Ionic Conductivity and Cycling Stability Improvement of PVDF/Nano-Clay Using PVP as Polymer Electrolyte Membranes for LiFePO4 Batteries
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphology Characterization
2.2. Surface Chemistry
2.3. Characterization of Crystallinity
2.4. Porosity and Electrolyte Uptake
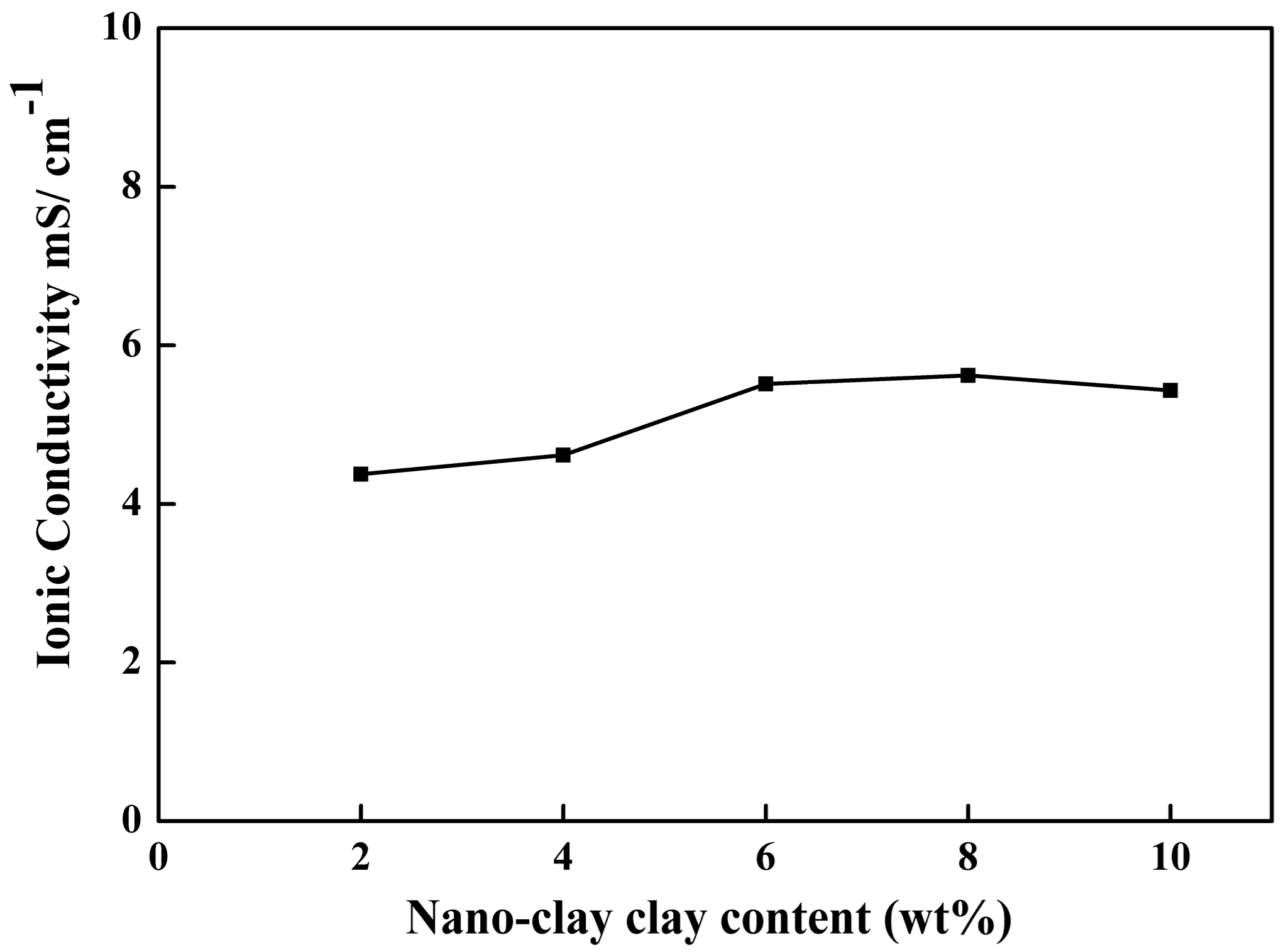
2.5. Ionic Conductivity
2.6. Rate Performance
3. Materials and Methods
3.1. Materials
3.2. Membrane Preparation
3.3. Membrane Characterization
3.4. Electrochemical and Battery Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, D.Y.; Wang, G.Z.; Li, W.S.; Li, G.L.; Tan, C.L.; Rao, M.M.; Liao, Y.H. Preparation and performances of porous polyacrylonitrile–methyl methacrylate membrane for lithium-ion batteries. J. Power Sources 2008, 184, 477–480. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, L.C.; Li, L.; Qu, Q.T.; Wu, Y.P.; Shimizu, M. Effects of preparation conditions on porous polymer membranes by microwave assisted effervescent disintegrable reaction and their electrochemical properties. J. Membr. Sci. 2010, 362, 113–118. [Google Scholar] [CrossRef]
- Liao, Y.; Chen, T.; Luo, X.; Fu, Z.; Li, X.; Li, W. Cycling performance improvement of polypropylene supported poly(vinylidene fluoride-co-hexafluoropropylene)/maleic anhydride-grated-polyvinylidene fluoride based gel electrolyte by incorporating nano-Al2O3 for full batteries. J. Membr. Sci. 2016, 507, 126–134. [Google Scholar] [CrossRef]
- Pu, W.; He, X.; Wang, L.; Tian, Z.; Jiang, C.; Wan, C. Preparation of P(AN–MMA) microporous membrane for Li-ion batteries by phase inversion. J. Membr. Sci. 2006, 280, 6–9. [Google Scholar] [CrossRef]
- Rao, M.M.; Liu, J.S.; Li, W.S.; Liang, Y.; Zhou, D.Y. Preparation and performance analysis of PE-supported P(AN-co-MMA) gel polymer electrolyte for lithium ion battery application. J. Membr. Sci. 2008, 322, 314–319. [Google Scholar] [CrossRef]
- Jarvis, C.R.; Macklin, W.J.; Macklin, A.J.; Mattingley, N.J.; Kronfli, E. Use of grafted PVdF-based polymers in lithium batteries. J. Power Sources 2001, 97–98, 664–666. [Google Scholar] [CrossRef]
- Rao, M.; Geng, X.; Liao, Y.; Hu, S.; Li, W. Preparation and performance of gel polymer electrolyte based on electrospun polymer membrane and ionic liquid for lithium ion battery. J. Membr. Sci. 2012, 399–400, 37–42. [Google Scholar] [CrossRef]
- Lee, H.; Yanilmaz, M.; Toprakci, O.; Fu, K.; Zhang, X. A review of recent developments in membrane separators for rechargeable lithium-ion batteries. Energy Environ. Sci. 2014, 7, 3857–3886. [Google Scholar] [CrossRef]
- Li, Z.H.; Zhang, P.; Zhang, H.P.; Wu, Y.P.; Zhou, X.D. A lotus root-like porous nanocomposite polymer electrolyte. Electrochem. Commun. 2008, 10, 791–794. [Google Scholar] [CrossRef]
- Appetecchi, G.B.; Croce, F.; Scrosati, B. Kinetics and stability of the lithium electrode in poly(methylmethacrylate)-based gel electrolytes. Electrochim. Acta 1995, 40, 991–997. [Google Scholar] [CrossRef]
- Yang, C.R.; Perng, J.T.; Wang, Y.Y.; Wan, C.C. Conductive behaviour of lithium ions in polyacrylonitrile. J. Power Sources 1996, 62, 89–93. [Google Scholar] [CrossRef]
- Liu, J.; Wu, X.; He, J.; Li, J.; Lai, Y. Preparation and performance of a novel gel polymer electrolyte based on poly(vinylidene fluoride)/graphene separator for lithium ion battery. Electrochim. Acta 2017, 235, 500–507. [Google Scholar] [CrossRef]
- Hao, J.; Xiao, Q.; Lei, G.; Li, Z.; Wu, L. A novel polyvinylidene fluoride/microfiber composite gel polymer electrolyte with an interpenetrating network structure for lithium ion battery. Electrochim. Acta 2014, 125, 450–456. [Google Scholar] [CrossRef]
- Li, H.; Chen, Y.-M.; Ma, X.-T.; Shi, J.-L.; Zhu, B.-K.; Zhu, L.-P. Gel polymer electrolytes based on active PVDF separator for lithium ion battery. I: Preparation and property of PVDF/poly(dimethylsiloxane) blending membrane. J. Membr. Sci. 2011, 379, 397–402. [Google Scholar] [CrossRef]
- Li, Z.; Su, G.; Gao, D.; Wang, X.; Li, X. Effect of Al2O3 nanoparticles on the electrochemical characteristics of P(VDF-HFP)-based polymer electrolyte. Electrochim. Acta 2004, 49, 4633–4639. [Google Scholar] [CrossRef]
- Raghavan, P.; Zhao, X.; Kim, J.-K.; Manuel, J.; Chauhan, G.S.; Ahn, J.-H.; Nah, C. Ionic conductivity and electrochemical properties of nanocomposite polymer electrolytes based on electrospun poly(vinylidene fluoride-co-hexafluoropropylene) with nano-sized ceramic fillers. Electrochim. Acta 2008, 54, 228–234. [Google Scholar] [CrossRef]
- Chung, S.H.; Wang, Y.; Persi, L.; Croce, F.; Greenbaum, S.G.; Scrosati, B.; Plichta, E. Enhancement of ion transport in polymer electrolytes by addition of nanoscale inorganic oxides. J. Power Sources 2001, 97–98, 644–648. [Google Scholar] [CrossRef]
- Raghavan, P.; Zhao, X.; Manuel, J.; Chauhan, G.S.; Ahn, J.-H.; Ryu, H.-S.; Ahn, H.-J.; Kim, K.-W.; Nah, C. Electrochemical performance of electrospun poly(vinylidene fluoride-co-hexafluoropropylene)-based nanocomposite polymer electrolytes incorporating ceramic fillers and room temperature ionic liquid. Electrochim. Acta 2010, 55, 1347–1354. [Google Scholar] [CrossRef]
- Riley, M.; Fedkiw, P.S.; Khan, S.A. Transport properties of lithium hectorite-based composite electrolytes. J. Electrochem. Soc. 2002, 149, A667–A674. [Google Scholar] [CrossRef]
- Deka, M.; Kumar, A. Enhanced electrical and electrochemical properties of PMMA–clay nanocomposite gel polymer electrolytes. Electrochim. Acta 2010, 55, 1836–1842. [Google Scholar] [CrossRef]
- Chen, H.-W.; Lin, T.-P.; Chang, F.-C. Ionic conductivity enhancement of the plasticized PMMA/LiClO4 polymer nanocomposite electrolyte containing clay. Polymer 2002, 43, 5281–5288. [Google Scholar] [CrossRef]
- Koh, M.J.; Hwang, H.Y.; Kim, D.J.; Kim, H.J.; Hong, Y.T.; Nam, S.Y. Preparation and characterization of porous PVdF-HFP/clay nanocomposite membranes. J. Mater. Sci. Technol. 2010, 26, 633–638. [Google Scholar] [CrossRef]
- Shukla, N.; Thakur, A.K. Ion transport model in exfoliated and intercalated polymer-clay nanocomposites. Solid State Ionics 2010, 181, 921–932. [Google Scholar] [CrossRef]
- Chang, X.; Wang, Z.; Quan, S.; Xu, Y.; Jiang, Z.; Shao, L. Exploring the synergetic effects of graphene oxide (GO) and polyvinylpyrrodione (PVP) on poly(vinylylidenefluoride) (PVDF) ultrafiltration membrane performance. Appl. Surf. Sci. 2014, 316, 537–548. [Google Scholar] [CrossRef]
- Xiao, W.; Li, X.; Wang, Z.; Guo, H.; Li, Y.; Yang, B. Performance of PVDF-HFP-based gel polymer electrolytes with different pore forming agents. Iran. Polym. J. 2012, 21, 755–761. [Google Scholar] [CrossRef]
- Susanto, H.; Ulbricht, M. Characteristics, performance and stability of polyethersulfone ultrafiltration membranes prepared by phase separation method using different macromolecular additives. J. Membr. Sci. 2009, 327, 125–135. [Google Scholar] [CrossRef]
- Xiao, W.; Miao, C.; Yin, X.; Zheng, Y.; Tian, M.; Li, H.; Mei, P. Effect of urea as pore-forming agent on properties of poly(vinylidene fluoride-co-hexafluoropropylene)-based gel polymer electrolyte. J. Power Sources 2014, 252, 14–20. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, W.; Li, X.; Miao, C.; Guo, H.; Wang, Z. Study on performance of a novel P(VDF-HFP)/SiO2 composite polymer electrolyte using urea as pore-forming agent. Ionics 2014, 20, 1217–1224. [Google Scholar] [CrossRef]
- Wang, Y.-P.; Gao, X.-H.; Li, H.-K.; Li, H.-J.; Liu, H.-G.; Guo, H.-X. Effect of active filler addition on the ionic conductivity of PVDF-PEG polymer electrolyte. J. Macromol. Sci. Part A 2009, 46, 461–467. [Google Scholar] [CrossRef]
- Zhang, H.P.; Zhang, P.; Li, G.C.; Wu, Y.P.; Sun, D.L. A porous poly(vinylidene fluoride) gel electrolyte for lithium ion batteries prepared by using salicylic acid as a foaming agent. J. Power Sources 2009, 189, 594–598. [Google Scholar] [CrossRef]
- Costa, C.M.; Rodrigues, L.C.; Sencadas, V.; Silva, M.M.; Rocha, J.G.; Lanceros-Méndez, S. Effect of degree of porosity on the properties of poly (vinylidene fluoride–trifluorethylene) for Li-ion battery separators. J. Membr. Sci. 2012, 407, 193–201. [Google Scholar] [CrossRef]
- Magistris, A.; Mustarelli, P.; Parazzoli, F.; Quartarone, E.; Piaggio, P.; Bottino, A. Structure, porosity and conductivity of PVdF films for polymer electrolytes. J. Power Sources 2001, 97–98, 657–660. [Google Scholar] [CrossRef]
- Arora, P.; Zhang, Z.J. Battery Separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-L.; Wang, D.-M.; Deratani, A.; Quémener, D.; Bouyer, D.; Lai, J.-Y. Insight into the preparation of poly(vinylidene fluoride) membranes by vapor-induced phase separation. J. Membr. Sci. 2010, 361, 154–166. [Google Scholar] [CrossRef]
- Dong, Z.; Zhang, Q.; Yu, C.; Peng, J.; Ma, J.; Ju, X.; Zhai, M. Effect of ionic liquid on the properties of poly(vinylidene fluoride)-based gel polymer electrolytes. Ionics 2013, 19, 1587–1593. [Google Scholar] [CrossRef]
- Mendes, S.F.; Costa, C.M.; Caparros, C.; Sencadas, V.; Lanceros-Méndez, S. Effect of filler size and concentration on the structure and properties of poly(vinylidene fluoride)/BaTiO3 nanocomposites. J. Mater. Sci. 2012, 47, 1378–1388. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, B.; Huang, X.; Chen, S.; Wang, G. Honeycomb-like porous gel polymer electrolyte membrane for lithium ion batteries with enhanced safety. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Fadaei, A.; Salimi, A.; Mirzataheri, M. Structural elucidation of morphology and performance of the PVDF/PEG membrane. J. Polym. Res. 2014, 21, 545. [Google Scholar] [CrossRef]
- Jeon, M.Y.; Kim, C.K. Phase behavior of polymer/diluent/diluent mixtures and their application to control microporous membrane structure. J. Membr. Sci. 2007, 300, 172–181. [Google Scholar] [CrossRef]
- Rajendran, S.; Mahendran, O.; Kannan, R. Lithium ion conduction in plasticized PMMA–PVdF polymer blend electrolytes. Mater. Chem. Phys. 2002, 74, 52–57. [Google Scholar] [CrossRef]
- Saikia, D.; Kumar, A. Ionic conduction in P (VDF-HFP)/PVDF–(PC + DEC)–LiClO4 polymer gel electrolytes. Electrochim. Acta 2004, 49, 2581–2589. [Google Scholar] [CrossRef]
- Kelarakis, A.; Hayrapetyan, S.; Ansari, S.; Fang, J.; Estevez, L.; Giannelis, E.P. Clay nanocomposites based on poly(vinylidene fluoride-co-hexafluoropropylene): Structure and properties. Polymer 2010, 51, 469–474. [Google Scholar] [CrossRef]
- Deka, M.; Kumar, A. Electrical and electrochemical studies of poly(vinylidene fluoride)–clay nanocomposite gel polymer electrolytes for Li-ion batteries. J. Power Sources 2011, 196, 1358–1364. [Google Scholar] [CrossRef]
- Prasanth, R.; Shubha, N.; Hng, H.H.; Srinivasan, M. Effect of nano-clay on ionic conductivity and electrochemical properties of poly(vinylidene fluoride) based nanocomposite porous polymer membranes and their application as polymer electrolyte in lithium ion batteries. Eur. Polym. J. 2013, 49, 307–318. [Google Scholar] [CrossRef]
- Martins, P.; Lopes, A.C.; Lanceros-Mendez, S. Electroactive phases of poly(vinylidene fluoride): Determination, processing and applications. Prog. Polym. Sci. 2014, 39, 683–706. [Google Scholar] [CrossRef]
- Kim, J.R.; Choi, S.W.; Jo, S.M.; Lee, W.S.; Kim, B.C. Characterization and properties of P (VdF-HFP)-based fibrous polymer electrolyte membrane prepared by electrospinning. J. Electrochem. Soc. 2005, 152, A295–A300. [Google Scholar] [CrossRef]







| Name of Sample | Composition | Heat Fusion ΔHm (J/g) | Degree of Crystallinity XC (%) | Melting Point Tm (°C) | ||
|---|---|---|---|---|---|---|
| PVDF | PVP | Nano-Clay | ||||
| C-2 | 10 | 7 | 2 | 38.99 | 37.27 | 164.75 |
| C-4 | 10 | 7 | 4 | 36.79 | 35.12 | 163.98 |
| C-6 | 10 | 7 | 6 | 25.42 | 24.31 | 163.36 |
| C-8 | 10 | 7 | 8 | 15.39 | 14.71 | 163.20 |
| C-10 | 10 | 7 | 10 | 22.96 | 27.71 | 163.54 |
| Polymer | Additive | Electrolyte | Ionic Conductivity (mS cm−1) | Ref |
|---|---|---|---|---|
| PVDF | PVP + nano-clay | LiPF6 in EC/DC/DMC | 5.610 | |
| PVDF | nano-clay | LiPF6 in EC/DEC | 3.080 | [44] |
| PVDF | SiO2-urea | LiPF6-EC/DMC/EMC | 3.652 | [28] |
| PVDF | PVP + graphene | LiPF6-EC/DMC/EMC | 3.610 | [13] |
| Sample | Discharge Capacity with Different Current Rate | |||||||
|---|---|---|---|---|---|---|---|---|
| 0.2 C | 0.4 C | 0.8 C | 1 C | 1.5 C | 2 C | 3 C | 4 C | |
| 10 C | 116.86 | 113.84 | 112.60 | 110.17 | 109.00 | 98.33 | 83.76 | 74.10 |
| 8 C | 125.85 | 122.48 | 121.26 | 118.67 | 117.38 | 90.21 | 90.21 | 76.57 |
| 6 C | 121.19 | 122.48 | 115.06 | 115.12 | 116.49 | 103.25 | 85.39 | 74.10 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dyartanti, E.R.; Purwanto, A.; Widiasa, I.N.; Susanto, H. Ionic Conductivity and Cycling Stability Improvement of PVDF/Nano-Clay Using PVP as Polymer Electrolyte Membranes for LiFePO4 Batteries. Membranes 2018, 8, 36. https://doi.org/10.3390/membranes8030036
Dyartanti ER, Purwanto A, Widiasa IN, Susanto H. Ionic Conductivity and Cycling Stability Improvement of PVDF/Nano-Clay Using PVP as Polymer Electrolyte Membranes for LiFePO4 Batteries. Membranes. 2018; 8(3):36. https://doi.org/10.3390/membranes8030036
Chicago/Turabian StyleDyartanti, Endah R., Agus Purwanto, I. Nyoman Widiasa, and Heru Susanto. 2018. "Ionic Conductivity and Cycling Stability Improvement of PVDF/Nano-Clay Using PVP as Polymer Electrolyte Membranes for LiFePO4 Batteries" Membranes 8, no. 3: 36. https://doi.org/10.3390/membranes8030036




