Application of Novel Anion-Exchange Blend Membranes (AEBMs) to Vanadium Redox Flow Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
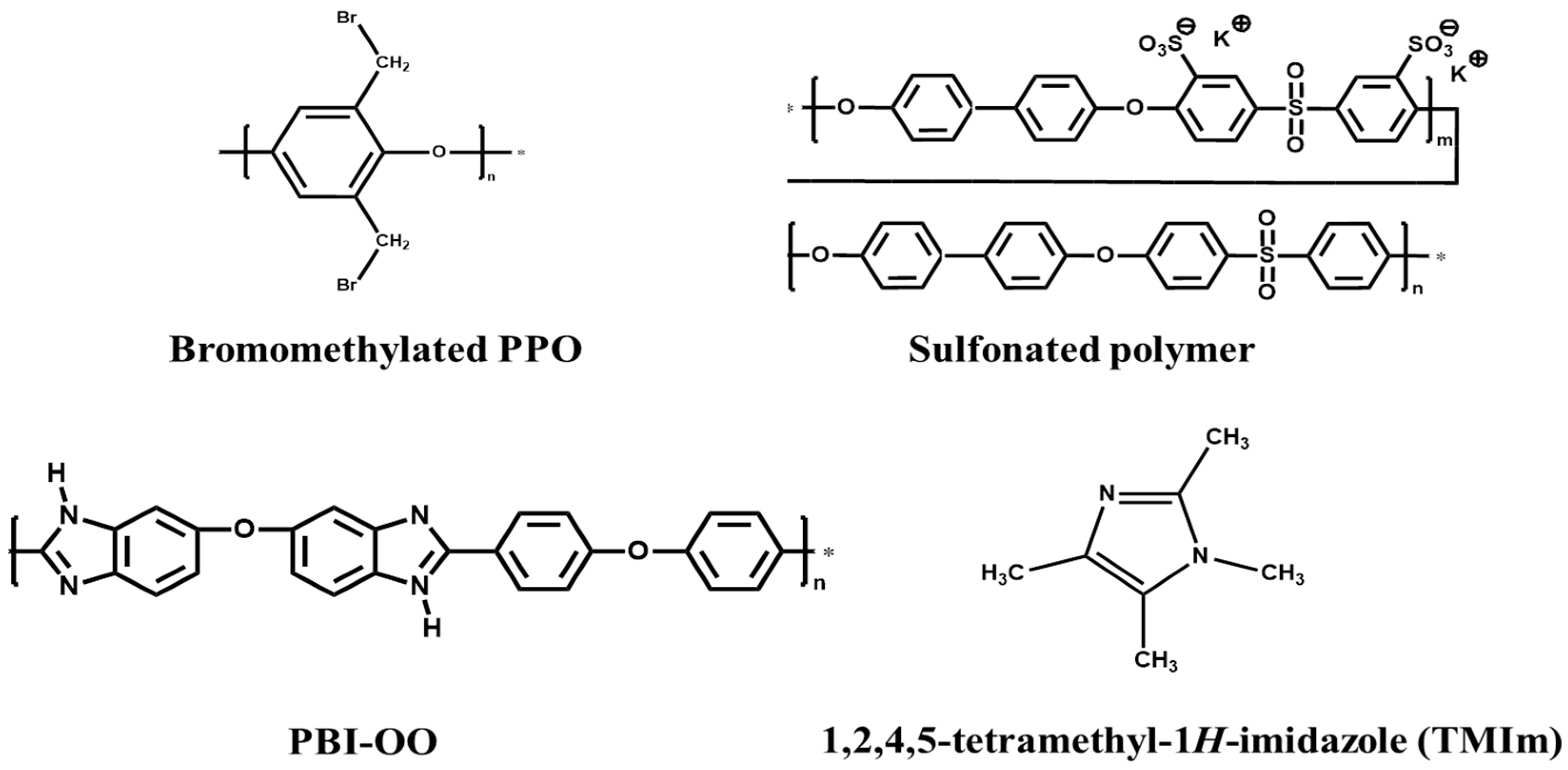
2.2. Membranes Preparation
2.3. Membrane Characterization
2.3.1. Ion Exchange Capacity
2.3.2. Conductivity
2.3.3. Water Uptake (WU) and Swelling Ratio (SR)
2.3.4. Fourier-Transform Infrared Spectroscopy (FT-IR)
2.3.5. Gel Content by Extraction
2.3.6. Thermal Stability
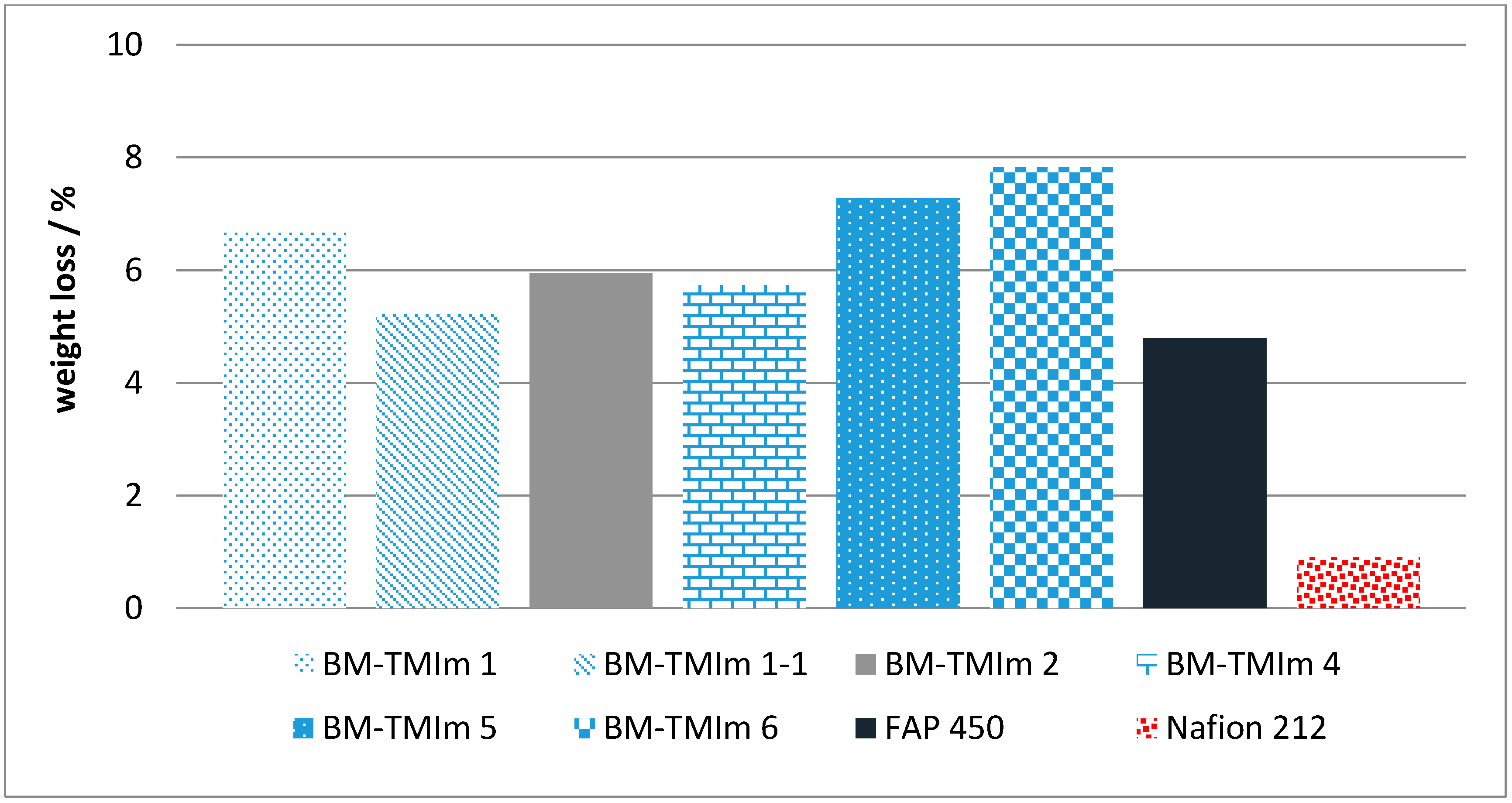
2.3.7. Chemical Stability
2.4. Vanadium Redox Flow Battery (VRFB) Test
3. Results and Discussion
3.1. Preparation and Properties of Anion-Exchange Blend Membranes
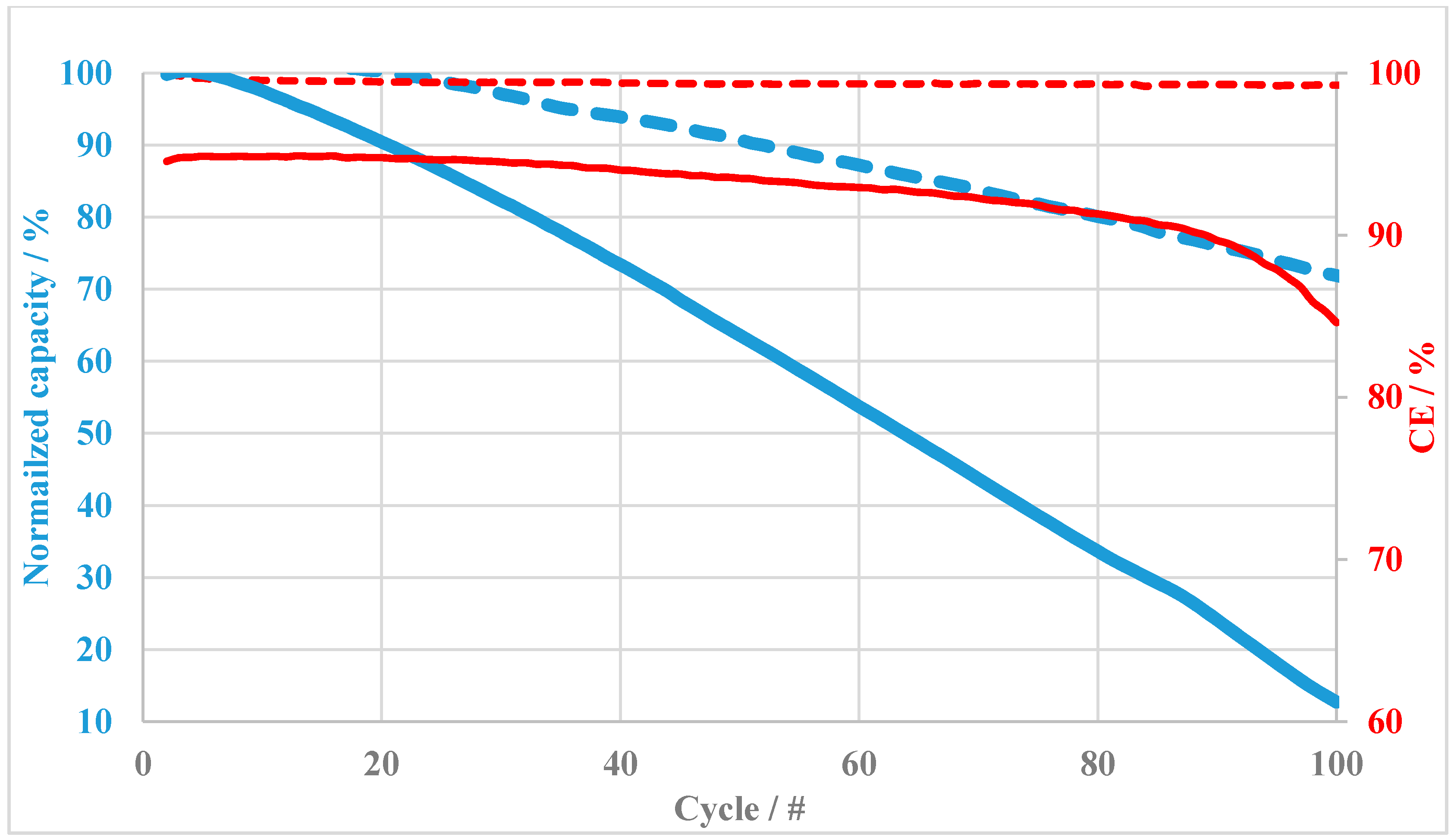
3.2. Vanadium Redox Flow Battery Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, K.J.; Park, M.-S.; Kim, Y.-J.; Kim, J.H.; Dou, S.X.; Skyllas-Kazacos, M. A technology review of electrodes and reaction mechanisms in vanadium redox flow batteries. J. Mater. Chem. A 2015, 3, 16913–16933. [Google Scholar] [CrossRef]
- Rychcik, M.; Robins, R.G.; Fane, A.G. THE New All-Vanadium Redox Flow Cell. J. Electrochem. Soc. 1986, 133, 1057–1058. [Google Scholar] [CrossRef]
- Schwenzer, B.; Zhang, J.; Kim, S.; Li, L.; Liu, J.; Yang, Z. Membrane development for vanadium redox flow batteries. ChemSusChem 2011, 4, 1388–1406. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, S.; Xiao, M.; Meng, Y. Preparation and properties of sulfonated poly(fluorenyl ether ketone) membrane for vanadium redox flow battery application. J. Power Sources 2010, 195, 2089–2095. [Google Scholar] [CrossRef]
- Luo, Q.; Zhang, H.; Chen, J.; Qian, P.; Zhai, Y. Modification of Nafion membrane using interfacial polymerization for vanadium redox flow battery applications. J. Memb. Sci. 2008, 311, 98–103. [Google Scholar] [CrossRef]
- Xi, J.; Wu, Z.; Teng, X.; Zhao, Y.; Chen, L.; Qiu, X. Self-assembled polyelectrolyte multilayer modified Nafion membrane with suppressed vanadium ion crossover for vanadium redox flow batteries. J. Mater. Chem. 2008, 18, 1232–1238. [Google Scholar] [CrossRef]
- Teng, X.; Zhao, Y.; Xi, J.; Wu, Z.; Qiu, X.; Chen, L. Nafion/organically modified silicate hybrids membrane for vanadium redox flow battery. J. Power Sources 2009, 189, 1240–1246. [Google Scholar] [CrossRef]
- Wang, N.; Peng, S.; Lu, D.; Liu, S.; Liu, Y.; Huang, K. Nafion/TiO2 hybrid membrane fabricated via hydrothermal method for vanadium redox battery. J. Solid State Electrochem. 2012, 16, 1577–1584. [Google Scholar] [CrossRef]
- Kim, S.; Tighe, T.B.; Schwenzer, B.; Yan, J.; Zhang, J.; Liu, J.; Yang, Z.; Hickner, M.A. Chemical and mechanical degradation of sulfonated poly(sulfone) membranes in vanadium redox flow batteries. J. Appl. Electrochem. 2011, 41, 1201–1213. [Google Scholar] [CrossRef]
- Luo, X.; Lu, Z.; Xi, J.; Wu, Z.; Zhu, W.; Chen, L.; Qiu, X. Influences of permeation of vanadium ions through PVDF-g-PSSA membranes on performances of vanadium redox flow batteries. J. Phys. Chem. B 2005, 109, 20310–20314. [Google Scholar] [CrossRef] [PubMed]
- Mai, Z.; Zhang, H.; Li, X.; Bi, C.; Dai, H. Sulfonated poly(tetramethydiphenyl ether ether ketone) membranes for vanadium redox flow battery application. J. Power Sources 2011, 196, 482–487. [Google Scholar] [CrossRef]
- Kim, S.; Yan, J.; Schwenzer, B.; Zhang, J.; Li, L.; Liu, J.; Yang, Z.; Hickner, M.A. Cycling performance and efficiency of sulfonated poly(sulfone) membranes in vanadium redox flow batteries. Electrochem. Commun. 2010, 12, 1650–1653. [Google Scholar] [CrossRef]
- Chen, D.; Hickner, M.A. V5+ degradation of sulfonated Radel membranes for vanadium redox flow batteries. Phys. Chem. Chem. Phys. 2013, 15, 11299–11305. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Kim, S.; Li, L.; Yang, G.; Hickner, M.A. Stable fluorinated sulfonated poly(arylene ether) membranes for vanadium redox flow batteries. RSC Adv. 2012, 2, 8087–8094. [Google Scholar] [CrossRef]
- Chen, D.; Hickner, M.A.; Wang, S.; Pan, J.; Xiao, M.; Meng, Y. Directly fluorinated polyaromatic composite membranes for vanadium redox flow batteries. J. Memb. Sci. 2012, 415–416, 139–144. [Google Scholar] [CrossRef]
- Chromik, A.; dos Santos, A.R.; Turek, T.; Kunz, U.; Häring, T.; Kerres, J. Stability of acid-excess acid-base blend membranes in all-vanadium redox-flow batteries. J. Memb. Sci. 2015, 476, 148–155. [Google Scholar] [CrossRef]
- Kerres, J.; Cui, W.U.S. Acid-Base Polymer Blends and Their Application in Membrane Processes. U.S. Patent 6194474 6 July 2004. [Google Scholar]
- Kerres, J.; Ullrich, A.; Häring, T. Engineering Ionomer Blends and Engineering Ionomer Blend Membranes. European Patent 1076676 28 January 2004. [Google Scholar]
- Kerres, J.; Zaidi, S.M.J.; Matsuura, T. Blend concepts for fuel cell membranes. In Polymer Membranes for Fuel Cells; Springer: Boston, MA, USA, 2008; pp. 185–221. [Google Scholar]
- Mohammadi, T.; Skyllas Kazacos, M. Modification of anion-exchange membranes for vanadium redox flow battery applications. J. Power Sources 1996, 63, 179–186. [Google Scholar] [CrossRef]
- Sun, C.N.; Tang, Z.; Belcher, C.; Zawodzinski, T.A.; Fujimoto, C. Evaluation of Diels-Alder poly(phenylene) anion exchange membranes in all-vanadium redox flow batteries. Electrochem. Commun. 2014, 43, 63–66. [Google Scholar] [CrossRef]
- Chen, D.; Hickner, M.A.; Agar, E.; Kumbur, E.C. Selective anion exchange membranes for high coulombic efficiency vanadium redox flow batteries. Electrochem. Commun. 2013, 26, 37–40. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, S.; Xing, D.; Han, R.; Yin, C.; Jian, X. Quaternized poly(phthalazinone ether ketone ketone) anion exchange membrane with low permeability of vanadium ions for vanadium redox flow battery application. J. Power Sources 2012, 217, 296–302. [Google Scholar] [CrossRef]
- Cha, M.S.; Jeong, H.Y.; Shin, H.Y.; Hong, S.H.; Kim, T.H.; Oh, S.G.; Lee, J.Y.; Hong, Y.T. Crosslinked anion exchange membranes with primary diamine-based crosslinkers for vanadium redox flow battery application. J. Power Sources 2017, 363, 78–86. [Google Scholar] [CrossRef]
- Ren, J.; Dong, Y.; Dai, J.; Hu, H.; Zhu, Y.; Teng, X. A novel chloromethylated/quaternized poly(sulfone)/poly(vinylidene fluoride) anion exchange membrane with ultra-low vanadium permeability for all vanadium redox flow battery. J. Memb. Sci. 2017, 544, 186–194. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, C.; Dai, Y.; Zheng, W.; Ruan, X.; He, G. A novel imidazolium-based amphoteric membrane for high-performance vanadium redox flow battery. J. Memb. Sci. 2017, 544, 98–107. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.; Li, D.; Liu, B.; Wang, J.; Song, Y. Novel amphoteric ion exchange membranes by blending sulfonated poly(ether ether ketone)/quaternized poly(ether imide) for vanadium redox flow battery applications. J. Mater. Chem. A 2015, 3, 17590–17597. [Google Scholar] [CrossRef]
- Katzfuß, A.; Gogel, V.; Jörissen, L.; Kerres, J. The application of covalently cross-linked BrPPO as AEM in alkaline DMFC. J. Memb. Sci. 2013, 425–426, 131–140. [Google Scholar] [CrossRef]
- Chromik, A.; Kerres, J.A. Degradation studies on acid-base blends for both LT and intermediate T fuel cells. Solid State Ionics 2013, 252, 140–151. [Google Scholar] [CrossRef]
- Li, Q.; Jensen, J.O.; Savinell, R.F.; Bjerrum, N.J. High temperature proton exchange membranes based on polybenzimidazoles for fuel cells. Prog. Polym. Sci. 2009, 34, 449–477. [Google Scholar] [CrossRef] [Green Version]
- Morandi, C.G.; Peach, R.; Krieg, H.M.; Kerres, J. Novel imidazolium-functionalized anion-exchange polymer PBI blend membranes. J. Memb. Sci. 2015, 476, 256–263. [Google Scholar] [CrossRef]
- Morandi, C.G.; Peach, R.; Krieg, H.M.; Kerres, J. Novel morpholinium-functionalized anion-exchange PBI–polymer blends. J. Mater. Chem. A 2015, 3, 1110–1120. [Google Scholar] [CrossRef]
- Ran, J.; Wu, L.; Varcoe, J.R.; Ong, A.L.; Poynton, S.D.; Xu, T. Development of imidazolium-type alkaline anion exchange membranes for fuel cell application. J. Memb. Sci. 2012, 415–416, 242–249. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.; Shao, Z.G.; Zhang, G.; Zhao, Y.; Yi, B. Crosslinked poly(vinylbenzyl chloride) with a macromolecular crosslinker for anion exchange membrane fuel cells. J. Power Sources 2014, 248, 905–914. [Google Scholar] [CrossRef]
- Merle, G.; Wessling, M.; Nijmeijer, K. Anion exchange membranes for alkaline fuel cells: A review. J. Memb. Sci. 2011, 377, 1–35. [Google Scholar] [CrossRef]
- Chen, D.; Hickner, M.A.; Agar, E.; Kumbur, E.C. Optimized anion exchange membranes for vanadium redox flow batteries. ACS Appl. Mater. Interfaces 2013, 5, 7559–7566. [Google Scholar] [CrossRef] [PubMed]
- Kerres, J.; Ullrich, A.; Hein, M.; Gogel, V.; Friedrich, K.A.; Jörissen, L. Cross-linked polyaryl blend membranes for polymer electrolyte fuel cells. Fuel Cells 2004, 4, 105–112. [Google Scholar] [CrossRef]
- Tian, B.; Yan, C.W.; Wang, F.H. Modification and evaluation of membranes for vanadium redox battery applications. J. Appl. Electrochem. 2004, 34, 1205–1210. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Q.; Yan, C.; Qiao, Y. Corrosion behavior of a positive graphite electrode in vanadium redox flow battery. Electrochim. Acta 2011, 56, 8783–8790. [Google Scholar] [CrossRef]
- Chen, D.; Hickner, M.A.; Agar, E.; Kumbur, E.C. Optimizing membrane thickness for vanadium redox flow batteries. J. Memb. Sci. 2013, 437, 108–113. [Google Scholar] [CrossRef]
- Cunha, Á.; Martins, J.; Rodrigues, N.; Brito, F.P. Vanadium redox flow batteries: A technology review. Int. J. Energy Res. 2015, 39, 889–918. [Google Scholar] [CrossRef]
- Sun, C.; Chen, J.; Zhang, H.; Han, X.; Luo, Q. Investigations on transfer of water and vanadium ions across Nafion membrane in an operating vanadium redox flow battery. J. Power Sources 2010, 195, 890–897. [Google Scholar] [CrossRef]








| Entry | Br-PPO **/wt % | PBI-OO ***/wt % | S-Polymer ****/wt % | TMIm ***** (Equivalent) |
|---|---|---|---|---|
| BM-TMIm * 1 | 52 | 35 | 13 | 3 |
| BM-TMIm 1-1 | 52 | 35 | 13 | 1 |
| BM-TMIm 2 | 60 | 26 | 15 | 1 |
| BM-TMIm 4 | 45 | 45 | 11 | 1 |
| BM-TMIm 5 | 36 | 55 | 9 | 1 |
| BM-TMIm 6 | 60 | 40 | 0 | 1 |
| Entry | IECs (OH Form) | Conductivities (mS/cm) | Gel (%) | Dimensional Stability | T onset (°C) | Thickness (µm, Wet in 1 M H2SO4) | |||
|---|---|---|---|---|---|---|---|---|---|
| WU (%) | SRL (%) | SRW (%) | SRT (%) | ||||||
| BM-TMIm 1 | 2.71 | 149 | 95 | 71 | 31 | 29 | 16 | 281 | 89 |
| BM-TMIm 1-1 | 3.26 | 40.9 | 95 | 47 | 19 | 18 | 15 | 314 | 71 |
| BM-TMIm 2 | 3.04 | 144 | 94 | 105 | n. a. ** | n. a. ** | n. a. ** | 320 | 92 |
| BM-TMIm 4 | 3.41 | 21.0 | 94 | 31 | 12 | 12 | 9 | 306 | 57 |
| BM-TMIm 5 | 2.93 | 13.7 | 92 | 33 | 12 | 11 | 8 | 306 | 66 |
| BM-TMIm 6 | 3.39 | 65.2 | 92 | 58 | 21 | 20 | 15 | 303 | 63 |
| FAP 450 * | 2.18 | 35.2 | - *** | 19 | 9 | 8 | 9 | 304 | 58 |
| Nafion® 212 | 0.88 (H form) | 98.5 | - | 8 | 7 | 9 | 3 | 299 | 53 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, H.; Krieg, H.M.; Kerres, J.A. Application of Novel Anion-Exchange Blend Membranes (AEBMs) to Vanadium Redox Flow Batteries. Membranes 2018, 8, 33. https://doi.org/10.3390/membranes8020033
Cho H, Krieg HM, Kerres JA. Application of Novel Anion-Exchange Blend Membranes (AEBMs) to Vanadium Redox Flow Batteries. Membranes. 2018; 8(2):33. https://doi.org/10.3390/membranes8020033
Chicago/Turabian StyleCho, Hyeongrae, Henning M. Krieg, and Jochen A. Kerres. 2018. "Application of Novel Anion-Exchange Blend Membranes (AEBMs) to Vanadium Redox Flow Batteries" Membranes 8, no. 2: 33. https://doi.org/10.3390/membranes8020033




