Performance of PES/LSMM-OGCN Photocatalytic Membrane for Phenol Removal: Effect of OGCN Loading
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials
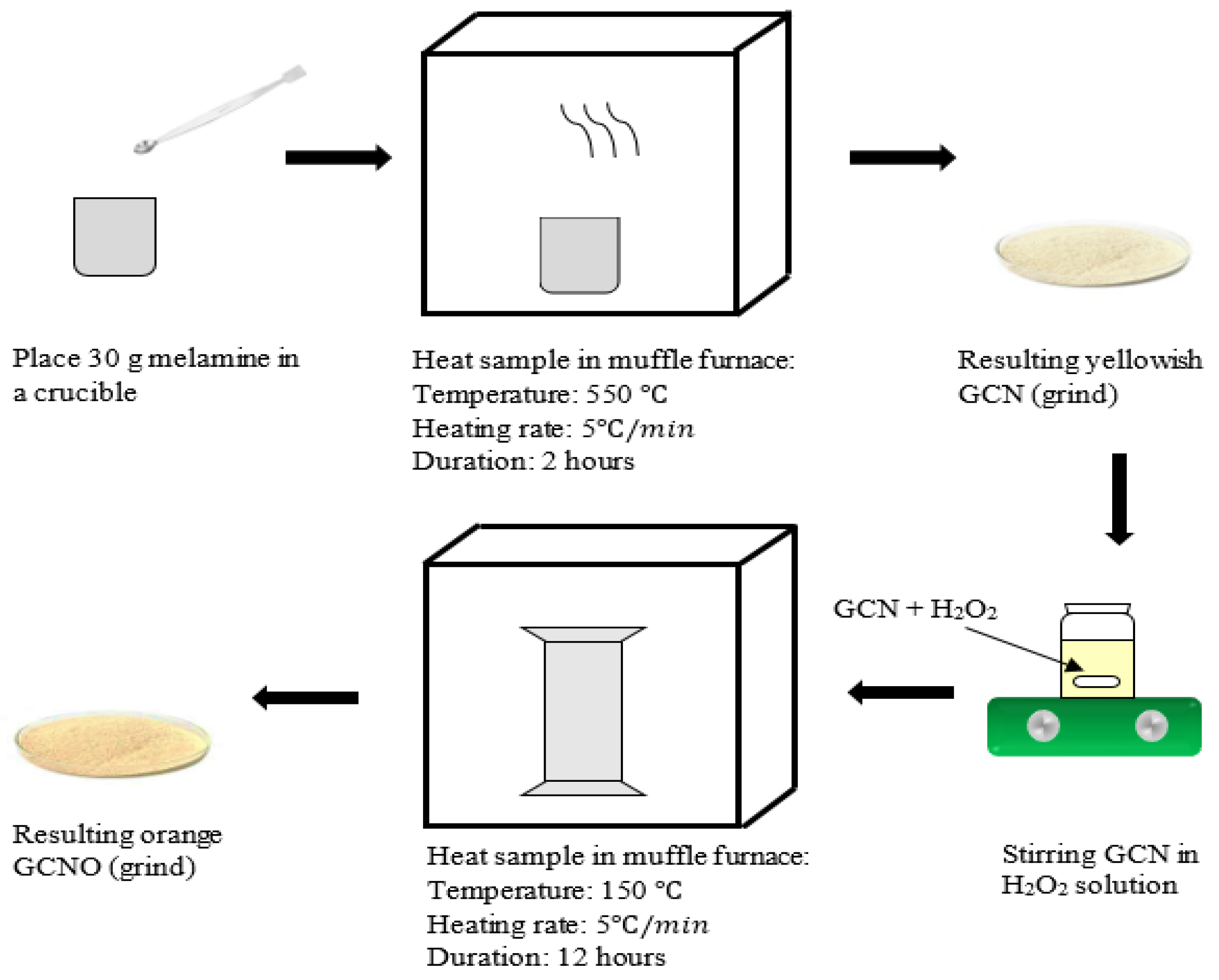
2.2. Synthesis of Photocatalyst
2.3. Preparation of PES/LSMM-OGCN Photocatalytic Membrane
2.4. Membrane Characterisation
2.5. Physical Characteristics of the Hybrid Photocatalytic Membrane
2.6. Adsorption and Photocatalytic Activity Measurement
3. Results and Discussion
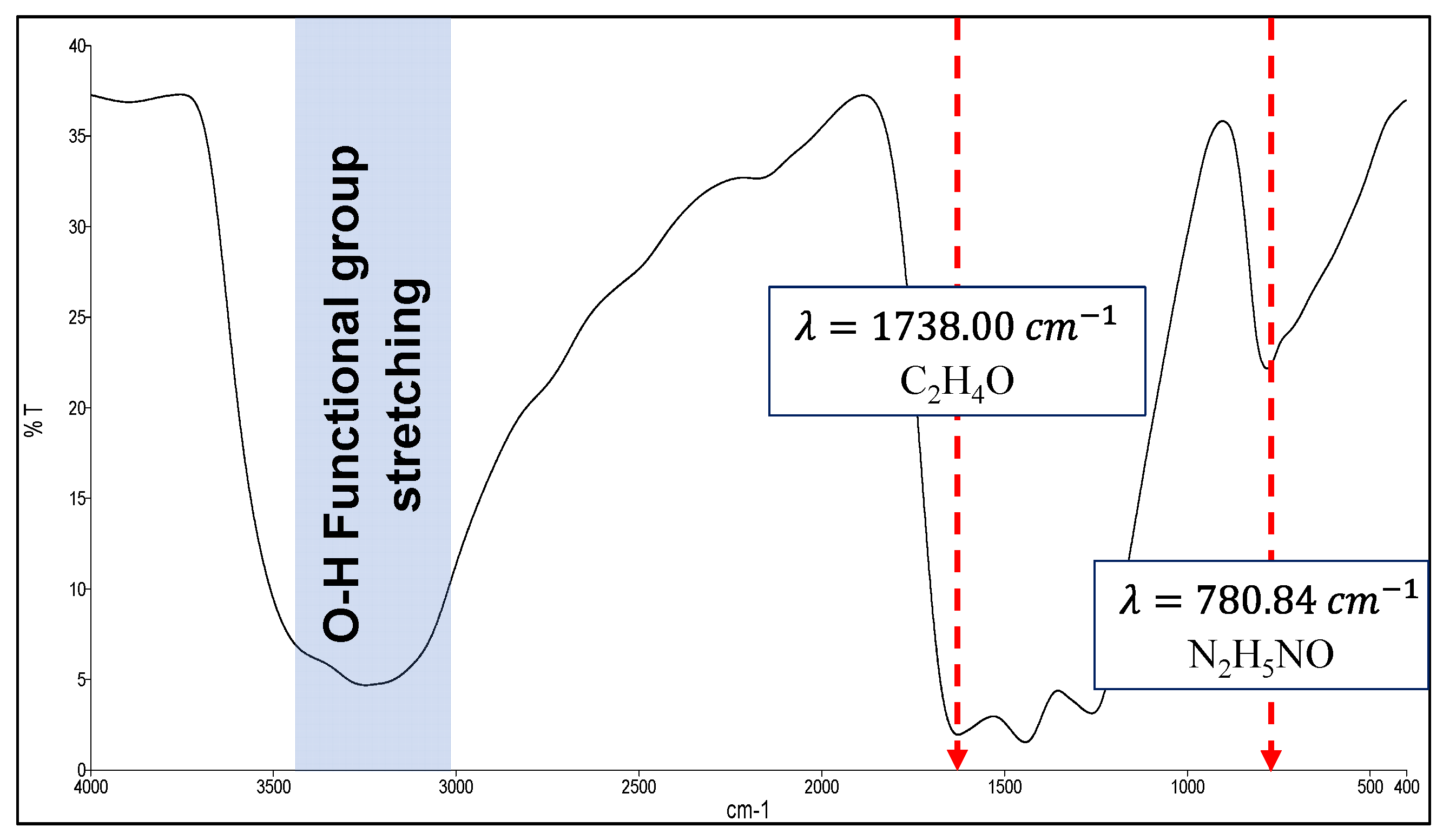
3.1. Oxygenated Graphitic Carbon Nitride (OGCN) Characterisations Study
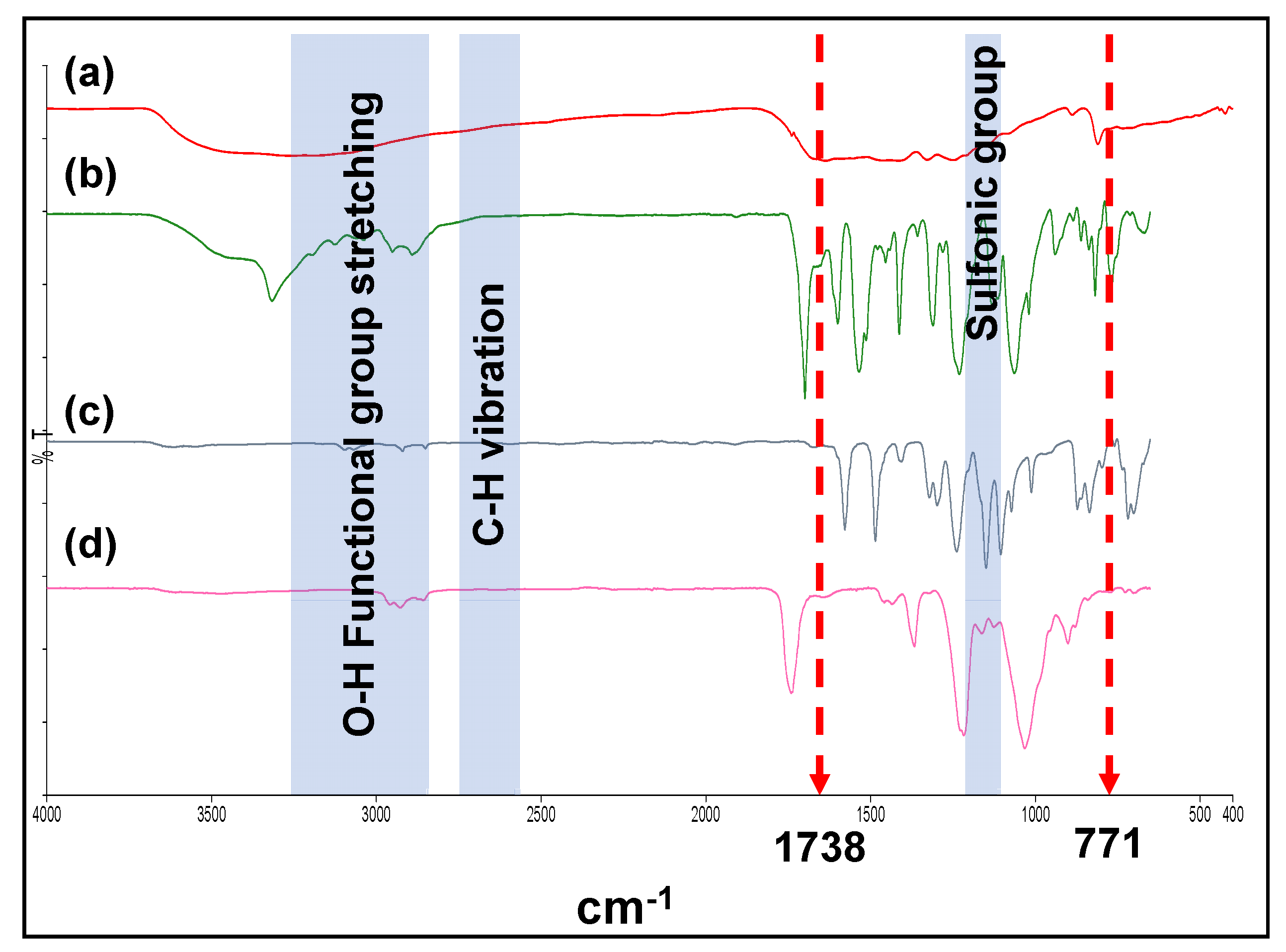
3.2. Confirmation of the Successfulness of OGCN and LSMM Incorporation in PES Matrix
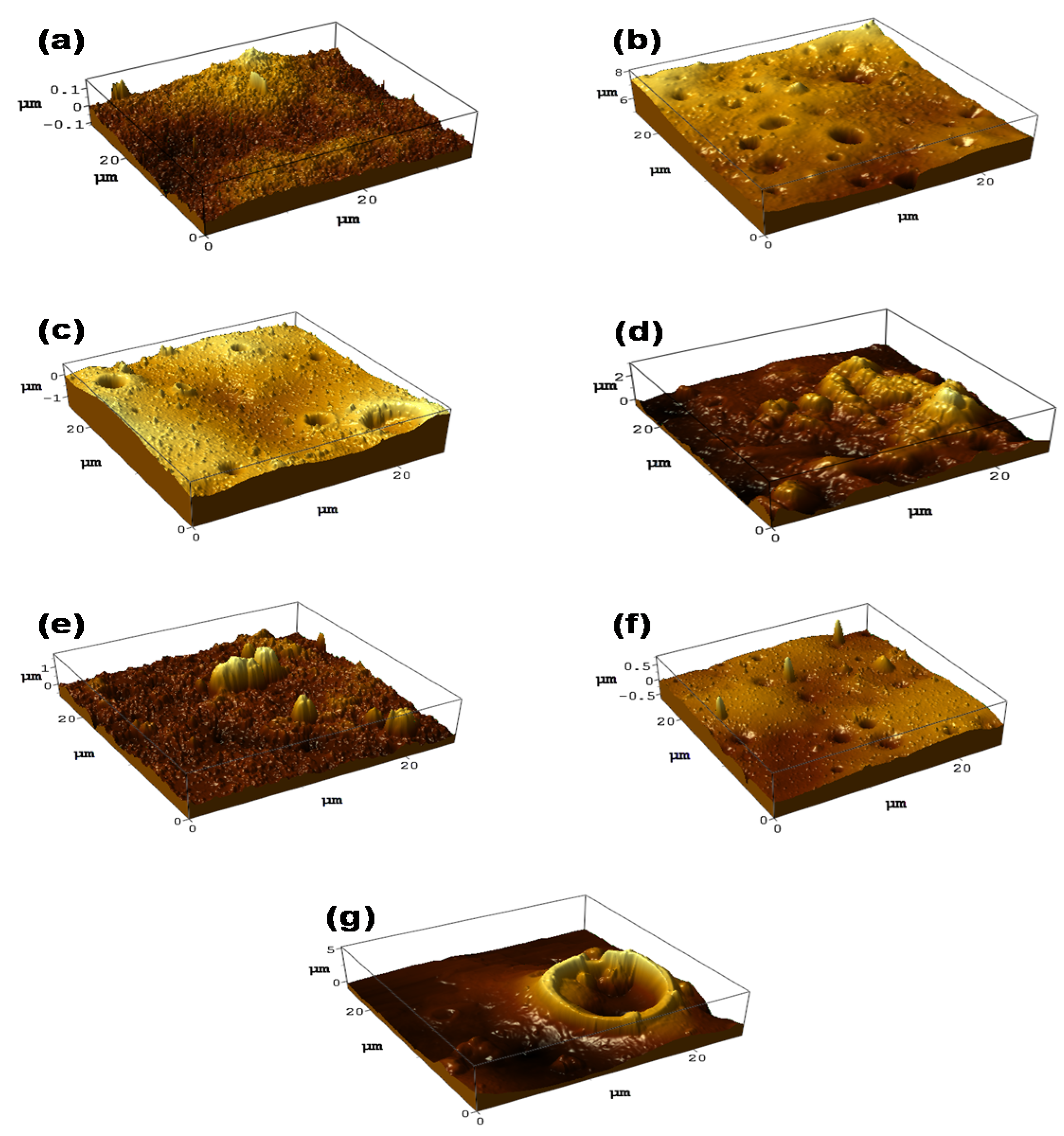
3.3. Morphology Analysis of PES/LSMM/OGCN Membrane
3.4. Surface Roughness Analysis of PES/LSMM/OGCN Membrane
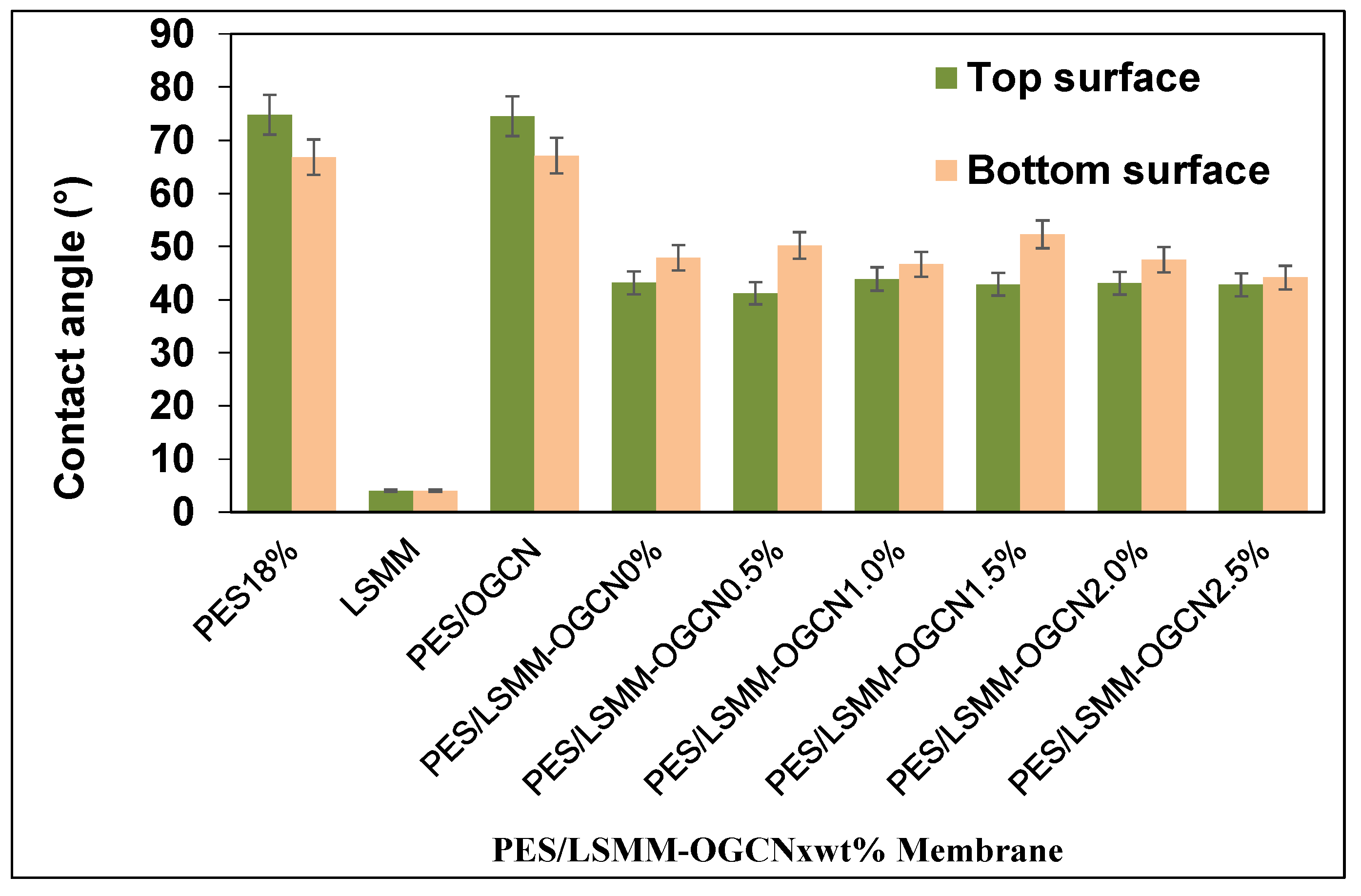
3.5. Hydrophilicity Analysis of PES/LSMM-OGCN Membrane
3.6. Pure Water Flux and Phenol Rejection Analysis of PES/LSMM-OGCN Membranes
3.7. Photocatalytic Activity of PES/LSMM-OGCN Membrane
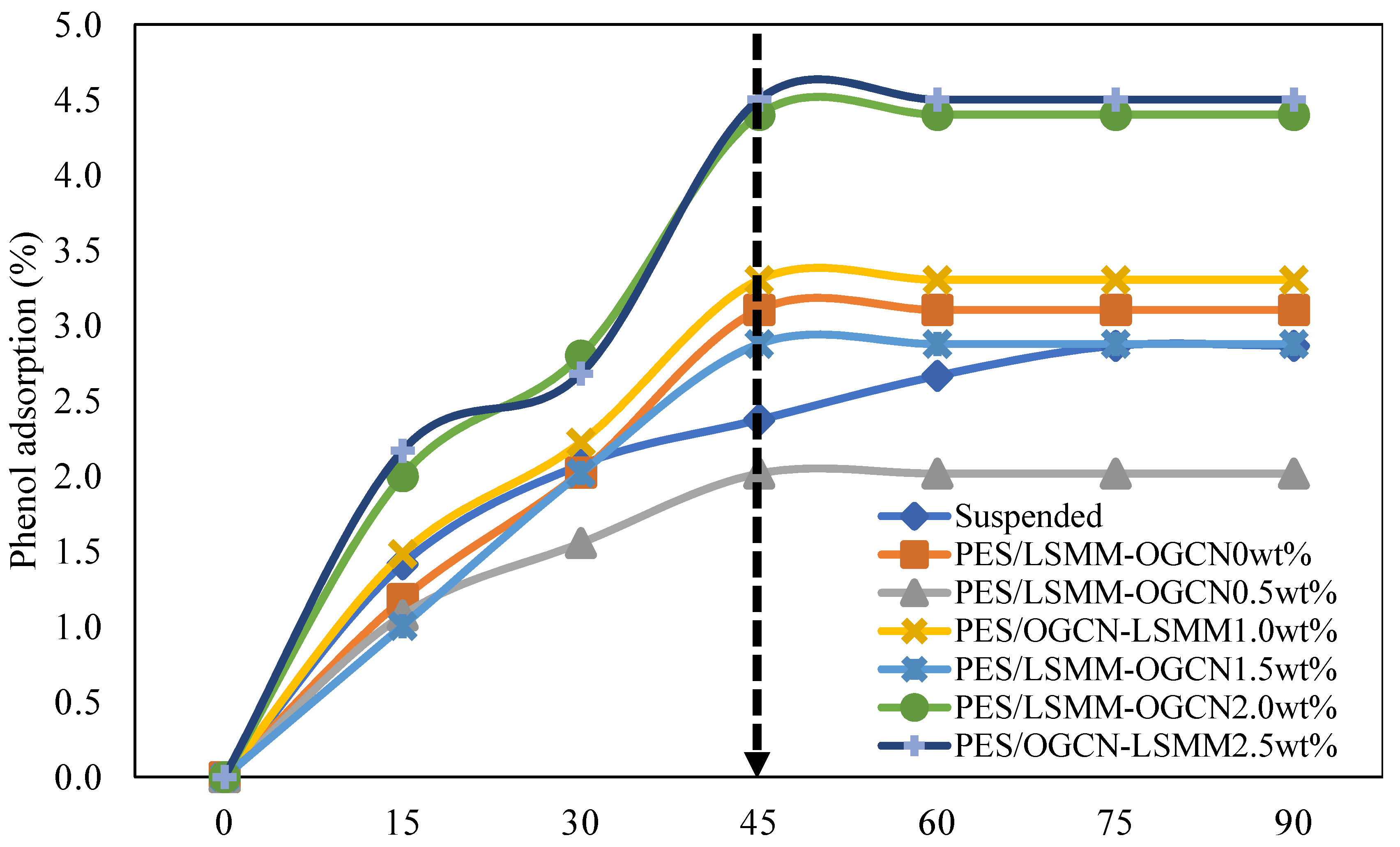
3.7.1. Adsorption Analysis of PES/LSMM-OGCN Membrane
3.7.2. Photocatalytic Degradation of Phenol by PES/LSMM-OGCN Membrane
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hoshi, M.; Ieshige, M.; Saitoh, T.; Nakagawa, T. Separation of aqueous phenol through polyurethane membranes by pervaporation. J. Appl. Polym. Sci. 1999, 71, 439–448. [Google Scholar] [CrossRef]
- Sanders, D.F.; Smith, Z.P.; Guo, R.; Robeson, L.M.; McGrath, J.E.; Paul, D.R.; Freeman, B.D. Energy-efficient polymeric gas separation membranes for a sustainable future: A review. Polymer 2013, 54, 4729–4761. [Google Scholar] [CrossRef]
- Le, N.L.; Nunes, S.P. Materials and membrane technologies for water and energy sustainability. Sustain. Mater. Technol. 2016, 7, 1–28. [Google Scholar] [CrossRef]
- Mu’azu, N.D.; Jarrah, N.; Zubair, M.; Alagha, O. Rmoval of phenolic compounds from water using sewage sludge-based activated carbon adsorption: A Review. Int. J. Environ. Res. Public Health 2017, 14, E1094. [Google Scholar] [CrossRef] [PubMed]
- Bodalo, A.; Gomez, J.L.; Gomez, M.; Leon, G.; Hidalgo, A.M.; Ruiz, M.A. Phenol removal from water by hybrid processes: Study of the membrane process step. Desalination 2008, 223, 323–329. [Google Scholar] [CrossRef]
- Akay, G.; Erhan, E.; Keskinler, B.; Algur, O.F. Removal of phenol from wastewater using membrane-immobilized enzymes: Part II. cross-flow filtration. J. Membr. Sci. 2002, 206, 61–68. [Google Scholar] [CrossRef]
- Villegas, L.G.C.; Mashhadi, N.; Chen, M.; Mukherjee, D.; Taylor, K.E.; Biswas, N. A short review of techniques for phenol removal from wastewater. Curr. Pollut. Rep. 2016, 2, 157–167. [Google Scholar] [CrossRef]
- Kujawski, W.; Warszawski, A.; Ratajczak, W.; Pore bski, T.; Capala, W.; Ostrowska, I. Removal of phenol from wastewater by different separation technique. Desalination 2004, 163, 287–296. [Google Scholar] [CrossRef]
- Yang, L.; Hakki, A.; Wang, F.; Macphee, D.E. Photocatalyst efficiencies in concrete technology: The effect of photocatalyst placement. Appl. Catal. B Environ. 2018, 222, 200–208. [Google Scholar] [CrossRef]
- Wang, T.; Xu, Z.; Wu, L.; Li, B.; Chen, M.; Xue, S.; Zhu, Y.; Cai, J. Enhanced photocatalytic activity for degrading phenol in seawater by TiO2-based catalysts under weak light irradiation. RSC Adv. 2017, 7, 31921–31929. [Google Scholar] [CrossRef]
- Bai, S.; Liu, H.; Sun, J.; Tian, Y.; Chen, S.; Song, J.; Liu, C.C. Improvement of TiO2 photocatalytic properties under visible light by WO3/TiO2 and MO3/TiO2 composites. Appl. Surf. Sci. 2015, 338, 61–68. [Google Scholar] [CrossRef]
- Guo, L.; Wan, B. Carbon dots decorated graphitic carbon nitride as an efficient metal-free photocatalyst for phenol degradation. Appl. Catal. B Environ. 2016, 180, 656–662. [Google Scholar] [Green Version]
- Zhu, J.; Xiao, P.; Li, H.; Carabineiro, A.C. Graphitic carbon nitride: Synthesis, properties, and applications in catalysis. Appl. Mater. Interfaces 2014, 6, 16449–16465. [Google Scholar] [CrossRef] [PubMed]
- Bee, S.; Hamid, A. Effect of band gap engineering in anionic-doped TiO2 photocatalyst effect of band gap engineering in anionic-doped TiO2 photocatalyst. Appl. Surf. Sci. 2016, 391, 326–336. [Google Scholar]
- Luo, X.L.; He, G.L.; Fang, Y.P.; Xu, Y.H. Nickel sulfide/graphitic carbon nitride/strontium titanate (NiS/g-C3N4/SrTiO3) composites with significantly enhanced photocatalytic hydrogen production activity. J. Colloid Interface Sci. 2018, 518, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Boonprakob, N.; Wetchakun, N.; Phanichphant, S.; Waxler, D.; Sherrell, P.; Nattested, A.; Chen, J.; Inceesungvorn, B. Enhanced visible-light photocatalytic activity of g-C3N4/TiO2 films. J. Colloid Interface Sci. 2014, 417, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Fischer, A.; Goettmann, F.; Antonietti, M.; Müller, J.-O.; Schlögl, R.; Carlsson, J.M. Graphitic carbon nitride materials: Variation of structure and morphology and their use as metal-free catalysts. J. Mater. Chem. 2008, 18, 4893–4908. [Google Scholar] [CrossRef]
- Lau, V.W.H.; Mesch, M.B.; Duppel, V.; Blum, V.; Senker, J.; Lotsch, B.V. Low molecular weight carbon nitrides for solar hydrogen evolution. J. Am. Chem. Soc. 2015, 137, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Zhang, L.; Wang, M.; Huang, W.; Zhou, Y.; Li, M.; Shi, J. Constructing carbon-nitride-based copolymers via schiff base chemistry for visible-light photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2015, 182, 68–73. [Google Scholar] [CrossRef]
- Cao, S.; Low, J.; Yu, J.; Jaroniec, M. Polymeric photocatalysts based on graphitic carbon nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef] [PubMed]
- Pandiselvi, K.; Fang, H.; Huang, X.; Wang, J.; Xu, X.; Li, T. Constructing a novel carbon nitride/polyanilinie/ZnO ternary heterostructure with enhanced photocatalytic performance using exfoliated carbon nitride nanosheets as supports. J. Hazard. Mater. 2016, 314, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sun, H.; Ang, H.M.; Tade, M.O.; Wang, S. Integrated oxygen-doping and dye sensitization of graphitic carbon nitride for enhanced visible light photodegradation. Colloid Interface Sci. 2016, 476, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Ming, L.; Yue, H.; Xu, L.; Chen, F. Hydrothermal synthesis of oxidized g-C3N4 and its regulation of photocatalytic activity. J. Mater. Chem. A 2014, 2, 19145–19149. [Google Scholar] [CrossRef]
- Liu, S.; Li, D.; Sun, H.; Ming, H.; Tadé, M.O.; Wang, S. Oxygen functional groups in graphitic carbon nitride for enhanced photocatalysis. Colloid Interface Sci. 2016, 468, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Peng, F.; Wang, H.; Yu, H.; Fang, Y. Carbon nitride polymer sensitized TiO2 nanotube arrays with enhanced visible light photoelectrochemical and photocatalytic performance. Chem. Commun. 2011, 47, 10323–10325. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Chen, H.; Jiang, F.; Wang, X. Enhanced visible-light photocatalytic nitrogen fixation over semi-crystalline graphitic carbon nitride: Oxygen and sulfur Co-doping for crystal and electronic structure modulation. J. Colloid Interface Sci. 2018, 509, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.F.; Song, J.; Pan, L.; Wang, Z.; Zhang, X.; Zou, J.J.; Wang, L. Carbon nitride with simultaneous porous network and O-doping for efficient solar-energy-driven hydrogen evolution. Nano Energy 2015, 12, 646–656. [Google Scholar] [CrossRef]
- Kochkodan, V.M.; Rolya, E.A.; Goncharuk, V.V. Photocatalytic membrane reactors for water treatment from organic pollutants. J. Water Chem. Technol. 2009, 31, 227–237. [Google Scholar] [CrossRef]
- Salim, N.E.; Jaafar, J.; Ismail, A.F.; Othman, M.H.D.; Rahman, M.A.; Yusof, N.; Qtaishat, M.; Matsuura, T.; Aziz, F.; Salleh, W.N.W. Preparation and characterization of hydrophilic surface modifier macromolecule modified poly(Ether Sulfone) photocatalytic membrane for phenol removal. Chem. Eng. J. 2018, 335, 236–247. [Google Scholar] [CrossRef]
- Bykov, I. Characterization of Natural and Technical Lignins. Master’s Thesis, Luleå University of Technology, Luleå, Sweden, 2008. [Google Scholar]
- Kim, I.C.; Yun, H.G.; Lee, K.H. Preparation of asymmetric polyacrylonitrile membrane with small pore size by phase inversion and post-treatment process. J. Membr. Sci. 2002, 199, 75–84. [Google Scholar] [CrossRef]
- Sotto, A.; Boromand, A.; Zhang, R.; Luis, P.; Arsuaga, J.M.; Kim, J.; Van der Bruggen, B. Effect of nanoparticle aggregation at low concentrations of TiO2 on the hydrophilicity, morphology, and fouling resistance of PES-TiO2 membranes. J. Colloid Interface Sci. 2011, 363, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, B.M.; Isloor, A.M.; Ismail, A.F. Enhanced hydrophilicity and salt rejection study of graphene oxide-polysulfone mixed matrix membrane. Desalination 2013, 313, 199–207. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, Z.; Guo, X.; Zhang, Z.; Guo, C. Structures and separation properties of PAN–Fe3O4 ultrafiltration membranes prepared under an orthogonal magnetic field. Ind. Eng. Chem. Res. 2006, 45, 7905–7912. [Google Scholar] [CrossRef]
- Xu, Z.; Zhuang, C.; Zou, Z.; Wang, J.; Xu, X.; Peng, T. Enhanced photocatalytic activity by the construction of a TiO2/Carbon nitride nanosheets heterostructure with high surface area via direct interfacial assembly. Nano Res. 2017, 10, 2193–2209. [Google Scholar] [CrossRef]
- Khayet, M. The effects of air hap length on the internal and external morphology of hollow fiber membranes. Chem. Eng. Sci. 2003, 58, 3091–3104. [Google Scholar] [CrossRef]
- Mulder, M. Basic Principle of Membrane Technology, 2nd ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996. [Google Scholar]
- Razmjou, A.; Mansouri, J.; Chen, V. The effects of mechanical and chemical modification of TiO2 nanoparticles on the surface chemistry, structure and fouling performance of PES ultrafiltration membranes. J. Membr. Sci. 2011, 378, 73–84. [Google Scholar] [CrossRef]
- Sitter, K.D.; Dotremont, C.; Genne, I.; Stoops, L. The use of nanoparticles as alternative pore former for the production of more sustainable polyethersulfone ultrafiltration membranes. J. Membr. Sci. 2014, 471, 168–178. [Google Scholar] [CrossRef]
- Zhang, Z.; An, Q.; Ji, Y.; Qian, J.; Gao, C. Effect of zero shear viscosity of the casting solution on the morphology and permeability of polysulfone membrane prepared via the phase inversion process. Desalination 2010, 260, 43–50. [Google Scholar] [CrossRef]
- Vijay, K.T.; Manju, K.T. Chemical Functionalization of Carbon Nanomaterials: Chemistry and Applications; Thakur, V.K., Thakur, M.K., Eds.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Teow, Y.H.; Ahmad, A.L.; Lim, J.K.; Ooi, B.S. Preparation and characterization of PVDF/TiO2 mixed matrix membrane via in situ colloidal precipitation method. Desalination 2012, 295, 61–69. [Google Scholar] [CrossRef]



| Element | C | H | N | O | S |
|---|---|---|---|---|---|
| Percentage (%) | 57.5938 | 2.1968 | 31.6516 | 8.0082 | <1 |
| Membrane | Total Surface Roughness, Ra (nm) |
|---|---|
| PES18wt% | 48.8 |
| PES/LSMM-OGCN0wt% | 30.8 |
| PES/LSMM-OGCN0.5wt% | 169.0 |
| PES/LSMM-OGCN1.0wt% | 463.1 |
| PES/LSMM-OGCN1.5wt% | 199.6 |
| PES/LSMM-OGCN2.0wt% | 101.0 |
| PES/LSMM-OGCN2.5wt% | 311.0 |
| Membranes | Pure Water Flux, Jw (L/m2·h) | Phenol Rejection (%) |
|---|---|---|
| PES18wt% | 55.50 | 0.52 |
| PES/LSMM-OGCN0wt% | 17.6 | 15.41 |
| PES/LSMM-OGCN0.5wt% | 3.5 | 2.65 |
| PES/LSMM-OGCN1.0wt% | 32.2 | 14.73 |
| PES/LSMM-OGCN1.5wt% | 264.0 | 2.65 |
| PES/LSMM-OGCN2.0wt% | 106.5 | 3.53 |
| PES/LSMM-OGCN2.5wt% | 176.5 | 3.26 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salim, N.E.; Nor, N.A.M.; Jaafar, J.; Ismail, A.F.; Matsuura, T.; Qtaishat, M.R.; Othman, M.H.D.; Rahman, M.A.; Aziz, F.; Yusof, N. Performance of PES/LSMM-OGCN Photocatalytic Membrane for Phenol Removal: Effect of OGCN Loading. Membranes 2018, 8, 42. https://doi.org/10.3390/membranes8030042
Salim NE, Nor NAM, Jaafar J, Ismail AF, Matsuura T, Qtaishat MR, Othman MHD, Rahman MA, Aziz F, Yusof N. Performance of PES/LSMM-OGCN Photocatalytic Membrane for Phenol Removal: Effect of OGCN Loading. Membranes. 2018; 8(3):42. https://doi.org/10.3390/membranes8030042
Chicago/Turabian StyleSalim, Noor Elyzawerni, Nor Azureen Mohamad Nor, Juhana Jaafar, Ahmad Fauzi Ismail, Takeshi Matsuura, Mohammed Rasool Qtaishat, Mohd Hafiz Dzarfan Othman, Mukhlis Abdul Rahman, Farhana Aziz, and Norhaniza Yusof. 2018. "Performance of PES/LSMM-OGCN Photocatalytic Membrane for Phenol Removal: Effect of OGCN Loading" Membranes 8, no. 3: 42. https://doi.org/10.3390/membranes8030042





