A Comparative Study on the Addition Methods of TiO2 Sintering Aid to the Properties of Porous Alumina Membrane Support
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Preparation
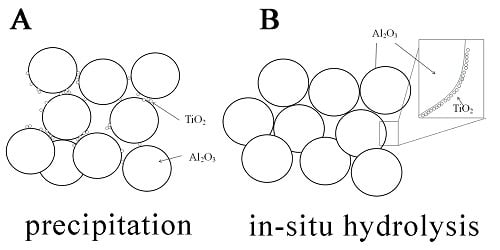
2.1.1. In-Situ Precipitation Method
2.1.2. In-Situ Hydrolysis Method
2.2. Characterization
3. Results and Discussion
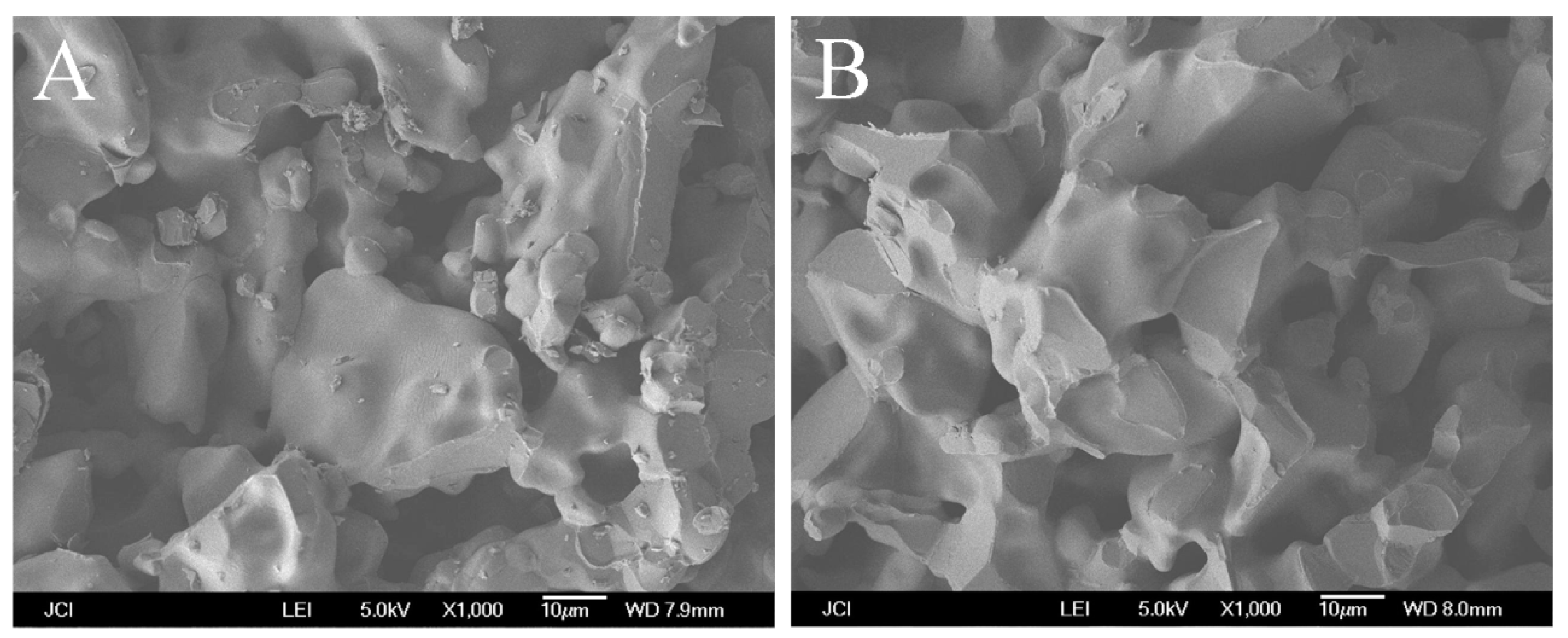
3.1. Effect of the Adding Method of TiO2 on the Bending Strength of Membrane Supports
3.2. Effect of the Adding Methods of TiO2 on the Pore Size Distribution
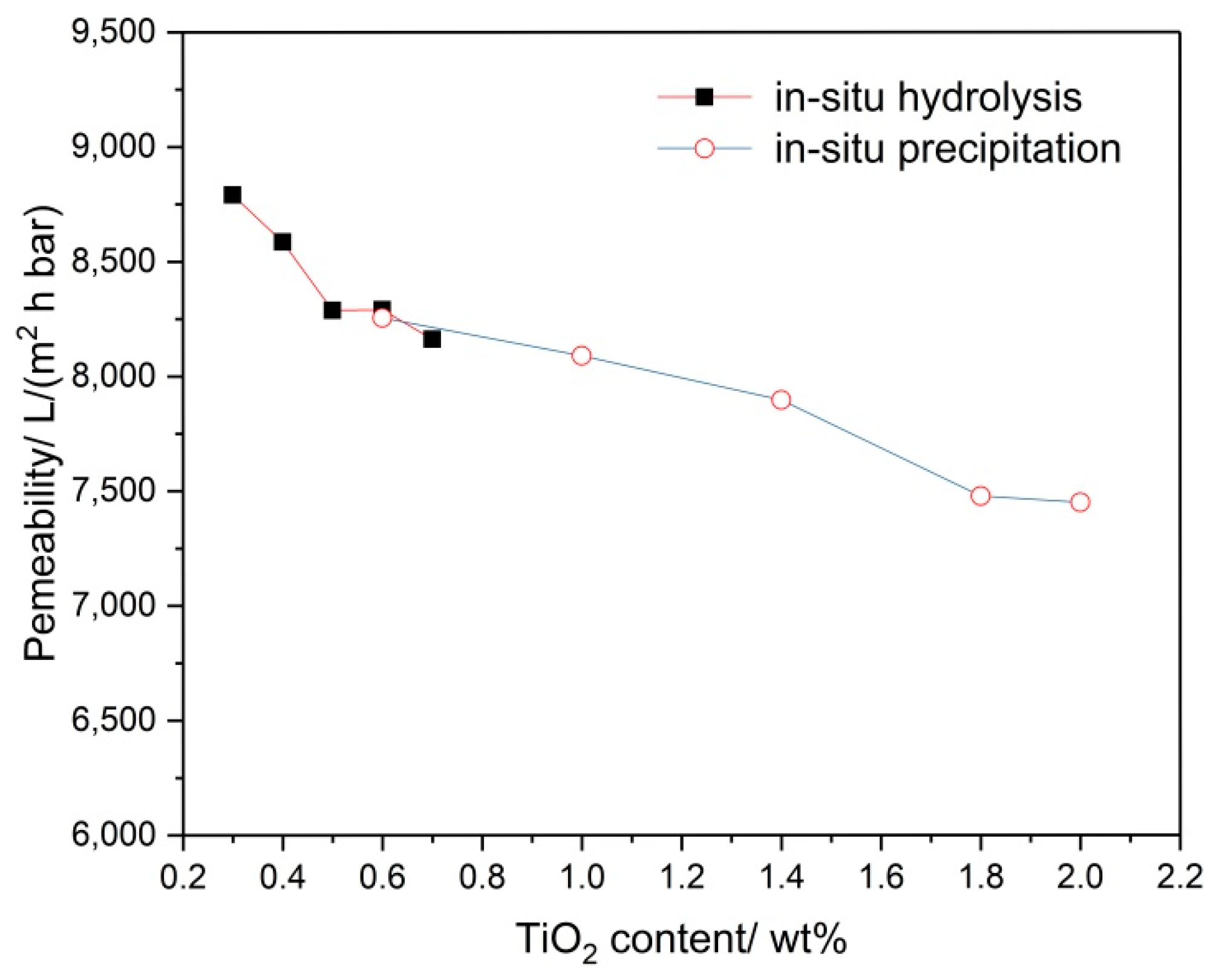
3.3. Effect of the Adding Methods of TiO2 on the Permeating Flux
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sarkar, S.; Bandyopadhyay, S.; Larbot, A.; Cerneaux, S. New clay–alumina porous capillary supports for filtration application. J. Membr. Sci. 2018, 392, 130–136. [Google Scholar] [CrossRef]
- Shkrabina, R.A.; Boneckamp, B.; Pex, P.; Veringa, H.; Ismagilov, Z.R. Porous structure of alumina ceramic support for gas separation membranes, II. Study of porous structure of ceramic composition. React. Kinet. Catal. Lett. 1995, 54, 193–201. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, S.; Zhang, X.; Yang, J.; Liu, X.; Meng, G. Fabrication and characterization of low cost tubular mineral-based ceramic membranes for micro-filtration from natural zeolite. J. Membr. Sci. 2006, 281, 592–599. [Google Scholar] [CrossRef]
- Bao, Q.; Dong, W.; Zhou, J.E.; Wang, Y.; Liu, Y. Effects of pore former on properties of alumina porous ceramic for application in micro-filtration membrane supports. Key Eng. Mater. 2015, 655, 97–102. [Google Scholar] [CrossRef]
- Zhou, J.E.; Yang, Y.; Chang, Q.; Wang, Y.; Ke, Y.; Bao, Q. Effect of particle size gradients of alumina powders on the pore size distribution and bending strength of ceramic membrane supports. J. Synth. Cryst. 2014, 43, 2125–2131. (In Chinese) [Google Scholar]
- Chang, Q.; Yang, Y.; Zhang, X.; Wang, Y.; Zhou, J.; Wang, X.; Cerneaux, S.; Dong, Y. Effect of particle size distribution of raw powders on pore size distribution and bending strength of Al2O3 microfiltration membrane supports. J. Eur. Ceram. Soc. 2014, 34, 3819–3825. [Google Scholar] [CrossRef]
- Goei, R.; Lim, T.T. Asymmetric TiO2, hybrid photocatalytic ceramic membrane with porosity gradient: Effect of structure directing agent on the resulting membranes architecture and performances. Ceram. Int. 2014, 40, 6747–6757. [Google Scholar] [CrossRef]
- Wei, L.; Huang, Y. Preparation of porous TiO2/stainless-steel membranes by carbon assisted solid-state particle sintering. J. Inorg. Mater. 2015, 30, 427–431. [Google Scholar]
- Baig, M.; Patel, F.; Alhooshani, K.; Muraza, O.; Wang, E.; Laoui, T. In-situ aging microwave heating synthesis of LTA zeolite layer on mesoporous TiO2 coated porous alumina support. J. Cryst. Growth 2015, 432, 123–128. [Google Scholar] [CrossRef]
- Kruft, J.; Bruck, H.; Shabana, Y. Effect of TiO2 nanopowder on the sintering behavior of nickel–alumina composites for functionally graded materials. J. Am. Ceram. Soc. 2008, 91, 2870–2877. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, J.; Li, C.; Wu, M.; Jia, D.; Li, Y. Optimization of alumina powder preparation conditions for ceramic membrane support sintering. J. Aust. Ceram. Soc. 2014, 50, 9–16. [Google Scholar]
- Wu, Z.; Zang, L.; Chen, Y.; Xie, Y. Gelcasting of Al2O3 with TiO2 added: the effects of sintering aid and dispersant. Chin. J. Process. Eng. 2001, 1, 398–401. (In Chinese) [Google Scholar]
- Yang, Y.; Zhou, J.E.; Wang, Y.; Chang, Q.; Yang, K. Effect of nano-TiO2 on sintering process of alumina porous ceramic membrane support. J. Synth. Cryst. 2015, 44, 2841–2846. (In Chinese) [Google Scholar]
- Chang, Q.; Wang, Y.; Cerneaux, S.; Zhou, J.E.; Zhang, X.; Wang, X. Preparation of microfiltration membrane supports using coarse alumina grains coated by nano TiO2, as raw materials. J. Eur. Ceram. Soc. 2014, 34, 4355–4361. [Google Scholar] [CrossRef]
- Qi, H.; Fan, Y.; Xing, W.; Winnubst, L. Effect of TiO2 doping on the characteristics of macroporous Al2O3/TiO2 membrane support. J. Eur. Ceram. Soc. 2010, 30, 1317–1325. [Google Scholar] [CrossRef]
- Li, D.; Zhu, Q.; Cui, S. Preparation and characterization of circular plate-shaped porous alumina ceramic membrane support. J. Environ. Eng. 2012, 06, 941–944. [Google Scholar]
- Wang, Y.; Chen, G.; Wang, Z.; Liu, J.; Luo, P. Improvement of microcracks resistance of porous aluminium titanate ceramic membrane support using attapulgite clay as additive clay as additive. Ceram. Int. 2018, 44, 2077–2084. [Google Scholar] [CrossRef]
- Oun, A.; Tahri, N.; Mahouche-Chergui, S.; Carbonnier, B.; Majumdar, S.; Sarkar, S. Tubular ultrafiltration ceramic membrane based on titania nanoparticles immobilized on macroporous clay-alumina support: Elaboration, characterization and application to dye removal. Sep. Purif. Technol. 2017, 188, 126–133. [Google Scholar] [CrossRef]
- Monash, P.; Pugazhenthi, G. Effect of TiO2 addition on the fabrication of ceramic membrane supports: A study on the separation of oil droplets and bovine serum albumin (BSA) from its solution. Desalination 2011, 279, 104–114. [Google Scholar] [CrossRef]
- Bissett, H.; Zah, J.; Krieg, H.M. Manufacture and optimization of tubular ceramic membrane supports. Powder Technol. 2008, 181, 57–66. [Google Scholar] [CrossRef]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Chang, Q.; Hu, Z.; Zhang, X. A Comparative Study on the Addition Methods of TiO2 Sintering Aid to the Properties of Porous Alumina Membrane Support. Membranes 2018, 8, 49. https://doi.org/10.3390/membranes8030049
Yang Y, Chang Q, Hu Z, Zhang X. A Comparative Study on the Addition Methods of TiO2 Sintering Aid to the Properties of Porous Alumina Membrane Support. Membranes. 2018; 8(3):49. https://doi.org/10.3390/membranes8030049
Chicago/Turabian StyleYang, Yulong, Qibing Chang, Zhiwen Hu, and Xiaozhen Zhang. 2018. "A Comparative Study on the Addition Methods of TiO2 Sintering Aid to the Properties of Porous Alumina Membrane Support" Membranes 8, no. 3: 49. https://doi.org/10.3390/membranes8030049