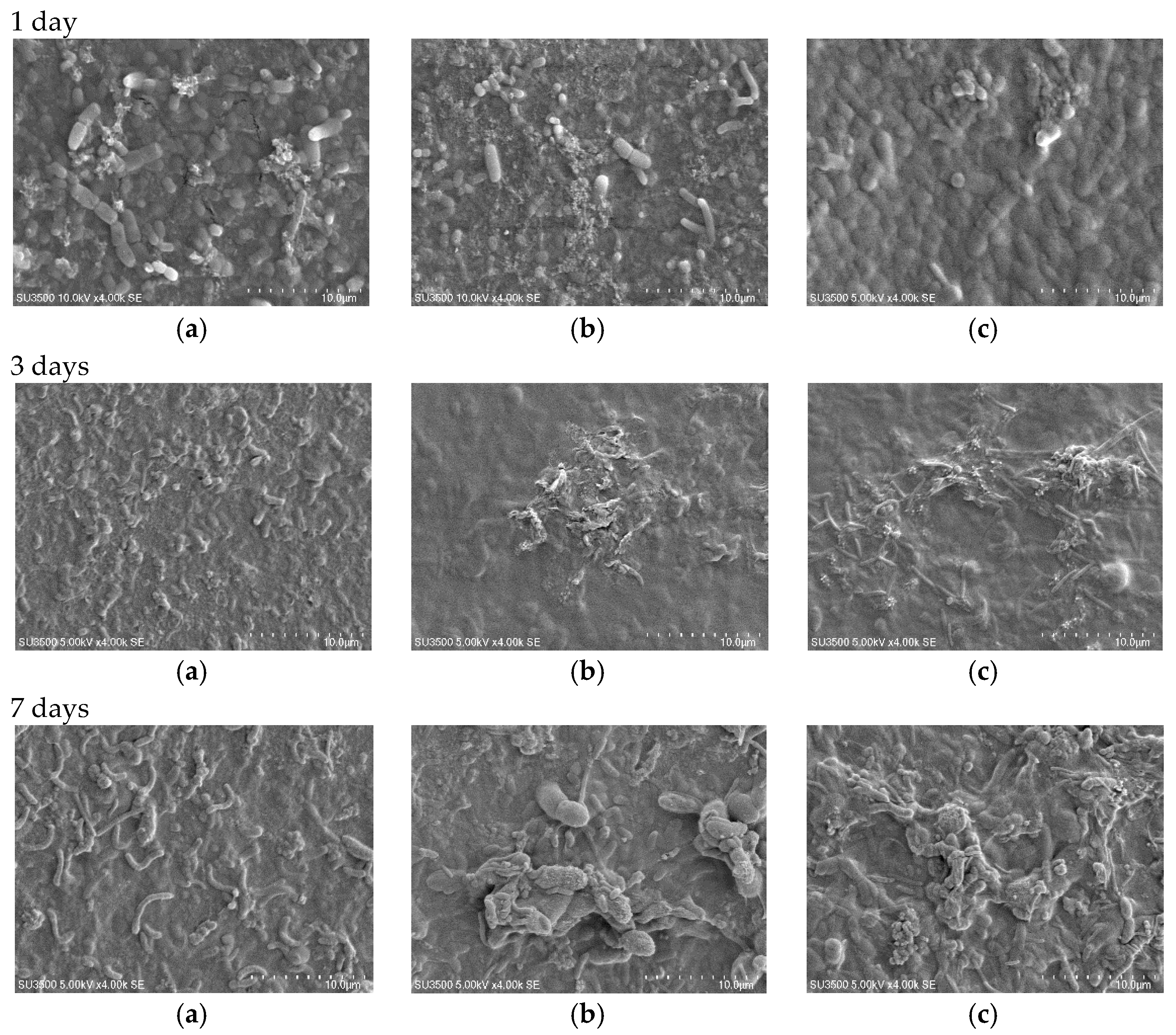
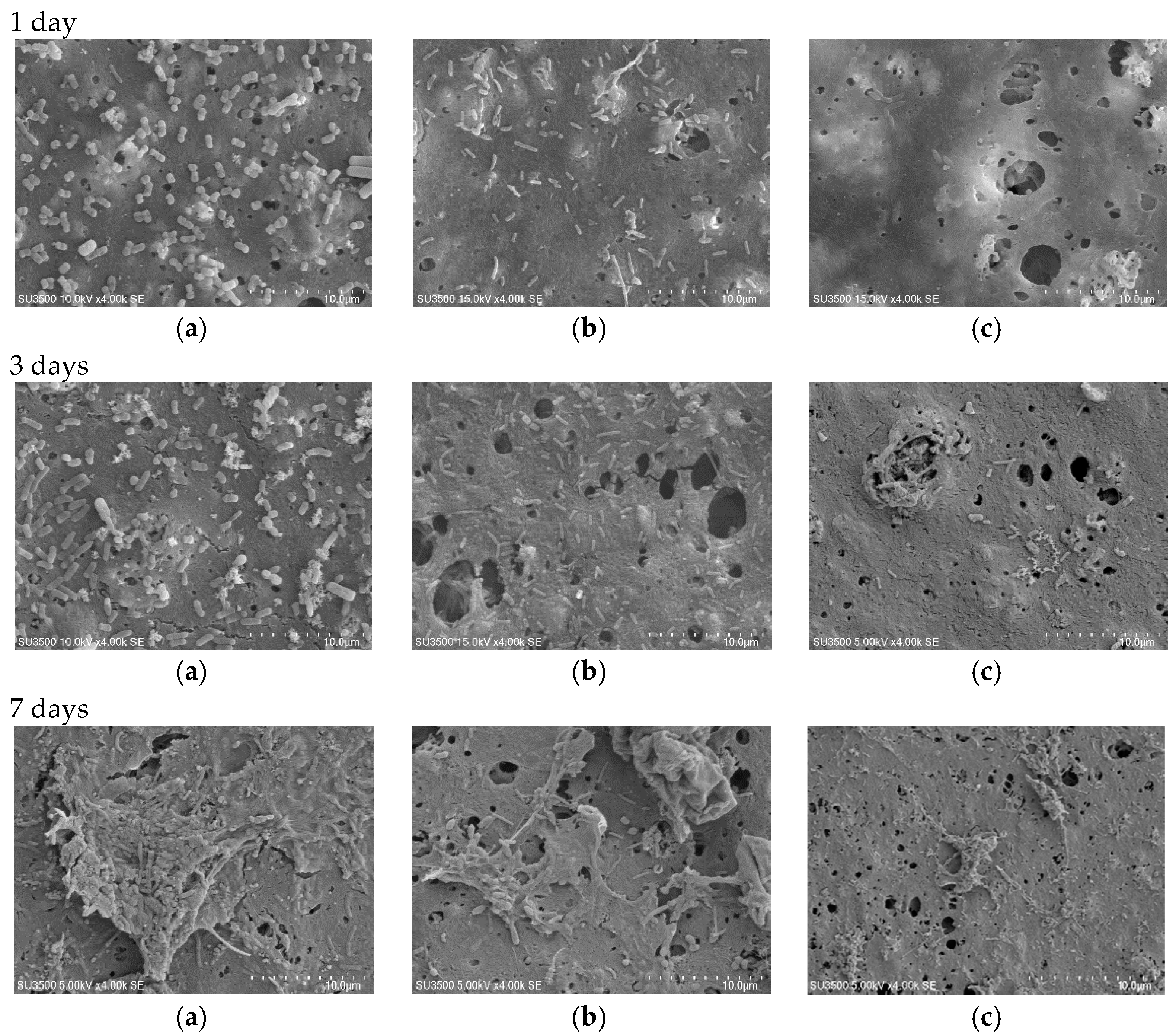
| Membrane Type | Thickness (µm) | Standard Deviation |
|---|---|---|
| Pure PVDF * | 475.625 | 8.551 |
| PVDF15 | 481.875 | 5.194 |
| PVDF30 | 509.75 | 9.750 |
| Pure PES ** | 64.5625 | 1.891 |
| PES15 | 71.4625 | 3.062 |
| PES30 | 74.3875 | 2.383 |
| Membrane Type | Contact Angle (°) |
|---|---|
| Pure PVDF | 75 ± 1 |
| PVDF15 | 108 ± 1 |
| PVDF30 | 120 ± 1 |
| Pure PES | 61 ± 1 |
| PES15 | 93 ± 1 |
| PES30 | 128 ± 1 |
| Contact Time | Silver Content | |||||||
|---|---|---|---|---|---|---|---|---|
| PVDF15 | PVDF30 | PES15 | PES30 | |||||
| Ave. | STDEV. | Ave. | STDEV. | Ave. | STDEV. | Ave. | STDEV. | |
| Before contact | 2.29 | 0.092 | 7.62 | 0.381 | 4.23 | 0.169 | 10.94 | 0.547 |
| 1 day | 2.12 | 0.085 | 7.06 | 0.282 | 3.58 | 0.143 | 9.83 | 0.393 |
| 3 days | 1.98 | 0.079 | 6.49 | 0.259 | 3.15 | 0.094 | 8.96 | 0.358 |
| 7 days | 1.66 | 0.065 | 6.29 | 0.314 | 2.85 | 0.085 | 8.76 | 0.307 |
| Membrane Type | RMS (nm) | Ra (nm) |
|---|---|---|
| Pure PVDF | 228.5 ± 8 | 203 ± 6.3 |
| PVDF15 | 196.3 ± 5.1 | 174 ± 4.8 |
| PVDF30 | 134.9 ± 4.7 | 118.5 ± 4.2 |
| Pure PES | 471.1 ± 13.8 | 409.53 ± 11.1 |
| PES15 | 358.2 ± 10.6 | 313.85 ± 8.7 |
| PES30 | 322.81 ± 7.1 | 283.32 ± 4.4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amouamouha, M.; Badalians Gholikandi, G. Characterization and Antibiofouling Performance Investigation of Hydrophobic Silver Nanocomposite Membranes: A Comparative Study. Membranes 2017, 7, 64. https://doi.org/10.3390/membranes7040064
Amouamouha M, Badalians Gholikandi G. Characterization and Antibiofouling Performance Investigation of Hydrophobic Silver Nanocomposite Membranes: A Comparative Study. Membranes. 2017; 7(4):64. https://doi.org/10.3390/membranes7040064
Chicago/Turabian StyleAmouamouha, Maryam, and Gagik Badalians Gholikandi. 2017. "Characterization and Antibiofouling Performance Investigation of Hydrophobic Silver Nanocomposite Membranes: A Comparative Study" Membranes 7, no. 4: 64. https://doi.org/10.3390/membranes7040064




