Impact of the Interaction between Aquatic Humic Substances and Algal Organic Matter on the Fouling of a Ceramic Microfiltration Membrane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of MF Feed Solutions
2.2. Microfiltration Tests
2.3. Analytical Methods
3. Results and Discussion
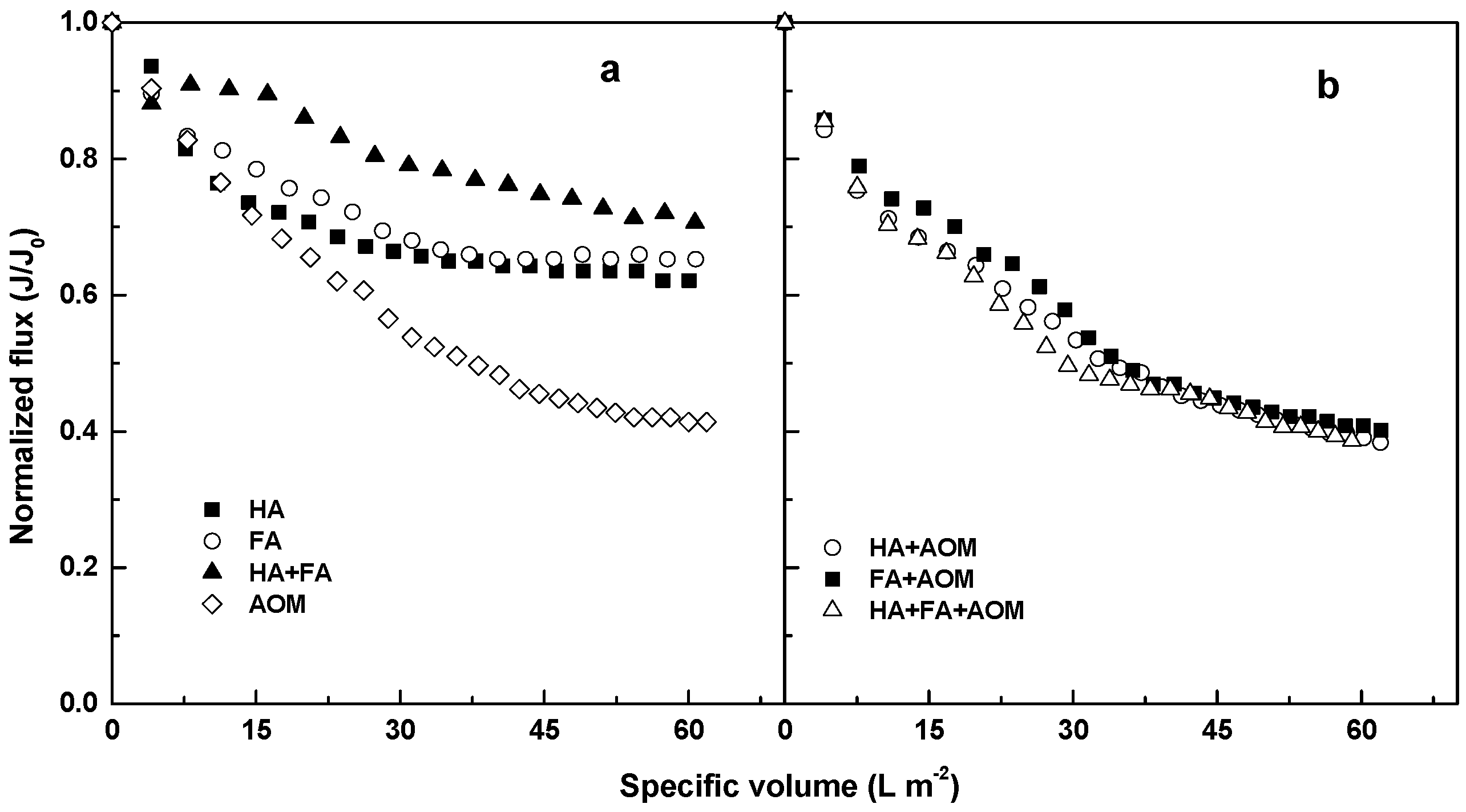
3.1. Microfiltration of the Solutions Containing Individual and Mixed Compounds
3.2. Characterization of the Feed Solutions
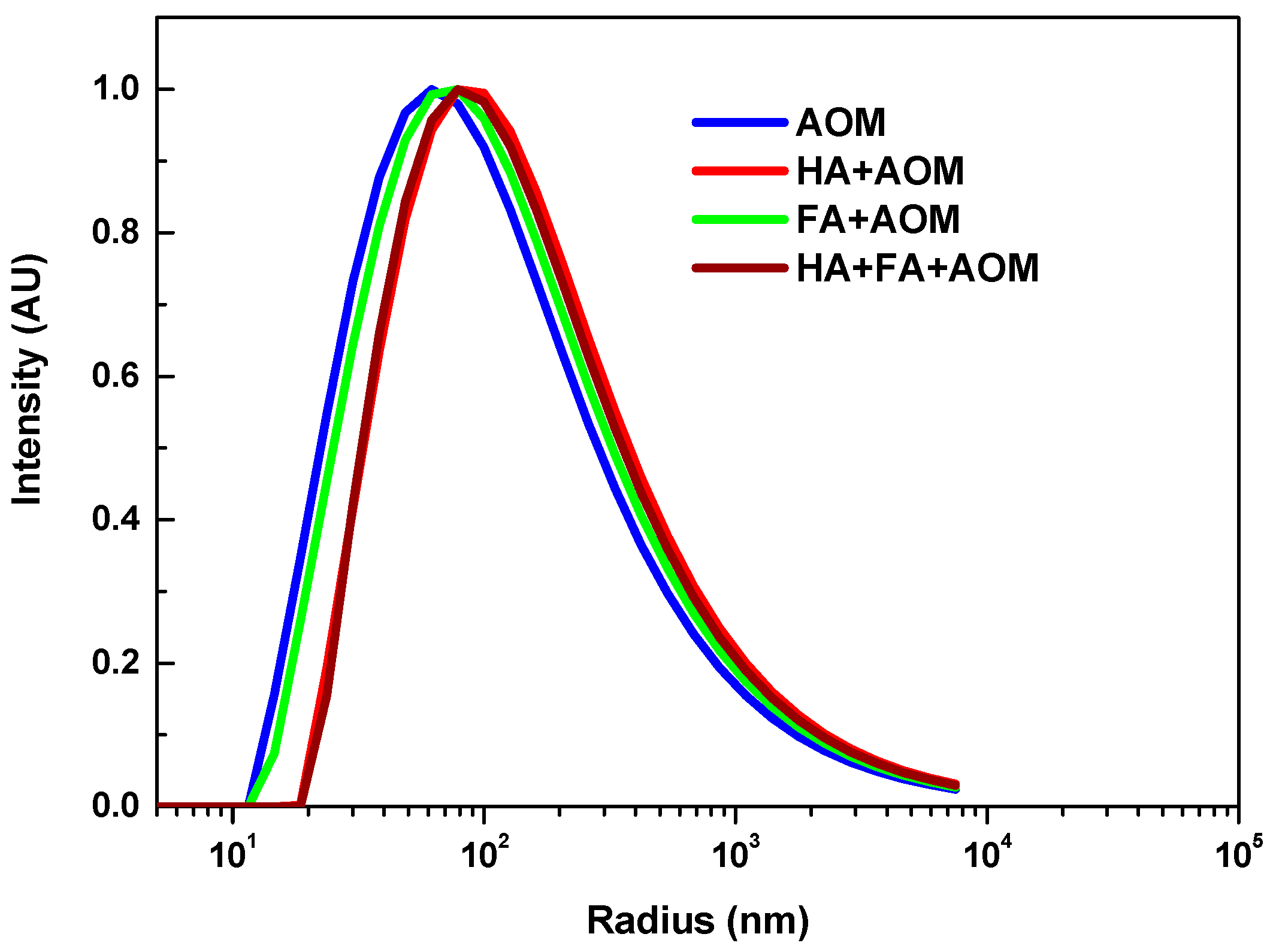
3.2.1. Hydrodynamic Molecular Size
3.2.2. Solution Zeta Potential
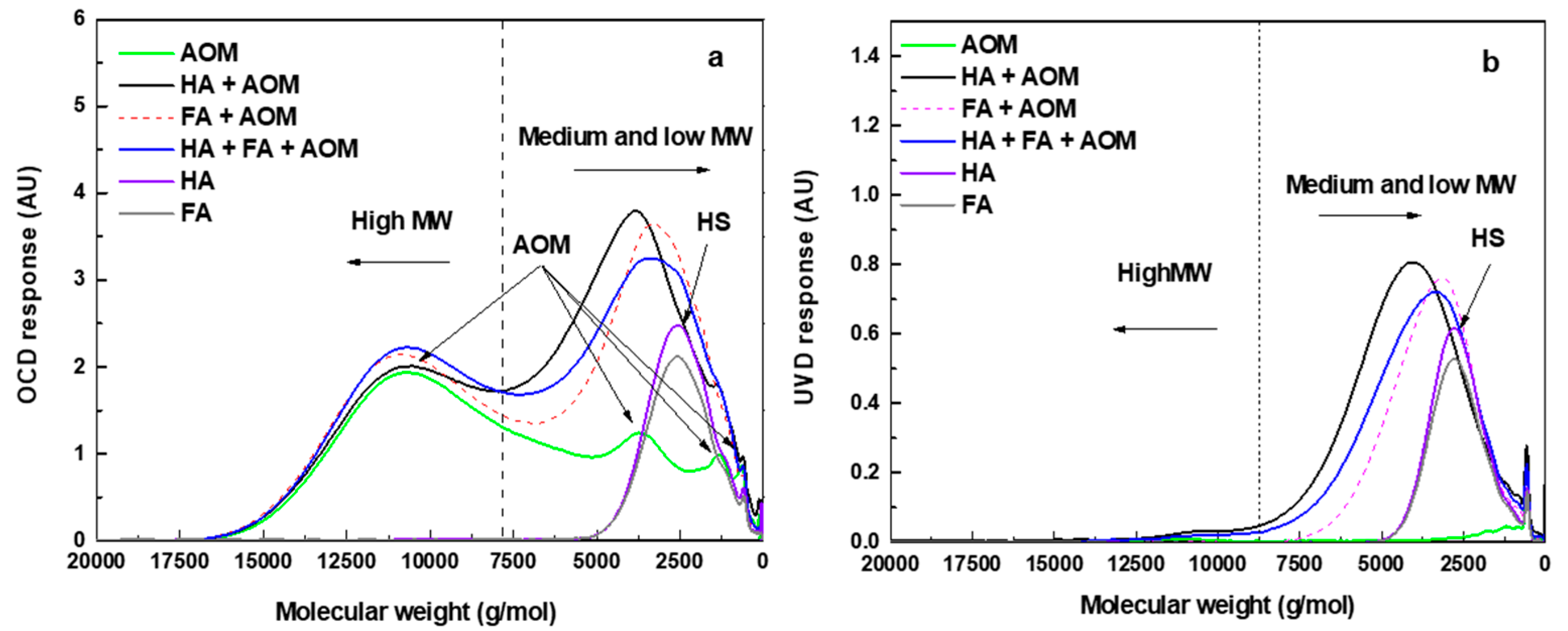
3.2.3. Molecular Weight Distribution
3.2.4. Fractionation of Organics in the Feed Solution
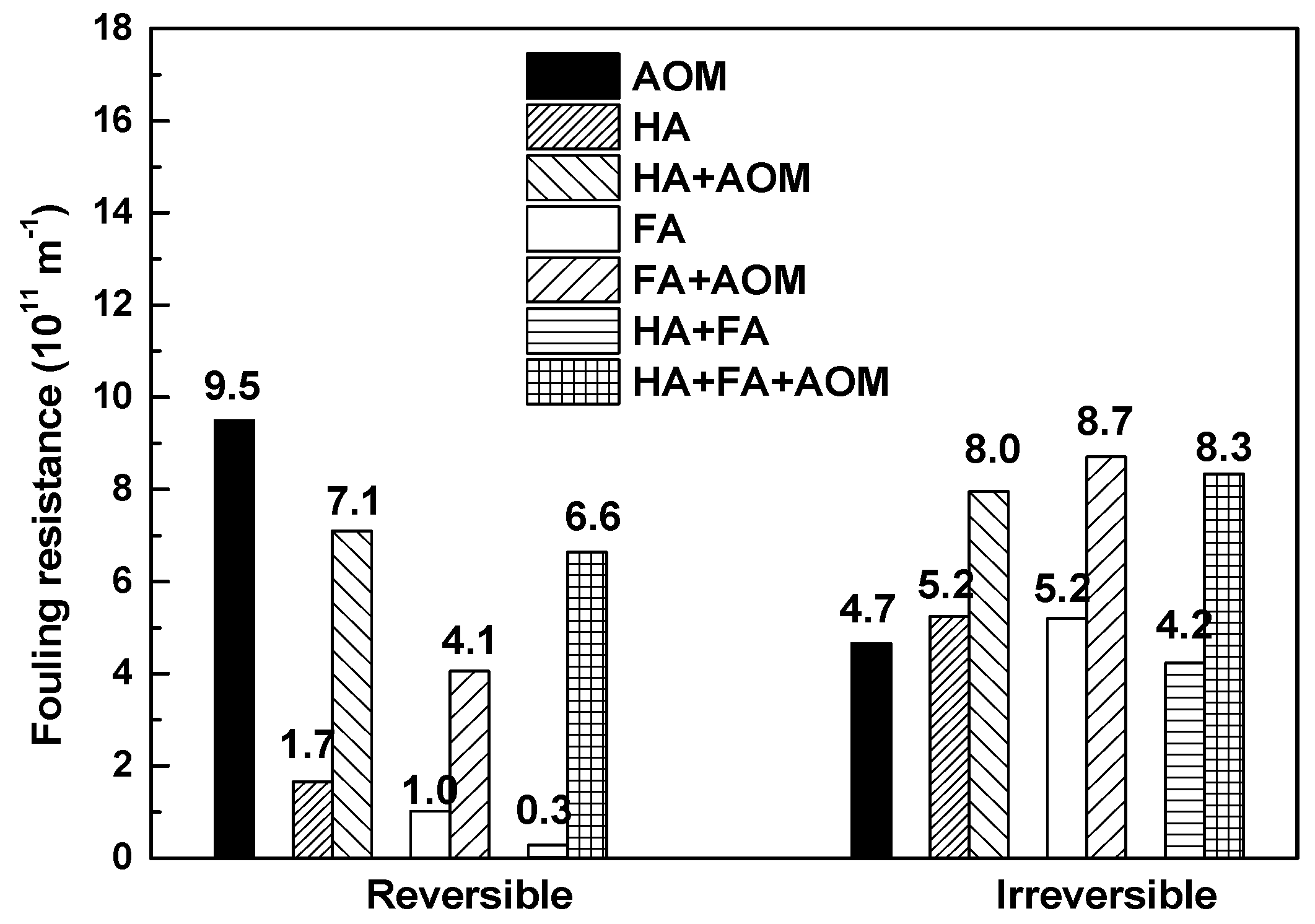
3.3. Membrane Fouling Mechanism
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hofs, B.; Ogier, J.; Vries, D.; Beerendonk, E.F.; Cornelissen, E.R. Comparison of ceramic and polymeric membrane permeability and fouling using surface water. Sep. Purif. Technol. 2011, 79, 365–374. [Google Scholar] [CrossRef]
- Ibn Abdul Hamid, K.; Sanciolo, P.; Gray, S.; Duke, M.; Muthukumaran, S. Impact of ozonation and biological activated carbon filtration on ceramic membrane fouling. Water Res. 2017, 126, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ding, A.; Gan, Z.; Qu, F.; Cheng, X.; Bai, L.; Guo, S.; Li, G.; Liang, H. Fluorescent natural organic matter responsible for ultrafiltration membrane fouling: Fate, contributions and fouling mechanisms. Chemosphere 2017, 182, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chang, C.-Y.; Hou, J.; Gao, C. Comparison of approaches to minimize fouling of a UF ceramic membrane in filtration of seawater. Chem. Eng. J. 2013, 223, 722–728. [Google Scholar] [CrossRef]
- Hang, H.; Liang, H.; Qu, F.; Liu, B.; Yu, H.; Du, X.; Li, G.; Snyder, S.A. Hydraulic backwashing for low-pressure membranes in drinking water treatment: A review. J. Membr. Sci. 2017, 540, 362–380. [Google Scholar]
- Fan, L.; Harris, J.L.; Roddick, F.A.; Booker, N.A. Influence of the characteristics of natural organic matter on the fouling of microfiltration membranes. Water Res. 2001, 35, 4455–4463. [Google Scholar] [CrossRef]
- Cho, J.; Amy, G.; Pellegrino, J. Membrane filtration of natural organic matter: Initial comparison of rejection and flux decline characteristics with ultrafiltration and nanofiltration membranes. Water Res. 1999, 33, 2517–2526. [Google Scholar] [CrossRef]
- Lee, N.; Amy, G.; Croué, J.-P. Low-pressure membrane (MF/UF) fouling associated with allochthonous versus autochthonous natural organic matter. Water Res. 2006, 40, 2357–2368. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Zydney, A.L. Humic acid fouling during microfiltration. J. Membr. Sci. 1999, 157, 1–12. [Google Scholar] [CrossRef]
- Yuan, W.; Zydney, A.L. Humic Acid Fouling during ultrafiltration. Environ. Sci. Technol. 2000, 34, 5043–5050. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, B.; Qu, F.; Ding, A.; Liang, H.; Zhao, Y.; Li, G. Control of ultrafiltration membrane fouling caused by algal extracellular organic matter (EOM) using enhanced Al coagulation with permanganate. Sep. Purif. Technol. 2017, 172, 51–58. [Google Scholar] [CrossRef]
- Huang, W.; Hu, M.; Qin, X.; Zhou, W.; Lv, W.; Dong, B. Fouling of extracellular algal organic matter during ultrafiltration: The influence of iron and the fouling mechanism. Algal Res. 2017, 25, 252–262. [Google Scholar] [CrossRef]
- Zaouri, N.; Gutierrez, L.; Dramas, L.; Garces, D.; Croue, J.-P. Interfacial interactions between Skeletonema costatum extracellular organic matter and metal oxides: Implications for ceramic membrane filtration. Water Res. 2017, 116, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Shao, Y.; Gao, N.; Li, L.; Deng, J.; Tan, C.; Zhu, M. Influence of hydrophobic/hydrophilic fractions of extracellular organic matters of Microcystis aeruginosa on ultrafiltration membrane fouling. Sci. Total Environ. 2014, 470, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Babel, S.; Takizawa, S. Microfiltration membrane fouling and cake behavior during algal filtration. Desalination 2010, 261, 46–51. [Google Scholar] [CrossRef]
- Goh, Y.; Harris, J.; Roddick, F. Impact of Microcystis aeruginosa on membrane fouling in a biologically treated effluent. Water Sci. Technol. 2011, 63, 2853–2859. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Liang, H.; Wang, Z.; Wang, H.; Yu, H.; Li, G. Ultrafiltration membrane fouling by extracellular organic matters (EOM) of Microcystis aeruginosa in stationary phase: Influences of interfacial characteristics of foulants and fouling mechanisms. Water Res. 2012, 46, 1490–1500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fan, L.; Roddick, F.A. Influence of the characteristics of soluble algal organic matter released from Microcystis aeruginosa on the fouling of a ceramic microfiltration membrane. J. Membr. Sci. 2013, 425–426, 23–29. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, L.; Roddick, F.A. Understanding the fouling of a ceramic microfiltration membrane caused by algal organic matter released from Microcystis aeruginosa. J. Membr. Sci. 2013, 447, 362–368. [Google Scholar] [CrossRef]
- Qu, F.; Liang, H.; Zhou, J.; Nan, J.; Shao, S.; Zhang, J.; Li, G. Ultrafiltration membrane fouling caused by extracellular organic matter (EOM) from Microcystis aeruginosa: Effects of membrane pore size and surface hydrophobicity. J. Membr. Sci. 2014, 449, 58–66. [Google Scholar] [CrossRef]
- Xiao, F.; Xiao, P.; Zhang, W.J.; Wang, D.S. Identification of key factors affecting the organic fouling on low-pressure ultrafiltration membranes. J. Membr. Sci. 2013, 447, 144–152. [Google Scholar] [CrossRef]
- Myat, D.T.; Stewart, M.B.; Mergen, M.; Zhao, O.; Orbell, J.D.; Gray, S. Experimental and computational investigations of the interactions between model organic compounds and subsequent membrane fouling. Water Res. 2014, 48, 108–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malcolm, R.L.; MacCarthy, P. Limitations in the use of commercial humic acids in water and soil research. Environ. Sci. Technol. 1986, 20, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Bolch, C.J.S.; Blackburn, S.I. Isolation and purification of Australian isolates of the toxic cyanobacterium Microcystis aeruginosa Kütz. J. Appl. Phycol. 1996, 8, 5–13. [Google Scholar] [CrossRef]
- Thurman, E.M.; Malcolm, R.L. Preparative isolation of aquatic humic substances. Environ. Sci. Technol. 1981, 15, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Serkiz, S.M.; Perdue, E.M. Isolation of dissolved organic matter from the Suwannee river using reverse osmosis. Water Res. 1990, 24, 911–916. [Google Scholar] [CrossRef]
- Ni, J.; Yu, Y.; Feng, W.; Yan, Q.; Pan, G.; Yang, B.; Zhang, X.; Li, X. Impacts of algal blooms removal by chitosan-modified soils on zooplankton community in Taihu Lake, China. J. Environ. Sci. 2010, 22, 1500–1507. [Google Scholar] [CrossRef]
- Gao, N.; Li, M.; Jing, W.; Fan, Y.; Xu, N. Improving the filtration performance of ZrO2 membrane in non-polar organic solvents by surface hydrophobic modification. J. Membr. Sci. 2011, 375, 276–283. [Google Scholar] [CrossRef]
- Huber, S.A.; Balz, A.; Abert, M.; Pronk, W. Characterisation of aquatic humic and non-humic matter with size-exclusion chromatography-organic carbon detection-organic nitrogen detection (LC-OCD-OND). Water Res. 2011, 45, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Aiken, G.R.; McKnight, D.M.; Thorn, K.A.; Thurman, E.M. Isolation of hydrophilic organic acids from water using nonionic macroporous resins. Org. Geochem. 1992, 18, 567–573. [Google Scholar] [CrossRef]
- Hanaor, D.; Michelazzi, M.; Leonelli, C.; Sorrell, C.C. The effects of carboxylic acids on the aqueous dispersion and electrophoretic deposition of ZrO2. J. Eur. Ceram. Soc. 2012, 32, 235–244. [Google Scholar] [CrossRef]
- Her, N.; Amy, G.; Foss, D.; Cho, J.; Yoon, Y.; Kosenka, P. Optimization of method for detecting and characterizing NOM by HPLC-size exclusion chromatography with UV and on-line DOC detection. Environ. Sci. Technol. 2002, 36, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.D.; Chun, H.K.; Yangali, V.A.Q.; Heijman, B.G.J.; Schippers, J.C. Natural organic matter (NOM) fouling of ultrafiltration membranes: Fractionation of NOM in surface water and characterisation by LC-OCD. Desalination 2005, 178, 73–83. [Google Scholar] [CrossRef]
- Kennedy, M.D.; Tobar, F.P.M.; Amy, G.; Schippers, J.C. Transparent exopolymer particle (TEP) fouling of ultrafiltration membrane systems. Desalin. Water Treat. 2009, 6, 169–176. [Google Scholar] [CrossRef]
- Tomaszewski, J.E.; Schwarzenbach, R.P.; Sander, M. Protein encapsulation by humic substances. Environ. Sci. Technol. 2011, 45, 6003–6010. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hessler, C.M.; Xue, Z.; Seo, Y. The role of extracellular polymeric substances on the sorption of natural organic matter. Water Res. 2012, 46, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
| Solution | Composition (mg DOC L−1) |
|---|---|
| HA | 2 |
| FA | 2 |
| HA+FA | 1 + 1 |
| AOM | 2 |
| HA+AOM | 2 + 2 |
| FA+AOM | 2 + 2 |
| HA+FA+AOM | 1 + 1 + 2 |
| Average ζ Potential | |
|---|---|
| AOM | −27 |
| HA | −43 |
| FA | −19 |
| HA+FA | −30 |
| HA+AOM | −44 |
| FA+AOM | −33 |
| HA+FA+AOM | −39 |
| HA | FA | HA+FA | AOM | |
|---|---|---|---|---|
| mg DOC L−1 | ||||
| HPO | 1.6 ± 0.1 | 1.5 ± 0.2 | 1.5 ± 0.2 | 0.6 ± 0.1 |
| TPI | ND | 0.07 ± 0.03 | 0.04 ± 0.02 | 0.4 ± 0.1 |
| HPI | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 1.0 ± 0.1 |
| HA+AOM | FA+AOM | HA+FA+AOM | |
|---|---|---|---|
| mg DOC L−1 | |||
| HPO | 2.0 ± 0.2 | 2.7 ± 0.1 | 2.9 ± 0.2 |
| TPI | 0.5 ± 0.1 | 0.6 ± 0.1 | 0.4 ± 0.1 |
| HPI | 1.5 ± 0.3 | 0.6 ± 0.1 | 0.6 ± 0.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Fan, L.; Roddick, F.A. Impact of the Interaction between Aquatic Humic Substances and Algal Organic Matter on the Fouling of a Ceramic Microfiltration Membrane. Membranes 2018, 8, 7. https://doi.org/10.3390/membranes8010007
Zhang X, Fan L, Roddick FA. Impact of the Interaction between Aquatic Humic Substances and Algal Organic Matter on the Fouling of a Ceramic Microfiltration Membrane. Membranes. 2018; 8(1):7. https://doi.org/10.3390/membranes8010007
Chicago/Turabian StyleZhang, Xiaolei, Linhua Fan, and Felicity A. Roddick. 2018. "Impact of the Interaction between Aquatic Humic Substances and Algal Organic Matter on the Fouling of a Ceramic Microfiltration Membrane" Membranes 8, no. 1: 7. https://doi.org/10.3390/membranes8010007





