Cortical Volume Alterations in Conduct Disordered Adolescents with and without Bipolar Disorder
Abstract
:1. Introduction
2. Methods
2.1. Subjects
| Factors | CD-BD (n = 24) | CD (n = 24) | Healthy Controls (n = 24) |
|---|---|---|---|
| Mean Age years (sd) | 15.83 (1.05) | 16.23 (1.05) | 15.3 (1.14) |
| Male Gender (%) | 16 (66) | 21 (84) | 16 (66) |
| Ethnicity | |||
| Non-Hispanic White (%) | 5 (21) | 3 (12) | 3 (12) |
| Hispanic (%) | 13 (54) | 21(87) | 20 (83) |
| African American (%) | 4 (17) | 0 | 1 (4) |
| Other Race/Ethnic (%) | 2 (8) | 0 | 0 |
| Hollingshead Socioeconomic Status | 34.31 (10.75) | 34.48 (10.08) | 37.04 (8.52) |
| IQ | 91.90 (15.45) | 97.75 (9.62) | 98.55 (10.74) |
| Mean YMRS (sd) | 6.00 (3.94) a | 2.60 (2.81) b | 0 |
| Mean CDRS (sd) | 25.62 (11.39) a | 22.71 (7.12) a | 17.14 (0.47) b |
| Mean PESQ (sd) | 39.48 (15.49) a | 41.83 (11.85) a | 18.91 (1.61) b |
| Mean Current C-GAS | 63.76 (7.60) a | 62.54 (7.31) a | 85.56 (3.31) b |
| Mean Past C-GAS | 30.00 (10.66) a | 43.40 (11.06) b | |
| Mean Highest Past C-GAS | 65.32 (6.58) | 63.61 (8.26) | |
| Lifetime Comorbid Condition | |||
| Alcohol Abuse or Dependence (%) | 7 (29) | 6 (24) | 0 |
| Cannabis Abuse or Dependence (%) | 17 (71) | 20 (83) | 0 |
| Early Onset CD (%) | 10 (42) | 6 (24) | 0 |
| ODD | 22 (96%) | 19 (76%) | 0 |
| PTSD (%) | 6 (25) | 6 (25) | 0 |
| GAD (%) | 6 (25) | 3 (12) | 0 |
| ADHD (%) | 18 (75) a | 11 (44) b | 0 |
| MDD (%) | 15 (63) a | 4 (16) b | 0 |
| Medications | |||
| Lithium | 6 (25) a | 0 b | 0 |
| Valproic Acid | 12 (50) a | 1 (4) b | 0 |
| Atypical Antipsychotic | 20 (83) a | 3 (12) b | 0 |
| Stimulants | 14 (58) | 9 (36) | 0 |
2.2. Imaging Methods
2.3. Pre-Processing
2.4. Analyses
3. Results
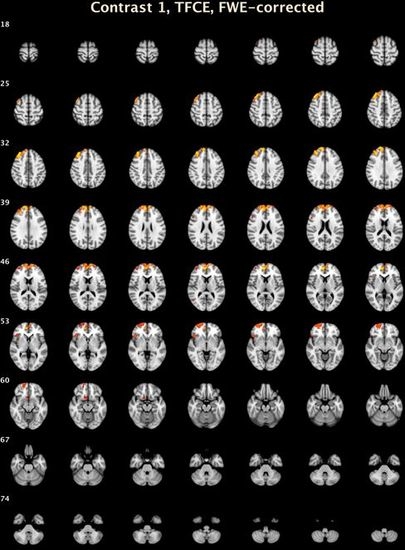
| Cluster Number | Size | Lowest p | X | Y | Z | Structure | Brodmann Area |
|---|---|---|---|---|---|---|---|
| CD-BD Compared to HC: Voxel Level | |||||||
| 1 | 23 | 0.002 | 10.2 | 51.8 | 8 | R Medial Frontal Gyrus | 10 |
| Threshold-Free Cluster Enhancement | |||||||
| 7 | 3273 | 0.005 | 15.2 | 49.4 | 26.6 | R Medial Frontal Gyrus | 9 |
| 6 | 449 | 0.027 | 23.2 | 60.8 | −6.8 | R Superior Frontal Gyrus | 10 |
| 5 | 135 | 0.031 | 45.2 | 23.4 | −2.6 | R Inferior Frontal Gyrus, B | 47 |
| 4 | 73 | 0.046 | 9 | 14 | −14.4 | R Anterior Cingulate | 25 |
| 3 | 26 | 0.044 | 49.8 | 21.4 | 21.6 | R Middle Frontal Gyrus | 46 |
| 2 | 11 | 0.047 | 49 | −65.6 | 9.8 | R Middle Temporal Gyrus | 37 |
| 1 | 1 | 0.05 | 42 | 24 | −20 | R Inferior Frontal Gyrus, B | 47 |

4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Raine, A.; Lenz, T.; Bihrle, S.; LaCasse, L.; Colletti, P. Reduced prefrontal grey matter volume and reduced autonomic activity in antisocial personality disorder. Arch. Gen. Psychiatry 2000, 57, 119–127. [Google Scholar]
- Yang, Y.; Raine, A.; Lencz, T.; Bihrle, S.; LaCasse, L.; Colletti, P. Volume reduction in prefrontal gray matter in unsuccessful criminal psychopaths. Biol. Psychiatry 2005, 57, 1103–1108. [Google Scholar]
- Oliveira-Souza, R.; Hare, R.D.; Bramati, I.E.; Garrido, G.J.; Azevedo, I.F.; Tovar-Moll, F.; Moll, J. Psychopathy as a disorder of the moral brain: Fronto-temporo-limbic grey matter reductions demonstrated by voxel-based morphometry. Neuroimage 2008, 40, 1202–1213. [Google Scholar] [CrossRef]
- Tiihonen, J.; Rossi, R.; Laakso, M.P.; Hodgins, S.; Testa, C.; Perez, J.; Repo-Tiihonen, E.; Vaurio, O.; Soininen, H.; Aronen, H.J.; et al. Brain anatomy of persistent violent offenders: More rather than less. Psychiatry Res. 2008, 163, 201–212. [Google Scholar]
- Dolan, M.C.; Deakin, J.F.; Roberts, N.; Anderson, I.M. Quantitative frontal and temporal structural MRI studies in personality-disordered offenders and control subjects. Psychiatry Res. 2002, 116, 133–149. [Google Scholar] [CrossRef]
- Kruesi, M.J.; Casanova, M.F.; Mannheim, G.; Johnson-Bilder, A. Reduced temporal lobe volume in early onset conduct disorder. Psychiatry Res. 2004, 132, 1–11. [Google Scholar]
- Bussing, R.; Grudnik, J.; Mason, D.; Wasiak, M.; Leonard, C. ADHD and conduct disorder: An MRI study in a community sample. World J. Biol. Psychiatry 2002, 3, 216–220. [Google Scholar] [CrossRef]
- Sterzer, P.; Stadler, C.; Poustka, F.; Kleinschmidt, A. A structural neural deficit in adolescents with conduct disorder and its association with lack of empathy. Neuroimage 2007, 37, 335–342. [Google Scholar] [CrossRef]
- Huebner, T.; Vloet, T.D.; Marx, I.; Konrad, K.; Fink, G.R.; Herpertz, S.C.; Herpertz-Dahlmann, B. Morphometric brain abnormalities in boys with conduct disorder. J. Am. Acad. Child Adolesc. Psychiatry 2008, 47, 540–547. [Google Scholar] [CrossRef]
- Hyatt, C.J.; Haney-Caron, E.; Stevens, M.C. Cortical thickness and folding deficits in conduct-disordered adolescents. Biol. Psychiatry 2012, 72, 207–214. [Google Scholar] [CrossRef]
- Hariri, A.R.; Bookheimer, S.Y.; Mazziotta, J.C. Modulating emotional responses: Effects of a neocortical network on the limbic system. Neuroreport 2000, 11, 43–48. [Google Scholar] [CrossRef]
- Phillips, M.L.; Drevets, W.C.; Rauch, S.L.; Lane, R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol. Psychiatry 2003, 54, 504–514. [Google Scholar] [CrossRef]
- Bechara, A.; Damasio, H.; Damasio, A.R.; Lee, G.P. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J. Neurosci. 1999, 19, 5473–5481. [Google Scholar]
- Anderson, S.W.; Bechara, A.; Damasio, H.; Tranel, D.; Damasio, A.R. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nat. Neurosci. 1999, 2, 1032–1037. [Google Scholar] [CrossRef]
- Connor, D.F.; Steeber, J.; McBurnett, K. A review of attention-deficit/hyperactivity disorder complicated by symptoms of oppositional defiant disorder or conduct disorder. J. Dev. Behav. Pediatr. 2010, 31, 427–440. [Google Scholar] [CrossRef]
- Aarons, G.A.; Brown, S.A.; Hough, R.L.; Garland, A.F.; Wood, P.A. Prevalence of adolescent substance use disorders across five sectors of care. J. Am. Acad. Child Adolesc. Psychiatry 2001, 40, 419–426. [Google Scholar] [CrossRef]
- Polier, G.G.; Vloet, T.D.; Herpertz-Dahlmann, B.; Laurens, K.R.; Hodgins, S. Comorbidity of conduct disorder symptoms and internalising problems in children: Investigating a community and a clinical sample. Eur. Child Adolesc. Psychiatry 2012, 21, 31–38. [Google Scholar] [CrossRef]
- Abram, K.M.; Teplin, L.A.; McClelland, G.M.; Dulcan, M.K. Comorbid psychiatric disorders in youth in juvenile detention. Arch. Gen. Psychiatry 2003, 60, 1097–1108. [Google Scholar]
- Garland, A.F.; Hough, R.L.; McCabe, K.M.; Yeh, M.; Wood, P.A.; Aarons, G.A. Prevalence of psychiatric disorders in youths across five sectors of care. J. Am. Acad. Child Adolesc. Psychiatry 2001, 40, 409–418. [Google Scholar] [CrossRef]
- Wasserman, G.A.; McReynolds, L.S.; Lucas, C.P.; Fisher, P.; Santos, L. The voice disc-IV with incarcerated male youths: Prevalence of disorder. J. Am. Acad. Child Adolesc. Psychiatry 2002, 41, 314–321. [Google Scholar] [CrossRef]
- Harzke, A.J.; Baillargeon, J.; Baillargeon, G.; Henry, J.; Olvera, R.L.; Torrealday, O.; Penn, J.V.; Parikh, R. Prevalence of psychiatric disorders in the texas juvenile correctional system. J. Correct Health Care 2012, 18, 143–157. [Google Scholar] [CrossRef]
- Baillargeon, J.; Binswanger, I.A.; Penn, J.V.; Williams, B.A.; Murray, O.J. Psychiatric disorders and repeat incarcerations: The revolving prison door. Am. J. Psychiatry 2009, 166, 103–109. [Google Scholar]
- Pliszka, S.R.; Sherman, J.O.; Barrow, M.V.; Irick, S. Affective disorders in juvenile offenders: A preliminary study. Am. J. Psychiatry 2000, 157, 130–132. [Google Scholar]
- Geller, B.; Zimerman, B.; Williams, M.; Bolhofner, K.; Craney, J.L.; DelBello, M.P.; Soutullo, C.A. Diagnostic characteristics of 93 cases of a prepubertal and early adolescent bipolar disorder phenotype by gender, puberty and comorbid attention deficit hyperactivity disorder. J. Child Adolesc. Psychopharmacol. 2000, 10, 157–164. [Google Scholar] [CrossRef]
- Faraone, S.V.; Biederman, J.; Wozniak, J.; Mundy, E.; Mennin, D.; O’Donnell, D. Is comorbidity with ADHD a marker for juvenile-onset mania? J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 1046–1055. [Google Scholar] [CrossRef]
- Axelson, D.; Birmaher, B.; Strober, M.; Gill, M.K.; Valeri, S.; Chiappetta, L.; Ryan, N.; Leonard, H.; Hunt, J.; Iyengar, S.; et al. Phenomenology of children and adolescents with bipolar spectrum disorders. Arch. Gen. Psychiatry 2006, 63, 1139–1148. [Google Scholar] [CrossRef]
- Birmaher, B.; Axelson, D.; Monk, K.; Kalas, C.; Goldstein, B.; Hickey, M.B.; Obreja, M.; Ehmann, M.; Iyengar, S.; Shamseddeen, W.; et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: The pittsburgh bipolar offspring study. Arch. Gen. Psychiatry 2009, 66, 287–296. [Google Scholar]
- Biederman, J.; Mick, E.; Wozniak, J.; Monuteaux, M.C.; Galdo, M.; Faraone, S.V. Can a subtype of conduct disorder linked to bipolar disorder be identified? Integration of findings from the massachusetts general hospital pediatric psychopharmacology research program. Biol. Psychiatry 2003, 53, 952–960. [Google Scholar] [CrossRef]
- Lish, J.D.; Dime-Meenan, S.; Whybrow, P.C.; Price, R.A.; Hirschfeld, R.M. The national depressive and manic-depressive association (DMDA) survey of bipolar members. J. Affect. Disord. 1994, 31, 281–294. [Google Scholar] [CrossRef]
- Carlson, G.A.; Bromet, E.J.; Sievers, S. Phenomenology and outcome of subjects with early- and adult-onset psychotic mania. Am. J. Psychiatry 2000, 157, 213–219. [Google Scholar] [CrossRef]
- Kovacs, M.; Pollock, M. Bipolar disorder and comorbid conduct disorder in childhood and adolescence. J. Am. Acad. Child Adolesc. Psychiatry 1995, 34, 715–723. [Google Scholar] [CrossRef]
- Olvera, R.L.; Semrud-Clikeman, M.; Pliszka, S.R.; O’Donnell, L. Neuropsychological deficits in adolescents with conduct disorder and comorbid bipolar disorder: A pilot study. Bipolar Disord. 2005, 7, 57–67. [Google Scholar] [CrossRef]
- Kaufman, J.; Birmaher, B.; Brent, D.; Rao, U.; Flynn, C.; Moreci, P.; Williamson, D.; Ryan, N. Schedule for affective disorders and schizophrenia for school age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 980–988. [Google Scholar] [CrossRef]
- Hollingshead, A.B. Four Factor Index of Social Status; Department of Sociology, Yale University: New Haven, CT, USA, 1975. [Google Scholar]
- Fristad, M.A.; Weller, E.B.; Weller, R.A. The mania rating scale: Can it be used in children? A preliminary report. J. Am. Acad. Child Adolesc. Psychiatry 1992, 31, 252–257. [Google Scholar] [CrossRef]
- Poznanski, E.O.; Mokros, H.B. Children’s Depression Rating Scale, Revised (Cdrs-R); Western Psychological Services: Los Angeles, CA, USA, 1996. [Google Scholar]
- Winters, K.C. Personal Experience Screening Questionnaire (PESQ); Western Psychological Services: Los Angeles, CA, USA, 1999. [Google Scholar]
- Elliott, C.D. Adminstration and Scoring Manual for the Differential Ability Scales (DAS); The Psychological Corporation: San Antonio, TX, USA, 1990. [Google Scholar]
- Shaffer, D.; Gould, M.S.; Brasic, J.; Ambrosini, P.; Fisher, P.; Bird, H.; Aluwahlia, S. A Children’s Global Assessment Scale (CGAS). Arch. Gen. Psychiatry 1983, 40, 1228–1231. [Google Scholar] [CrossRef]
- Wright, I.C.; McGuire, P.K.; Poline, J.B.; Travere, J.M.; Murray, R.M.; Frith, C.D.; Frackowiak, R.S.; Friston, K.J. A voxel-based method for the statistical analysis of gray and white matter density applied to schizophrenia. Neuroimage 1995, 2, 244–252. [Google Scholar] [CrossRef]
- Bullmore, E.T.; Suckling, J.; Overmeyer, S.; Rabe-Hesketh, S.; Taylor, E.; Brammer, M.J. Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans. Med. Imaging 1999, 18, 32–42. [Google Scholar] [CrossRef]
- Ashburner, J.; Friston, K.J. Voxel-based morphometry-the methods. Neuroimage 2000, 11, 805–821. [Google Scholar] [CrossRef]
- Good, C.D.; Johnsrude, I.S.; Ashburner, J.; Henson, R.N.; Friston, K.J.; Frackowiak, R.S. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 2001, 14, 21–36. [Google Scholar] [CrossRef]
- Zhang, Y.; Brady, M.; Smith, S. Segmentation of brain MR images through a hidden markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging 2001, 20, 45–57. [Google Scholar]
- Smith, S.M.; Nichols, T.E. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 2009, 44, 83–98. [Google Scholar] [CrossRef]
- Jenkinson, M.; Smith, S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001, 5, 143–156. [Google Scholar] [CrossRef]
- Andersson, J.; Jenkinson, M.; Smith, S. FMRIB’s Non-Linear Image Registration Tool; FMRIB Centre: Oxford, UK, 2007. [Google Scholar]
- Holmes, A.P.; Blair, R.C.; Watson, J.D.; Ford, I. Nonparametric analysis of statistic images from functional mapping experiments. J. Cereb. Blood Flow. Metab. 1996, 16, 7–22. [Google Scholar]
- Nichols, T.E.; Holmes, A.P. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum. Brain Mapp. 2002, 15, 1–25. [Google Scholar] [CrossRef]
- Steiner, H. Practice parameters for the assessment and treatment of children and adolescents with conduct disorder. American academy of child and adolescent psychiatry. J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 122–139. [Google Scholar] [CrossRef]
- Fuster, J.M. The prefrontal cortex- an update: Time is of the essence. Neuroimage 2001, 30, 319–333. [Google Scholar]
- Narumoto, J.; Yamada, H.; Iidaka, T.; Sadato, N.; Fukui, K.; Itoh, H.; Yonekura, Y. Brain regions involved in verbal or non-verbal aspects of facial emotion recognition. Neuroreport 2000, 11, 2571–2576. [Google Scholar] [CrossRef]
- Goldapple, K.; Segal, Z.; Garson, C.; Lau, M.; Bieling, P.; Kennedy, S.; Mayberg, H. Modulation of cortical-limbic pathways in major depression: Treatment-specific effects of cognitive behavior therapy. Arch. Gen. Psychiatry 2004, 61, 34–41. [Google Scholar] [CrossRef]
- Lopez-Larson, M.P.; DelBello, M.P.; Zimmerman, M.E.; Schwiers, M.L.; Strakowski, S.M. Regional prefrontal gray and white matter abnormalities in bipolar disorder. Biol. Psychiatry 2002, 52, 93–100. [Google Scholar] [CrossRef]
- McIntosh, A.M.; Job, D.E.; Moorhead, T.W.; Harrison, L.K.; Forrester, K.; Lawrie, S.M.; Johnstone, E.C. Voxel-based morphometry of patients with schizophrenia or bipolar disorder and their unaffected relatives. Biol. Psychiatry 2004, 56, 544–552. [Google Scholar] [CrossRef]
- Dickstein, D.P.; Milham, M.P.; Nugent, A.C.; Drevets, W.C.; Charney, D.S.; Pine, D.S.; Leibenluft, E. Frontotemporal alterations in pediatric bipolar disorder: Results of a voxel-based morphometry study. Arch. Gen. Psychiatry 2005, 62, 734–741. [Google Scholar] [CrossRef]
- Frazier, J.A.; Breeze, J.L.; Makris, N.; Giuliano, A.S.; Herbert, M.R.; Seidman, L.; Biederman, J.; Hodge, S.M.; Dieterich, M.E.; Gerstein, E.D.; et al. Cortical gray matter differences identified by structural magnetic resonance imaging in pediatric bipolar disorder. Bipolar Disord. 2005, 7, 555–569. [Google Scholar] [CrossRef]
- Frangou, S.; Donaldson, S.; Hadjulis, M.; Landau, S.; Goldstein, L.H. The maudsley bipolar disorder project: Executive dysfunction in bipolar disorder I and its clinical correlates. Biol. Psychiatry 2005, 58, 859–864. [Google Scholar] [CrossRef]
- Houenou, J.; Frommberger, J.; Carde, S.; Glasbrenner, M.; Diener, C.; Leboyer, M.; Wessa, M. Neuroimaging-based markers of bipolar disorder: Evidence from two meta-analyses. J. Affect. Disord. 2011, 132, 344–355. [Google Scholar] [CrossRef]
- Ochsner, K.N.; Ray, R.D.; Cooper, J.C.; Robertson, E.R.; Chopra, S.; Gabrieli, J.D.E.; Gross, J.J. For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage 2004, 23, 483–499. [Google Scholar] [CrossRef]
- Blair, K.S.; Smith, B.W.; Mitchell, D.G.V.; Morton, J.; Vythilingam, M.; Pessoa, L.; Fridberg, D.; Zametkin, A.; Nelson, E.E.; Drevets, W.C.; et al. Modulation of emotion by cognition and cognition by emotion. Neuroimage 2007, 35, 430–440. [Google Scholar] [CrossRef]
- Price, J.L.; Drevets, W.C. Neurocircuitry of mood disorders. Neuropsychopharmacology 2010, 35, 192–216. [Google Scholar] [CrossRef]
- Strakowski, S.M.; DelBello, M.P.; Adler, C.M. The functional neuroanatomy of bipolar disorder: A review of neuroimaging findings. Mol. Psychiatry 2005, 10, 105–116. [Google Scholar] [CrossRef]
- Adler, C.M.; DelBello, M.P.; Strakowski, S.M. Brain network dysfunction in bipolar disorder. CNS Spectr. 2006, 11, 312–320. [Google Scholar]
- Phillips, M.L.; Ladouceur, C.D.; Drevets, W.C. Neural systems underlying voluntary and automatic emotion regulation: Toward a neural model of bipolar disorder. Mol. Psychiatry 2008, 13, 829–857. [Google Scholar] [CrossRef]
- Bechara, A.; Damasio, H.; Damasio, A.R. Emotion, decision making and the orbitofrontal cortex. Cereb. Cortex 2000, 10, 295–307. [Google Scholar] [CrossRef]
- Scarpa, A.; Raine, A. The psychophysiology of child misconduct. Pediatr. Ann. 2004, 33, 296–304. [Google Scholar]
- Herpertz, S.C.; Mueller, B.; Qunaibi, M.; Lichterfeld, C.; Konrad, K.; Herpertz-Dahlmann, B. Response to emotional stimuli in boys with conduct disorder. Am. J. Psychiatry 2005, 162, 1100–1107. [Google Scholar] [CrossRef]
- Herpertz, S.C.; Vloet, T.; Mueller, B.; Domes, G.; Willmes, K.; Herpertz-Dahlmann, B. Similar autonomic responsivity in boys with conduct disorder and their fathers. J. Am. Acad. Child Adolesc. Psychiatry 2007, 46, 535–544. [Google Scholar] [CrossRef]
- Damasio, A.R.; Tranel, D.; Damasio, H. Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behav. Brain Res. 1990, 41, 81–94. [Google Scholar] [CrossRef]
- Bechara, A.; Tranel, D.; Damasio, H.; Damasio, A.R. Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cereb. Cortex 1996, 6, 215–225. [Google Scholar]
- Raine, A. Annotation: The role of prefrontal deficits, low autonomic arousal, and early health factors in the development of antisocial and aggressive behavior in children. J. Child Psychol. Psychiatry 2002, 43, 417–434. [Google Scholar] [CrossRef]
- Marsh, A.A.; Finger, E.C.; Mitchell, D.G.; Reid, M.E.; Sims, C.; Kosson, D.S.; Towbin, K.E.; Leibenluft, E.; Pine, D.S.; Blair, R.J. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. Am. J. Psychiatry 2008, 165, 712–720. [Google Scholar] [CrossRef]
- Cabeza, R.; Nyberg, L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J. Cogn. Neurosci. 2000, 12, 1–47. [Google Scholar] [CrossRef]
- Narumoto, J.; Okada, T.; Sadato, N.; Fukui, K.; Yonekura, Y. Attention to emotion modulates fMRI activity in human right superior temporal sulcus. Brain Res. Cogn. Brain Res. 2001, 12, 225–231. [Google Scholar] [CrossRef]
- Aichhorn, M.; Perner, J.; Weiss, B.; Kronbichler, M.; Staffen, W.; Ladurner, G. Temporo-parietal junction activity in theory-of-mind tasks: Falseness, beliefs, or attention. J. Cogn. Neurosci. 2009, 21, 1179–1192. [Google Scholar] [CrossRef]
- Vollm, B.A.; Taylor, A.N.; Richardson, P.; Corcoran, R.; Stirling, J.; McKie, S.; Deakin, J.F.; Elliott, R. Neuronal correlates of theory of mind and empathy: A functional magnetic resonance imaging study in a nonverbal task. Neuroimage 2006, 29, 90–98. [Google Scholar] [CrossRef]
- Saxe, R.; Wexler, A. Making sense of another mind: The role of the right temporo-parietal junction. Neuropsychologia 2005, 43, 1391–1399. [Google Scholar] [CrossRef]
- Glahn, D.C.; Thompson, P.M.; Blangero, J. Neuroimaging endophenotypes: Strategies for finding genes influencing brain structure and function. Hum. Brain Mapp. 2007, 28, 488–501. [Google Scholar] [CrossRef]
- Glahn, D.C.; Knowles, E.E.; McKay, D.R.; Sprooten, E.; Raventos, H.; Blangero, J.; Gottesman, I.I.; Almasy, L. Arguments for the sake of endophenotypes: Examining common misconceptions about the use of endophenotypes in psychiatric genetics. Am. J. Med. Gen. 2014. [Google Scholar] [CrossRef]
- Friedman, L.; Findling, R.L.; Kenny, J.T.; Swales, T.P.; Stuve, T.A.; Jesberger, J.A.; Lewin, J.S.; Schulz, S.C. An MRI study of adolescent patients with either schizophrenia or bipolar disorder as compared to healthy control subjects. Biol. Psychiatry 1999, 46, 78–88. [Google Scholar] [CrossRef]
- Strakowski, S.M.; DelBello, M.P.; Zimmerman, M.E.; Getz, G.E.; Mills, N.P.; Ret, J.; Shear, P.; Adler, C.M. Ventricular and periventricular structural volumes in first- versus multiple-episode bipolar disorder. Am. J. Psychiatry 2002, 159, 1841–1847. [Google Scholar]
- DelBello, M.P.; Zimmerman, M.E.; Mills, N.P.; Getz, G.E.; Strakowski, S.M. Magnetic resonance imaging analysis of amygdala and other subcortical brain regions in adolescents with bipolar disorder. Bipolar Disord. 2004, 6, 43–52. [Google Scholar] [CrossRef]
- Chang, K.; Karchemskiy, A.; Barnea-Goraly, N.; Garrett, A.; Simeonova, D.I.; Reiss, A. Reduced amygdalar gray matter volume in familial pediatric bipolar disorder. J. Am. Acad. Child Adolesc. Psychiatry 2005, 44, 565–573. [Google Scholar] [CrossRef]
- Chang, K.; Barnea-Goraly, N.; Karchemskiy, A.; Simeonova, D.I.; Barnes, P.; Ketter, T.; Reiss, A.L. Cortical magnetic resonance imaging findings in familial pediatric bipolar disorder. Biol. Psychiatry 2005, 58, 197–203. [Google Scholar] [CrossRef]
- Hafeman, D.M.; Chang, K.D.; Garrett, A.S.; Sanders, E.M.; Phillips, M.L. Effects of medication on neuroimaging findings in bipolar disorder: An updated review. Bipolar Disord. 2012, 14, 375–410. [Google Scholar] [CrossRef]
- Ashburner, J.; Friston, K.J. Why voxel-based morphometry should be used. Neuroimage 2001, 14, 1238–1243. [Google Scholar] [CrossRef]
- Konarski, J.Z.; McIntyre, R.S.; Kennedy, S.H.; Rafi-Tari, S.; Soczynska, J.K.; Ketter, T.A. Volumetric neuroimaging investigations in mood disorders: Bipolar disorder versus major depressive disorder. Bipolar Disord. 2008, 10, 1–37. [Google Scholar]
- Rubia, K. “Cool” inferior frontostriatal dysfunction in attention-deficit/hyperactivity disorder versus “hot” ventromedial orbitofrontal-limbic dysfunction in conduct disorder: A review. Biol. Psychiatry 2011, 69, 69–87. [Google Scholar] [CrossRef]
- Gillihan, S.J.; Parens, E. Should we expect “neural signatures” for DSM diagnoses? J. Clin. Psychiatry 2011, 72, 1383–1389. [Google Scholar] [CrossRef]
- Connor, D.F.; Doerfler, L.A. ADHD with comorbid oppositional defiant disorder or conduct disorder: Discrete or nondistinct disruptive behavior disorders? J. Atten. Disord. 2008, 12, 126–134. [Google Scholar] [CrossRef]
- Anderson, D.; Ardekani, B.A.; Burdick, K.E.; Robinson, D.G.; John, M.; Malhotra, A.K.; Szeszko, P.R. Overlapping and distinct gray and white matter abnormalities in schizophrenia and bipolar I disorder. Bipolar Disord. 2013, 15, 680–693. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Olvera, R.L.; Glahn, D.C.; O'Donnell, L.; Bearden, C.E.; Soares, J.C.; Winkler, A.M.; Pliszka, S.R. Cortical Volume Alterations in Conduct Disordered Adolescents with and without Bipolar Disorder. J. Clin. Med. 2014, 3, 416-431. https://doi.org/10.3390/jcm3020416
Olvera RL, Glahn DC, O'Donnell L, Bearden CE, Soares JC, Winkler AM, Pliszka SR. Cortical Volume Alterations in Conduct Disordered Adolescents with and without Bipolar Disorder. Journal of Clinical Medicine. 2014; 3(2):416-431. https://doi.org/10.3390/jcm3020416
Chicago/Turabian StyleOlvera, Rene L., David C. Glahn, Louise O'Donnell, Carrie E. Bearden, Jair C. Soares, Anderson M. Winkler, and Steven R. Pliszka. 2014. "Cortical Volume Alterations in Conduct Disordered Adolescents with and without Bipolar Disorder" Journal of Clinical Medicine 3, no. 2: 416-431. https://doi.org/10.3390/jcm3020416