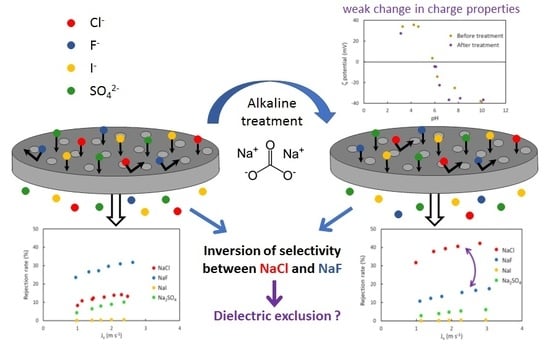
Modification of the Selectivity Properties of Tubular Ceramic Membranes after Alkaline Treatment
Abstract
:1. Introduction
2. Materials and Methods
- Zetametry measurements: all tests were repeated three times, the pH was measured with a numerical pHmeter (+/− 0.01), the observed maximal Zeta potential error was 3.6 mV.
- Rejection experiments: the permeation flux was calculated from the mass (error ≤ 0.2%) and the time measurements (error ≤ 1.7%), with a relative error less than 2%. Neutral solute rejection (Vitamin B12) was calculated from absorbance measurements according to Equation (3). The results are given in Table 1.
3. Modeling Part
4. Results and Discussion
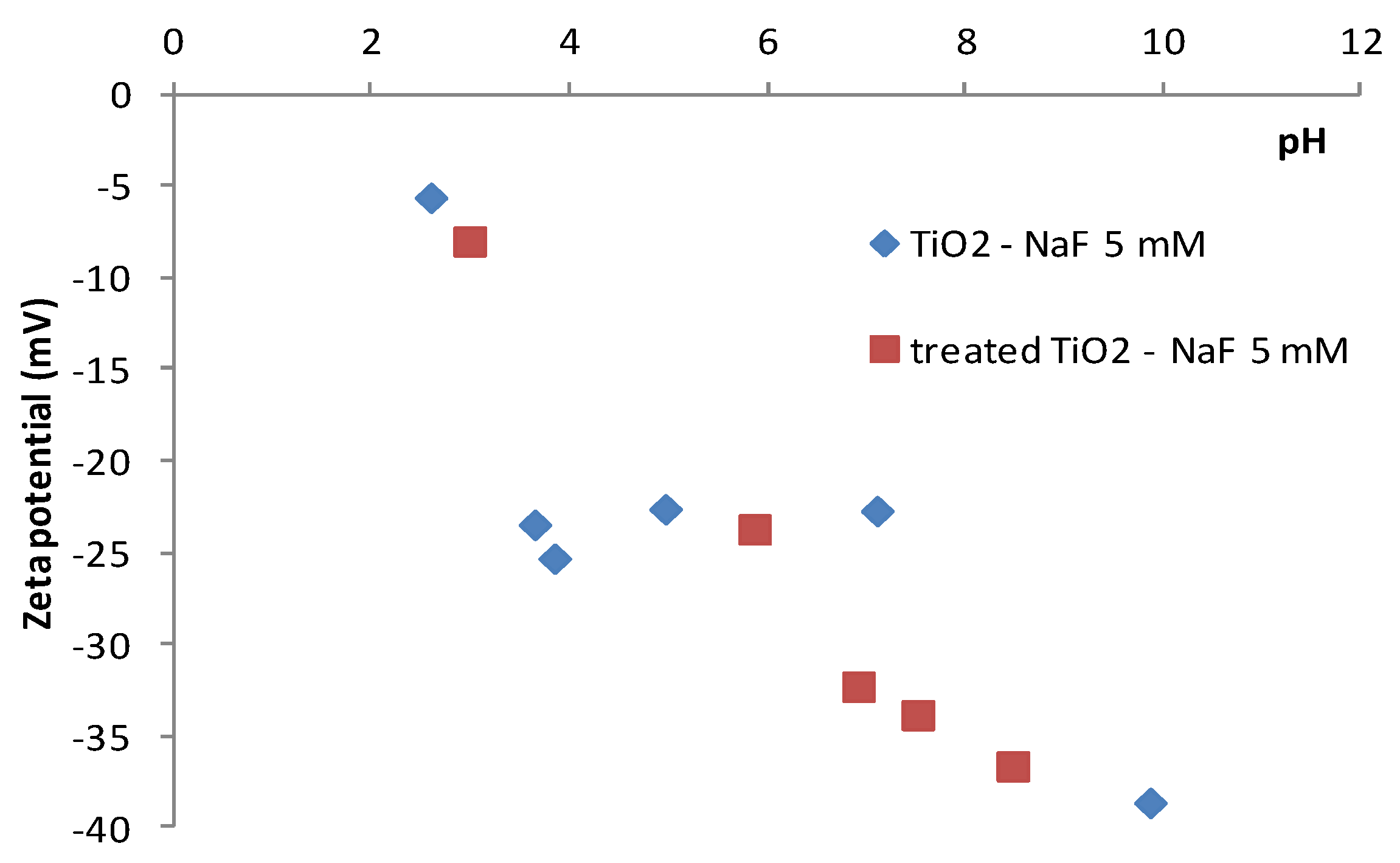
4.1. Surface Properties
4.2. Hydraulic Performances, Average Pore Radius
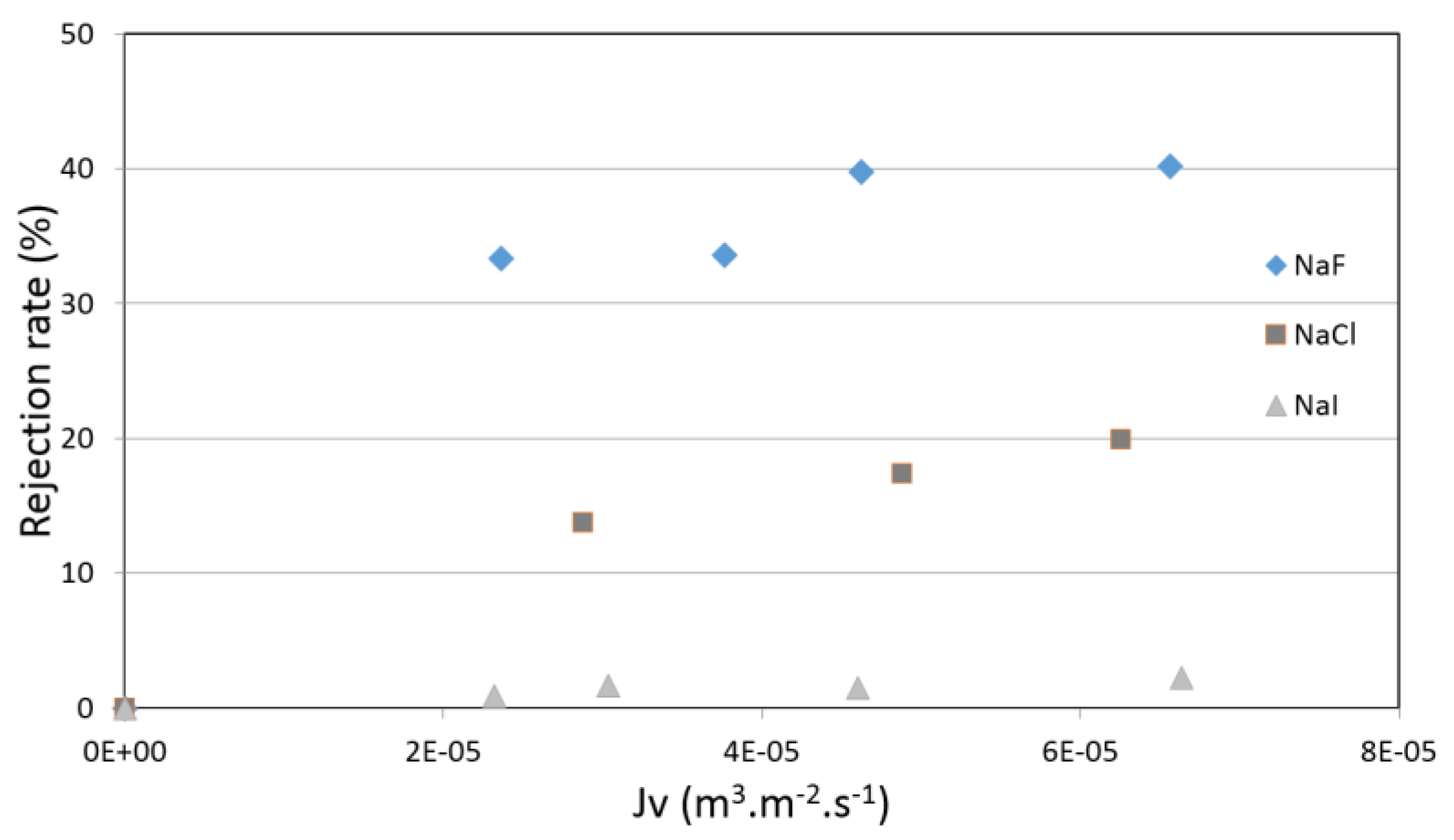
4.3. Pure Salt-Water Filtration Experiments
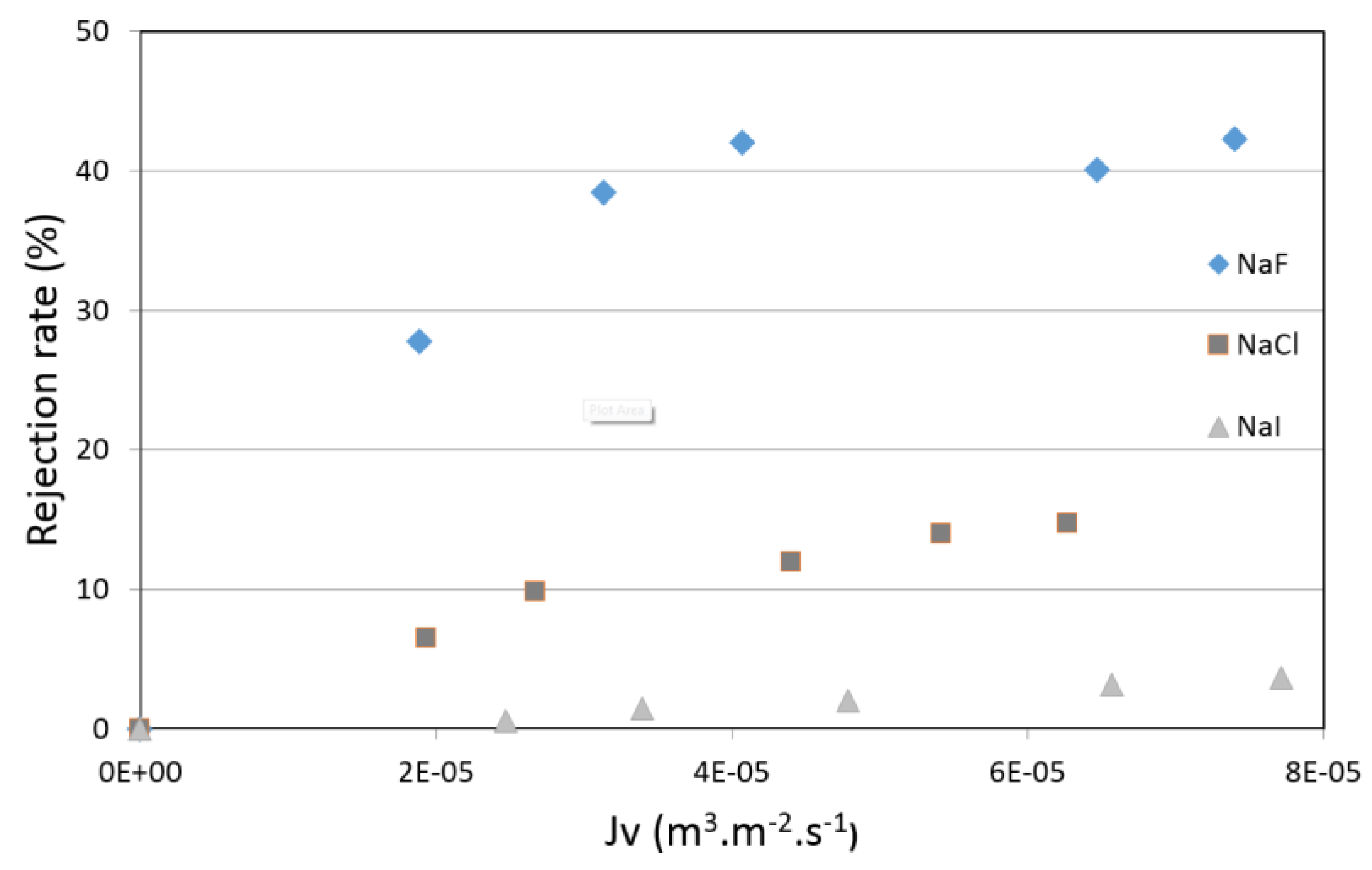
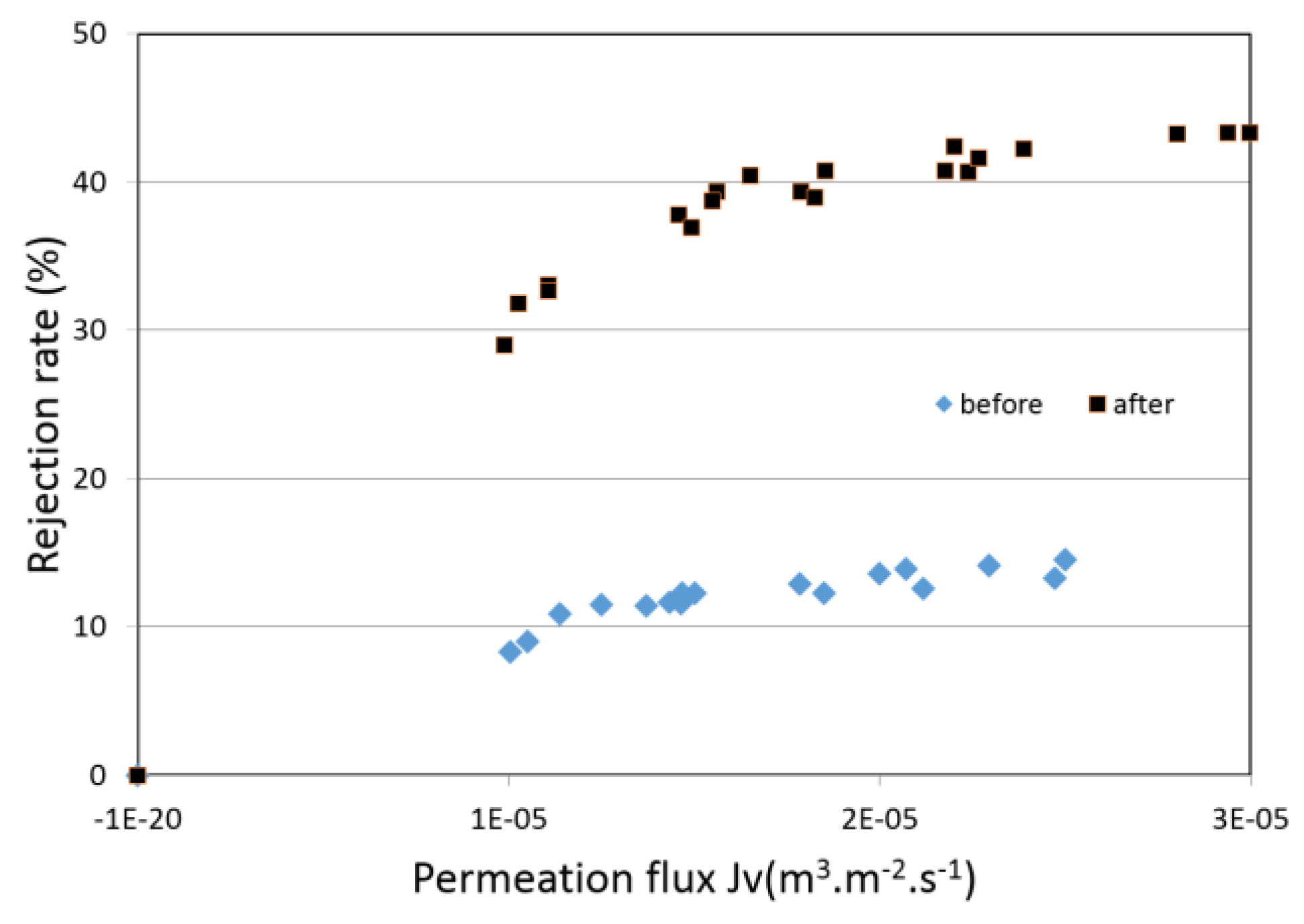
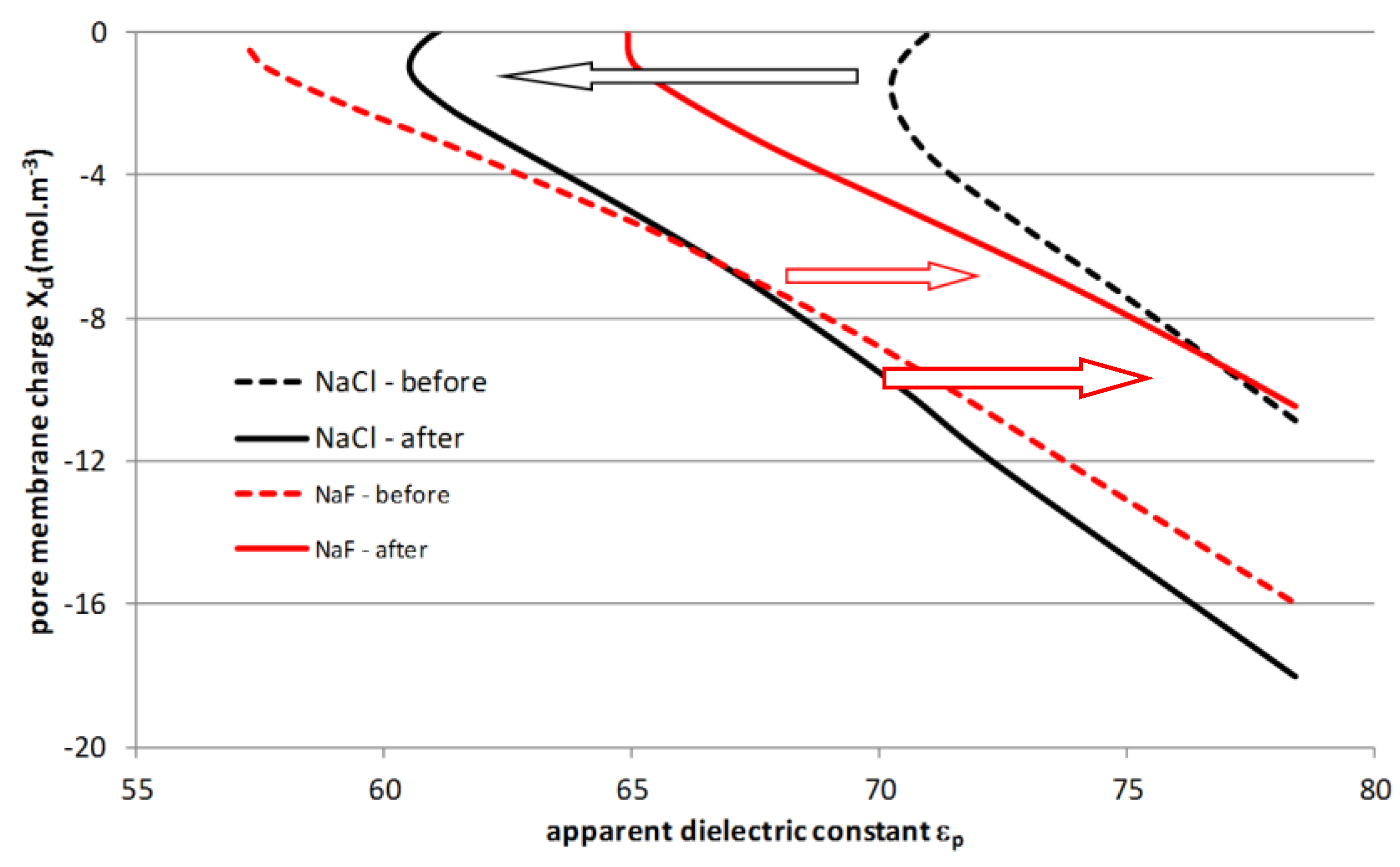
4.4. Filtration Experiments after Alkaline Treatment
5. Conclusions
Author Contributions
Conflicts of Interest
Glossary
| ci | concentration of ion i within the pore (mol·m−3) |
| Ci,p | permeate concentration of ion i (mol·m−3) |
| Ci,r | bulk concentration of ion i (mol·m−3) |
| Di,∞ | molecular diffusion coefficient of ion i at infinite dilution (m2·s−1) |
| e | electronic charge (1.602 × 10−19 C) |
| F | Faraday constant (96,487 C·mol−1) |
| ji | ionic flux of ion i (mol·m−2·s−1) |
| Jv | volumetric permeation flux (m3 m−2·s−1) |
| Jw | volumetric permeation flux of pure water (m3·m−2·s−1) |
| kB | Boltzmann constant (96,487 C·mol−1) |
| Ki,c | ionic hindrance factor for convection (dimensionless) |
| Ki,d | ionic hindrance factor for diffusion (dimensionless) |
| Lp | water permeability (m3·m−2) |
| P | pressure (Pa) |
| R | universal gas constant (8.314 J·mol−1·K−1) |
| rS(i) | Stokes radius of ion i (m) |
| Ri | observed rejection of ion i (dimensionless) |
| rp | average pore radius (m) |
| T | temperature (K) |
| V | solvent velocity in the pore (m·s−1) |
| x | axial position within the pore (m) |
| Xd | membrane effective charge density (eq·m−3) |
| zi | valence of ion i (dimensionless) |
| Greek Letters | |
| γi,p | activity coefficient of ion i in the pore (dimensionless) |
| γi,s | activity coefficient of ion i in the solution side of the interface (dimensionless) |
| ΔP | applied pressure (Pa) |
| ΔWi | dielectric exclusion energy (J) |
| ΔyD | Donnan potential (V) |
| Δp | osmotic pressure difference (Pa) |
| ε0 | permittivity of free space (8.85419 × 10−12 F·m−1) |
| εb | bulk dielectric constant (dimensionless) |
| εp | pore dielectric constant (dimensionless) |
| µ | dynamic viscosity (Pa·s) |
| φi | steric partition coefficient (dimensionless) |
| ψ | electrical potential within the pore (V) |
| Subscript | |
| i | ion i |
| p | pore |
References
- Baker, R.W. Membrane Technology and Applications, 3rd ed.; Wiley: London, UK, 2012. [Google Scholar]
- Efligenir, A.; Déon, S.; Fievet, P.; Druart, C.; Morin-Crini, N.; Crini, G. Decontamination of polluted discharge waters from surface treatment industries by pressure-driven membranes: Removal performances and environmental impact. Chem. Eng. J. 2014, 258, 298–309. [Google Scholar] [CrossRef]
- Hong, S.U.; Miller, M.D.; Bruening, M.L. Removal of dyes, sugars, and amino acids from NaCl solutions using multilayer polyelectrolyte nanofiltration membranes. Ind. Eng. Chem. Res. 2006, 45, 6284–6288. [Google Scholar] [CrossRef]
- Knorr, D.; Froehling, A.; Jaeger, H.; Reineke, K.; Schlueter, O.; Schoessler, K. Emerging technologies in food processing. Annu. Rev. Food Sci. Technol. 2011, 2, 203–235. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.H.; Todaro, C.M. Fermentation and Biochemical. In Engineering Handbook, 3rd ed.; Elsevier: Waltham, MA, USA, 2014. [Google Scholar]
- Weber, R.; Chmiel, H.; Mavrov, V. Characteristics and application of new ceramic nanofiltration membranes. Desalination 2003, 157, 113–125. [Google Scholar] [CrossRef]
- Ha, J.H.; Jung, D.W.; Song, I.H. The effect of an alumina coating on the pore characteristics of a diatomite-kaolin composite support layer. Ceram. Int. 2014, 40, 12961–12967. [Google Scholar] [CrossRef]
- Anderson, M.A.; Gieselmann, M.J.; Xu, Q. Titania and alumina ceramic membranes. J. Membr. Sci. 1988, 39, 243–258. [Google Scholar] [CrossRef]
- Nandi, B.K.; Uppaluri, R.; Purkait, M.K. Treatment of oily waste water using low-cost ceramic membrane: Flux decline mechanism and economic feasibility. Sep. Sci. Technol. 2009, 44, 2840–2869. [Google Scholar] [CrossRef]
- Li, L.; Lee, R. Purification of produced water by ceramic membranes: Material screening, process design and economics. Sep. Sci. Technol. 2009, 44, 3455–3484. [Google Scholar] [CrossRef]
- Condom, S.; Larbot, A.; Younssi, S.A.; Persin, M. Use of ultra- and nanofiltration ceramic membranes for desalination. Desalination 2004, 168, 207–213. [Google Scholar] [CrossRef]
- Song, Z.; Fathizadeh, M.; Huang, Y.; Chu, K.H.; Yoon, Y.; Wang, L.; Xu, W.L.; Yu, M. TiO2 nanofiltration membranes prepared by molecular deposition for water purification. J. Memb. Sci. 2016, 510, 72–78. [Google Scholar] [CrossRef]
- Rahimi, N.; Pax, R.A.; Gray, E.A. Review of functional titanium oxides. I: TiO2 and its modifications. Prog. Solid State Chem. 2016, 44, 86–105. [Google Scholar] [CrossRef]
- Mozia, S.; Szymański, K.; Michalkiewicz, B.; Tryba, B.; Toyoda, M.; Morawski, A.W. Effect of process parameters on fouling and stability of MF/UF TiO2 membranes in a photocatalytic membrane reactor. Sep. Purif. Technol. 2015, 142, 137–148. [Google Scholar] [CrossRef]
- Dutournié, P.; Limousy, L.; Déon, S.; Bourseau, P. Unsteady transport of divalent salt through a mineral membrane of ultrafiltration: Evolution of ionic adsorption equilibrium. Desalination 2011, 265, 184–189. [Google Scholar]
- Shang, R.; Verliefde, A.R.D.; Hu, J.; Zeng, Z.; Lu, J.; Kemperman, A.J.B.; Deng, H.; Nijmeijer, K.; Heijman, S.G.J.; Rietveld, L.C. Tight ceramic UF membrane as RO pre-treatment: The role of electrostatic interactions on phosphate rejection. Water Res. 2014, 48, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Albo, J.; Hagiwara, H.; Yanagishita, H.; Ito, K.; Tsuru, T. Structural characterization of thin-film polyamide reverse osmosis membranes. Ind. Eng. Chem. Res. 2014, 53, 1442–1451. [Google Scholar] [CrossRef]
- Albo, J.; Wang, J.; Tsuru, T. Gas transport properties of interfacially polymerized polyamide compsite membranes under different pre-treatments and temperatures. J. Membr. Sci. 2014, 449, 109–118. [Google Scholar] [CrossRef]
- Albo, J.; Wang, J.; Tsuru, T. Application of interfacially polymerized polyamide composite membranes to isopropanol dehydration: Effect of membrane pre-treatment and temperature. J. Membr. Sci. 2014, 453, 384–393. [Google Scholar] [CrossRef]
- Kim, J.; Van der Bruggen, B. The use of nanoparticles in polymeric and ceramic membrane structures: Review of manufacturing procedures and performance improvment for water treatment. Environ. Pollut. 2010, 158, 2335–2349. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.C.C.; Li, C.J. Tubular TiO2/Al2O3 composite membranes: Preparation, characterization, and performance in electrofiltration of oxide-CMP wastewater. Desalination 2008, 234, 354–361. [Google Scholar] [CrossRef]
- Dutournié, P.; Said, A.; Daou, T.J.; Bikaï, J.; Limousy, L. Hydraulic Performance Modifications of a Zeolite Membrane after an Alkaline Treatment: Contribution of Polar and Apolar Surface Tension Components. Adv. Mater. Sci. Eng. 2015. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, D.; Jiang, L.; Jin, J. Recent progress in developing advanced membranes for emulsified oil/water separation. NPG Asia Mater. 2014, 6, e101. [Google Scholar] [CrossRef]
- Luo, J.; Wan, Y. Effects of pH and salt on nanofiltration—a critical review. J. Membr. Sci. 2013, 438, 18–28. [Google Scholar] [CrossRef]
- Tanioka, A.; Matsumoto, H.; Ohshima, H. Membrane potential as a function of dielectric constant. In Encyclopedia of Biocolloid and Biointerface Science, 1st ed.; Wiley & sons, Inc.: Hoboken, NJ, USA, 2016; Volume 2. [Google Scholar]
- Bikaï, J.; Limousy, L.; Dutournié, P.; Josien, L.; Blel, W. Stabilisation of the water permeability of mineral ultrafiltration membranes: An empirical modelling of surface and pore hydration. C R Chim. 2015, 18, 56–62. [Google Scholar] [CrossRef]
- Bowen, W.R.; Welfoot, J.S. Modelling the performance of membrane nanofiltration-critical assessment and model development. Chem. Eng. Sci. 2002, 57, 1121–1137. [Google Scholar] [CrossRef]
- Déon, S.; Dutournié, P.; Limousy, L.; Bourseau, P. The two-dimensional pore and polarization transport model to describe mixture separation by nanofiltration: Model validation. AIChE J. 2011, 57, 985–995. [Google Scholar] [CrossRef]
- Bandini, S.; Vezzani, D. Nanofiltration modeling: The role of dielectric exclusion in membrane characterization. Chem. Eng. Sci. 2003, 58, 3303–3326. [Google Scholar] [CrossRef]
- Chevereau, E.; Zouaoui, N.; Limousy, L.; Dutournié, P.; Déon, S.; Bourseau, P. Surface properties of ceramic ultrafiltration TiO2 membranes: Effects of surface equilibriums on salt retention. Desalination 2010, 255, 1–8. [Google Scholar] [CrossRef]
- Bowen, W.R.; Welfoot, J.S.; Williams, P.M. Linearized transport model for nanofiltration: Development and assessment. AIChE J. 2002, 48, 760–773. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 87th ed.; Taylor & Francis: Boca Raton, FL, USA, 2007. [Google Scholar]
- Srivastava, K.; Kumar, S.; Kumar, S. Studies with Inorganic Ion-exchange Membranes. Effect of Binding Material on the Permeation of Electrolyte Solutions across Titanium tungstoarsenate Membranes. Bull. Chem. Soc. Jpn. 1985, 58, 2376–2379. [Google Scholar] [CrossRef]
- Lin, Y.L.; Lee, C.H. Elucidating the rejection mechanisms of PPCPs by nanofiltration and reverse osmosis membranes. Ind. Eng. Chem. Res. 2014, 53, 6798–6806. [Google Scholar] [CrossRef]
- Mickols, W. Substantial Changes in the transport model of reverse osmosis and nanofiltration by incorporating accurate activity data of electrolytes. Ind. Eng. Chem. Res. 2016, 55, 11139–11149. [Google Scholar] [CrossRef]
- Szymczyk, A.; Fievet, P. Investigating transport properties of nanofiltration membranes by means of a steric, electric and dielectric exclusion model. J. Membr. Sci. 2005, 252, 77–88. [Google Scholar] [CrossRef]
- Dutournié, P.; Limousy, L.; Blel, W.; Déon, S.; Fievet, P. Understanding of ion transport in a Na-Mordenite membrane: Use of numerical modeling to estimate surface-solute interactions in the pore. Ind. Eng. Chem. Res. 2014, 53, 8221–8227. [Google Scholar] [CrossRef]
- Tessman, J.R.; Kahn, A.H.; Shockley, W. Electronic polarizabilities of ions in crystals. Phys. Rev. 1953, 92, 890–895. [Google Scholar] [CrossRef]
- Jungwirth, P.; Curtis, J.E.; Tobias, D.J. Polarizability and aqueous solvation of the sulphate dianion. Chem. Phys. Lett. 2003, 367, 704–710. [Google Scholar] [CrossRef]
- Dutournié, P.; Limousy, L.; Zouaoui, N.; Mahzoul, H.; Chevereau, E. Facilitated transport of monovalent salt mixture through ultrafiltration Na-Mordenite membrane: Numerical investigations of electric and dielectric contributions. Desalination 2011, 280, 397–402. [Google Scholar] [CrossRef]
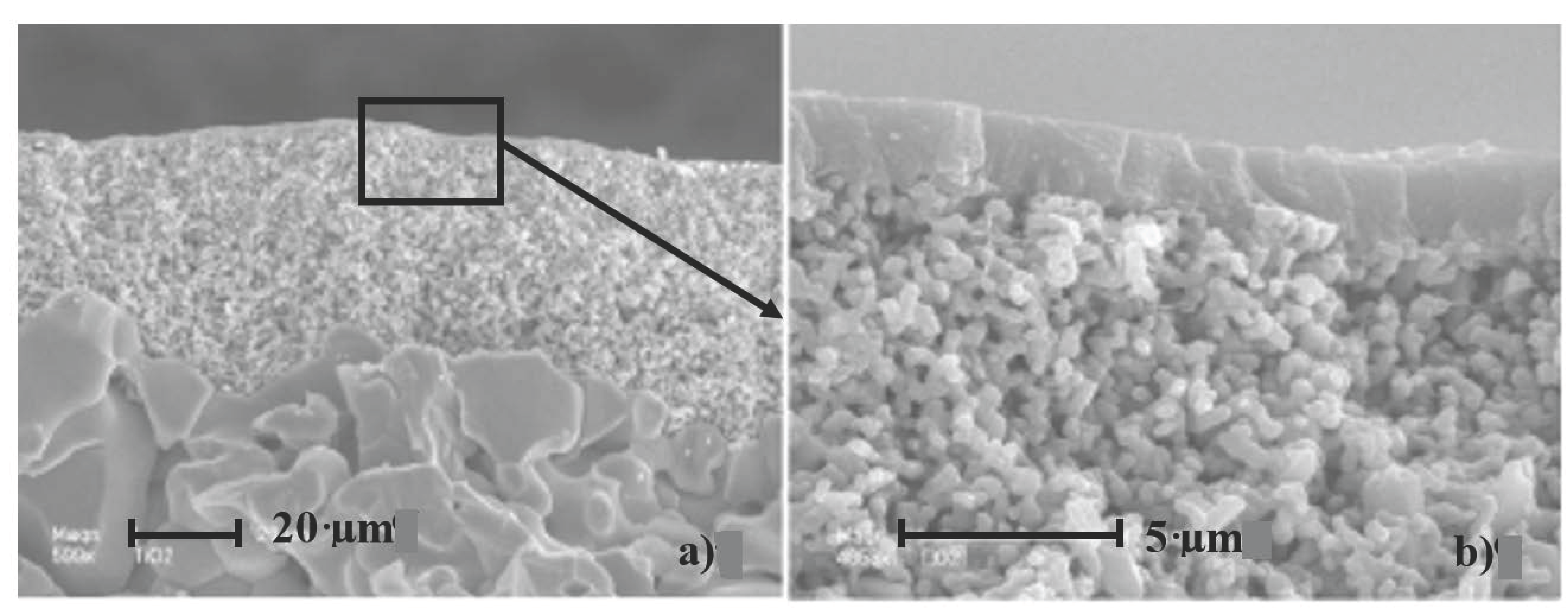
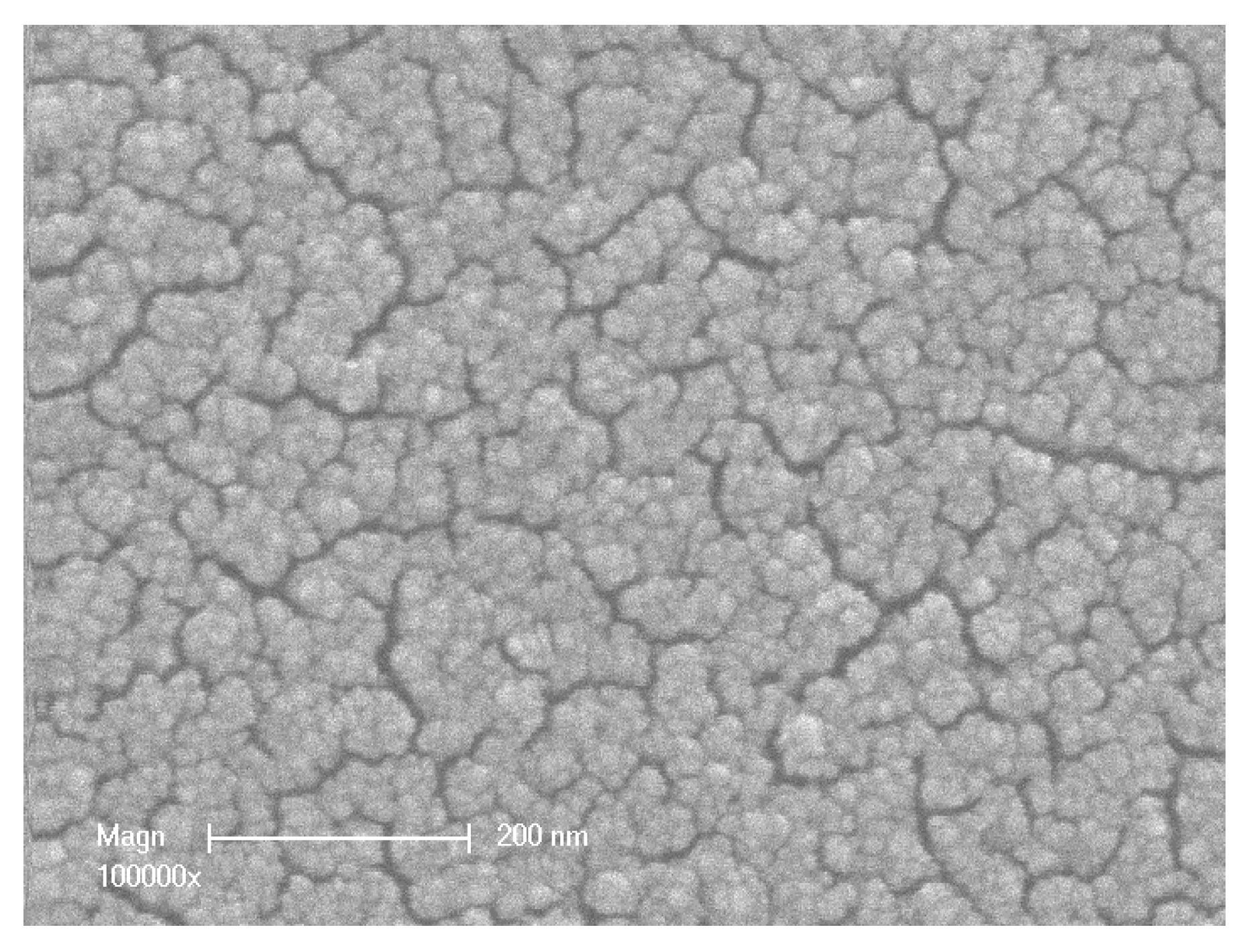
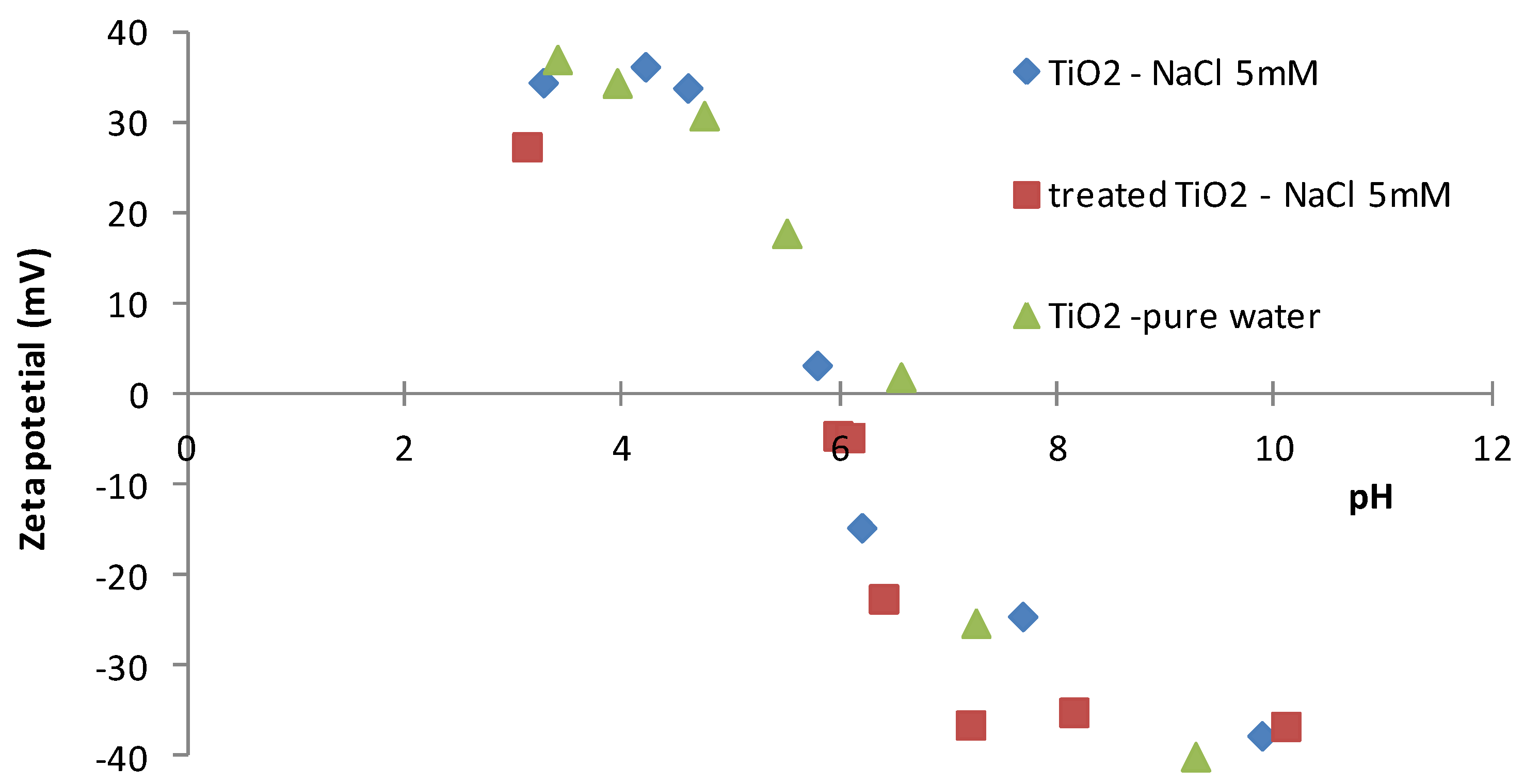

| Membrane Performances | Membrane 1 | Membrane 2 | Membrane 3 |
|---|---|---|---|
| 1014 Lp (m3·m−2memb) | 2.95 | 3.8 | 5.7 |
| Rejection rate (%) | 69 (±1.9) | 60 (±2.4) | 33 (±4.0) |
| Average pore radius (nm) | 1.4 | 1.5 | 2.3 |
| Maximal Rejection Rate (%) | Membrane 1 | Membrane 2 | Membrane 3 |
|---|---|---|---|
| NaCl (5 mM) | 14 | 20 | 15 |
| NaF (5 mM) | 32 | 40 | 42 |
| NaI (5 mM) | ≈0 | 2 | 3 |
| Na2SO4 (2.5 mM) | 10 | 7 | 4 |
| NaBr (5 mM) | -- | 17 | -- |
| Membrane Performances | Membrane 1 | Membrane 2 | Membrane 3 | |||
|---|---|---|---|---|---|---|
| before | after | before | after | before | after | |
| 1014 Lp (m3·m−2memb) | 2.95 | 3.1 | 3.8 | 4.3 | 5.7 | 6.0 |
| VB 12 rejection rate (%) | 69 | -- | 60 | 56 | 33 | 56 |
| Average pore radius (nm) | 1.4 | -- | 1.5 | 1.6 | 2.3 | 1.6 |
| Maximal Rejection Rate (%) | Membrane 1 | Membrane 2 | Membrane 3 | |||
|---|---|---|---|---|---|---|
| before | after | before | after | before | after | |
| NaCl (5 mM) | 14 | 42 | 20 | 35 | 15 | 36 |
| NaF (5 mM) | 32 | 18 | 40 | 32 | 42 | 26 |
| NaI (5 mM) | 0 | 0 | 2 | 3 | 3 | 8 |
| Na2SO4 (2.5 mM) | 10 | 8 | 7 | 7 | 4 | 2 |
| NaBr (5 mM) | -- | -- | 17 | 29 | -- | -- |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutournié, P.; Limousy, L.; Anquetil, J.; Déon, S. Modification of the Selectivity Properties of Tubular Ceramic Membranes after Alkaline Treatment. Membranes 2017, 7, 65. https://doi.org/10.3390/membranes7040065
Dutournié P, Limousy L, Anquetil J, Déon S. Modification of the Selectivity Properties of Tubular Ceramic Membranes after Alkaline Treatment. Membranes. 2017; 7(4):65. https://doi.org/10.3390/membranes7040065
Chicago/Turabian StyleDutournié, Patrick, Lionel Limousy, Jérôme Anquetil, and Sébastien Déon. 2017. "Modification of the Selectivity Properties of Tubular Ceramic Membranes after Alkaline Treatment" Membranes 7, no. 4: 65. https://doi.org/10.3390/membranes7040065