Myocardial Expression of Macrophage Migration Inhibitory Factor in Patients with Heart Failure
Abstract
:1. Introduction
2. Methods
2.1. Study Setting and Population
2.2. Sample Preparation
2.3. RNA Isolation and Quantitative Real-Time PCR
3. Statistics
4. Results
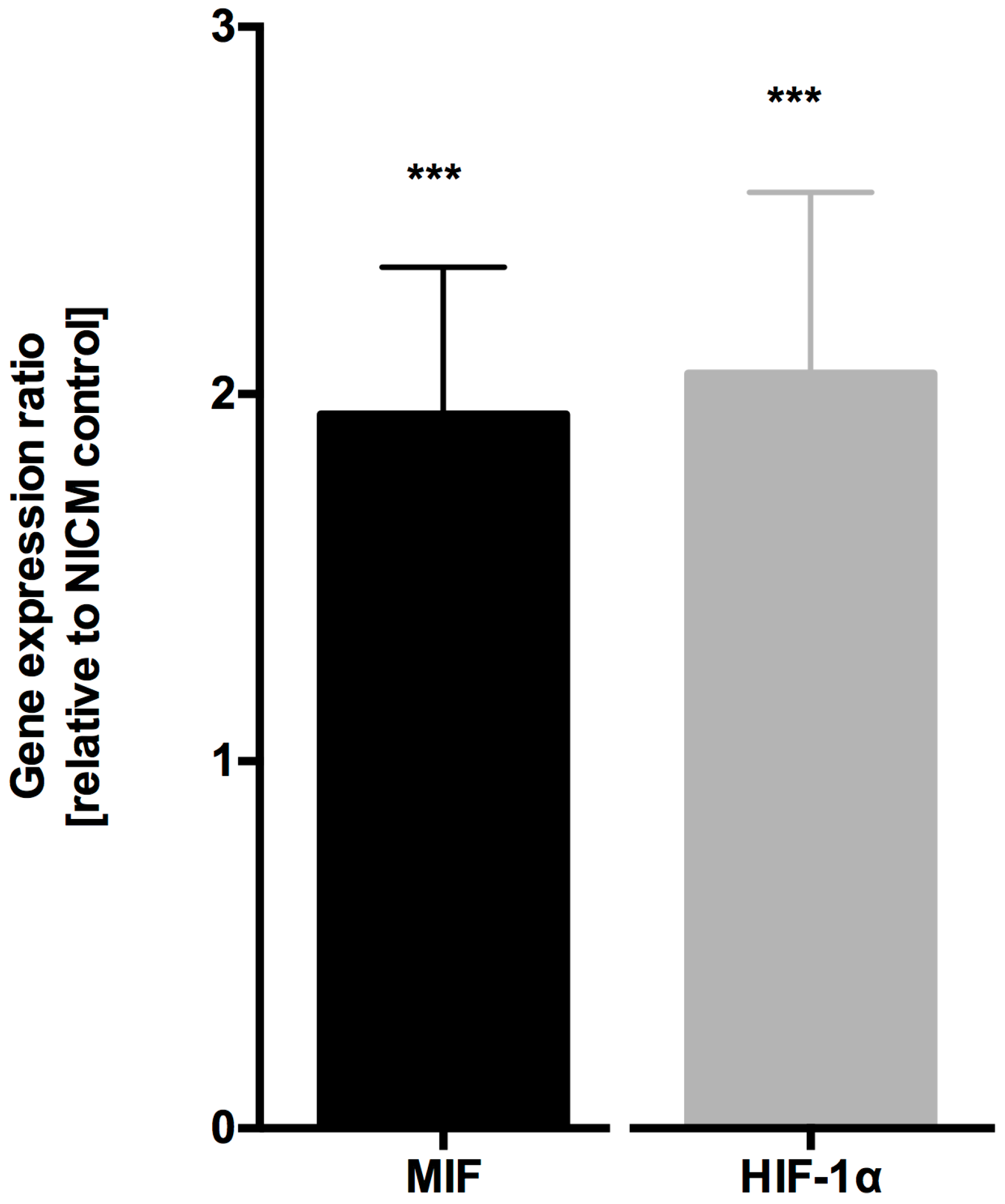
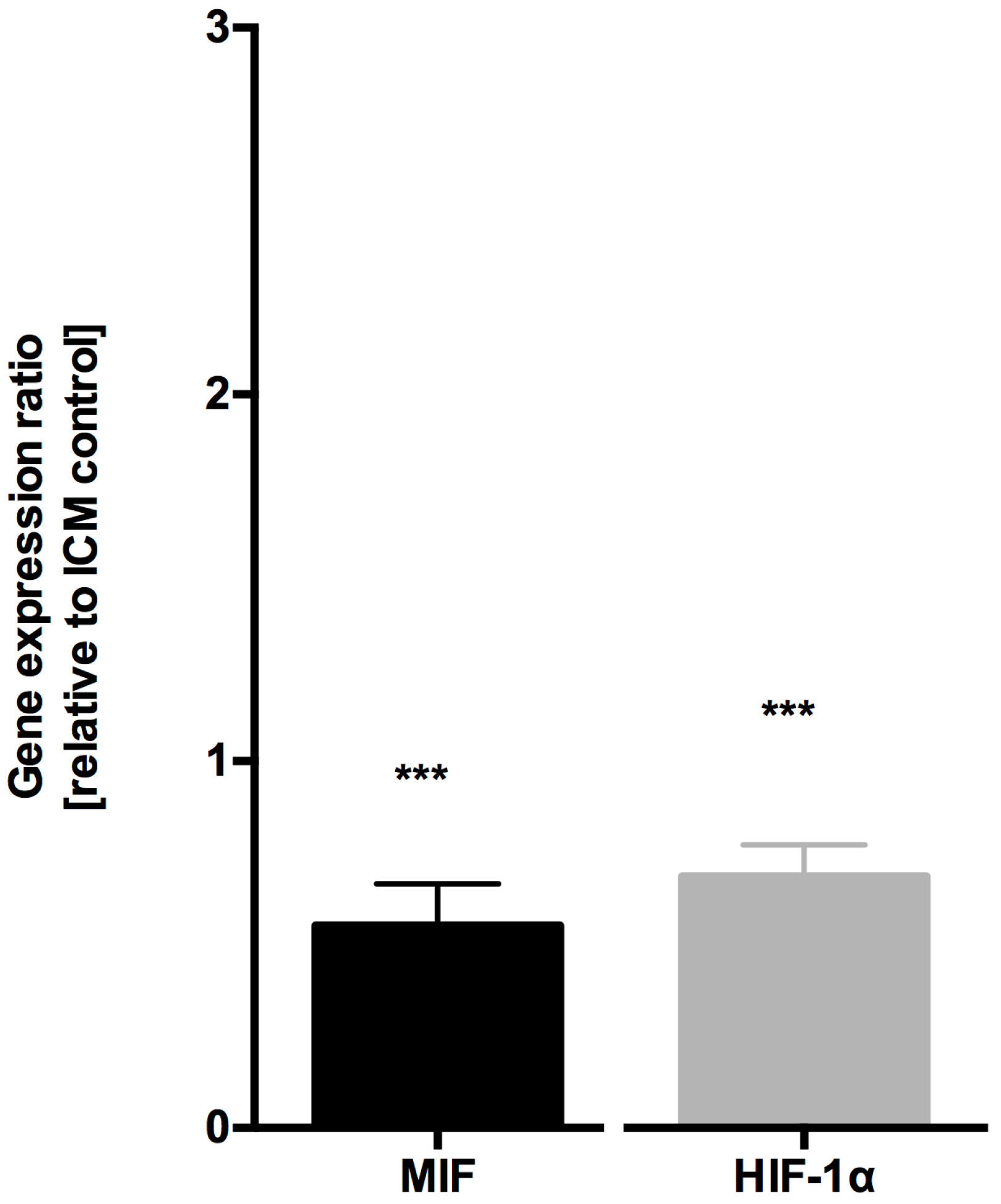
4.1. MIF and HIF-1α Expression is Increased in Ischemic Cardiomyopathy
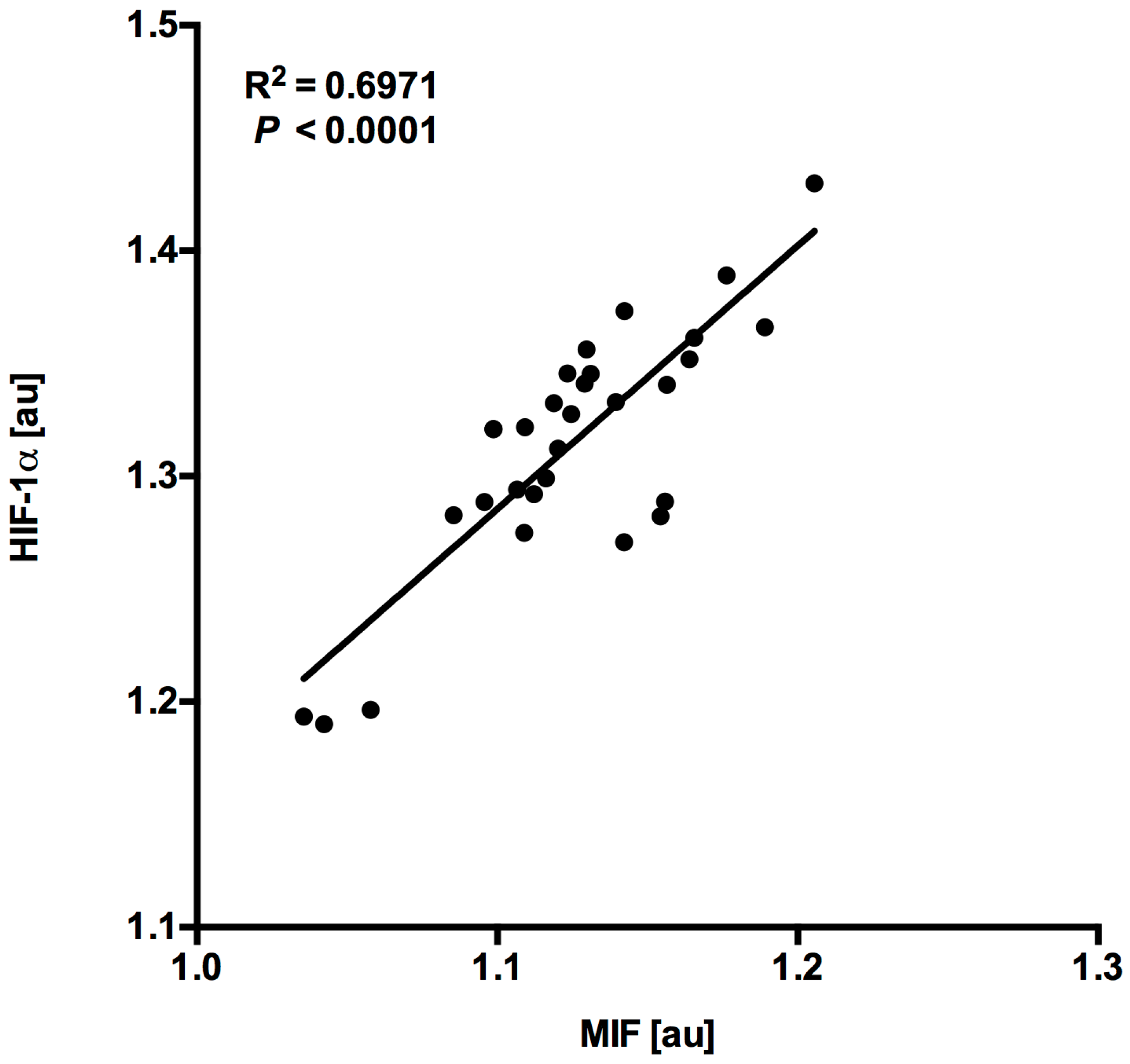
4.2. MIF and HIF-1α Expression Show a Close Correlation in Myocardial Tissue Samples
5. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, E. The war against heart failure: The Lancet lecture. Lancet 2014, 385, 812. [Google Scholar] [CrossRef]
- Braunwald, E. Biomarkers in heart failure. N. Engl. J. Med. 2008, 358, 2148–2159. [Google Scholar] [CrossRef] [PubMed]
- Kelkar, A.A.; Butler, J.; Schelbert, E.B.; Greene, S.J.; Quyyumi, A.A.; Bonow, R.O.; Cohen, I.; Gheorghiade, M.; Lipinski, M.J.; Sun, W.; et al. Mechanisms Contributing to the Progression of Ischemic and Nonischemic Dilated Cardiomyopathy: Possible Modulating Effects of Paracrine Activities of Stem Cells. J. Am. Coll. Cardiol. 2015, 66, 2038–2047. [Google Scholar] [CrossRef] [PubMed]
- Shioi, T.; Matsumori, A.; Kihara, Y.; Inoko, M.; Ono, K.; Iwanaga, Y.; Yamada, T.; Iwasaki, A.; Matsushima, K.; Sasayama, S. Increased expression of interleukin-1 beta and monocyte chemotactic and activating factor/monocyte chemoattractant protein-1 in the hypertrophied and failing heart with pressure overload. Circ. Res. 1997, 81, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Singal, P.K.; Khaper, N.; Palace, V.; Kumar, D. The role of oxidative stress in the genesis of heart disease. Cardiovasc. Res. 1998, 40, 426–432. [Google Scholar] [CrossRef]
- Chen, D.; Assad-Kottner, C.; Orrego, C.; Torre-Amione, G. Cytokines and acute heart failure. Crit. Care Med. 2008, 36, S9–S16. [Google Scholar] [CrossRef] [PubMed]
- Asare, Y.; Schmitt, M.; Bernhagen, J. The vascular biology of macrophage migration inhibitory factor (MIF). Expression and effects in inflammation, atherogenesis and angiogenesis. Thromb. Haemost. 2013, 109, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Pohl, J.; Papathanasiou, M.; Heisler, M.; Stock, P.; Kelm, M.; Hendgen-Cotta, U.B.; Rassaf, T.; Luedike, P. Renal replacement therapy neutralizes elevated MIF levels in septic shock. J. Intensive Care 2016, 4, 39. [Google Scholar] [CrossRef] [PubMed]
- Pohl, J.; Rammos, C.; Totzeck, M.; Stock, P.; Kelm, M.; Rassaf, T.; Luedike, P. MIF reflects tissue damage rather than inflammation in post-cardiac arrest syndrome in a real life cohort. Resuscitation 2016, 100, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Luedike, P.; Rammos, C.; Pohl, J.; Heisler, M.; Totzeck, M.; Kleophas, W.; Hetzel, G.R.; Kelm, M.; Hendgen-Cotta, U.; Rassaf, T. Filtration of Macrophage Migration Inhibitory Factor (MIF) in Patients with End Stage Renal Disease Undergoing Hemodialysis. PLoS ONE 2015, 10, e0140215. [Google Scholar] [CrossRef] [PubMed]
- Rammos, C.; Hendgen-Cotta, U.B.; Pohl, J.; Totzeck, M.; Luedike, P.; Schulze, V.T.; Kelm, M.; Rassaf, T. Modulation of circulating macrophage migration inhibitory factor in the elderly. Biomed. Res. Int. 2014, 2014, 582586. [Google Scholar] [CrossRef] [PubMed]
- Burger-Kentischer, A.; Goebel, H.; Seiler, R.; Fraedrich, G.; Schaefer, H.E.; Dimmeler, S.; Kleemann, R.; Bernhagen, J.; Ihling, C. Expression of macrophage migration inhibitory factor in different stages of human atherosclerosis. Circulation 2002, 105, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Mueller, K.A.; Schwille, J.; Vollmer, S.; Ehinger, E.; Kandolf, R.; Klingel, K.; Kramer, U.; Gawaz, M.; Geisler, T.; Mueller, I.I. Prognostic impact of macrophage migration inhibitory factor in patients with non-ischemic heart failure undergoing endomyocardial biopsy. Int. J. Cardiol. 2016, 203, 656–659. [Google Scholar] [CrossRef] [PubMed]
- Rassaf, T.; Weber, C.; Bernhagen, J. Macrophage migration inhibitory factor in myocardial ischaemia/reperfusion injury. Cardiovasc. Res. 2014, 102, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Luedike, P.; Hendgen-Cotta, U.B.; Sobierajski, J.; Totzeck, M.; Reeh, M.; Dewor, M.; Lue, H.; Krisp, C.; Wolters, D.; Kelm, M.; et al. Cardioprotection through S-nitros(yl)ation of macrophage migration inhibitory factor. Circulation 2012, 125, 1880–1889. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.J.; Li, J.; Leng, L.; McDonald, C.; Atsumi, T.; Bucala, R.; Young, L.H. Macrophage migration inhibitory factor stimulates AMP-activated protein kinase in the ischaemic heart. Nature 2008, 451, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Koga, K.; Kenessey, A.; Powell, S.R.; Sison, C.P.; Miller, E.J.; Ojamaa, K. Macrophage migration inhibitory factor provides cardioprotection during ischemia/reperfusion by reducing oxidative stress. Antioxid. Redox. Signal. 2011, 14, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Pohl, J.; Hendgen-Cotta, U.B.; Rammos, C.; Luedike, P.; Mull, E.; Stoppe, C.; Jülicher, K.; Lue, H.; Merx, M.W.; Kelm, M.; et al. Targeted intracellular accumulation of macrophage migration inhibitory factor in the reperfused heart mediates cardioprotection. Thromb. Haemost. 2016, 115, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Nishihira, J.; Katsuki, T.; Kobayashi, E.; Ikeda, U.; Shimada, K. Elevation of plasma levels of macrophage migration inhibitory factor in patients with acute myocardial infarction. Am. J. Cardiol. 2002, 89, 248–249. [Google Scholar] [CrossRef]
- Yu, C.M.; Lau, C.P.; Lai, K.W.H.; Huang, X.R.; Chen, W.H.; Lan, H.Y. Elevation of plasma level of macrophage migration inhibitory factor in patients with acute myocardial infarction. Am. J. Cardiol. 2001, 88, 774–777. [Google Scholar] [CrossRef]
- Simons, D.; Grieb, G.; Hristov, M.; Pallua, N.; Weber, C.; Bernhagen, J.; Steffens, G. Hypoxia-induced endothelial secretion of macrophage migration inhibitory factor and role in endothelial progenitor cell recruitment. J. Cell Mol. Med. 2011, 15, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Heinl-Green, A.; Radke, P.W.; Munkonge, F.M.; Frass, O.; Zhu, J.; Vincent, K.; Geddes, D.M.; Alton, E.W. The efficacy of a ‘master switch gene’ HIF-1alpha in a porcine model of chronic myocardial ischaemia. Eur. Heart J. 2005, 26, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Luo, F.; Yang, L.; Wu, W.; Liu, X. Hypoxia stimulates the expression of macrophage migration inhibitory factor in human vascular smooth muscle cells via HIF-1alpha dependent pathway. BMC Cell Biol. 2010, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Wang, J.; Thomas, D.P.; Tong, C.; Leng, L.; Wang, W.; Merk, M.; Zierow, S.; Bernhagen, J.; Ren, J.; et al. Impaired macrophage migration inhibitory factor-AMP-activated protein kinase activation and ischemic recovery in the senescent heart. Circulation 2010, 122, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.; Tazzyman, S.; Lund, E.L.; Junker, N.; Lewis, C.E.; Kristjansen, P.E.G.; Murdoch, C. Hypoxia-induced secretion of macrophage migration-inhibitory factor from MCF-7 breast cancer cells is regulated in a hypoxia-inducible factor-independent manner. Cancer Lett. 2008, 265, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.; Winters, G.L.; Fishbein, M.C.; Tazelaar, H.D.; Kobashigawa, J.; Abrams, J.; Andersen, C.B.; Angelini, A.; Berry, G.J.; Burke, M.M.; et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J. Heart Lung Transplant. 2005, 24, 1710–1720. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef] [PubMed]
- Belaiba, R.S.; Bonello, S.; Zähringer, C.; Schmidt, S.; Hess, J.; Kietzmann, T.; Görlach, A. Hypoxia up-regulates hypoxia-inducible factor-1alpha transcription by involving phosphatidylinositol 3-kinase and nuclear factor kappaB in pulmonary artery smooth muscle cells. Mol. Biol. Cell 2007, 18, 4691–4697. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Hu, X.; Wu, X.; Merk, M.; Leng, L.; Bucala, R.; Young, L.H. Cardiac macrophage migration inhibitory factor inhibits JNK pathway activation and injury during ischemia/reperfusion. J. Clin. Investig. 2009, 119, 3807–3816. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.A.; Volpert, O.V.; Woods, J.M.; Kumar, P.; Harlow, L.A.; Koch, A.E. Migration inhibitory factor mediates angiogenesis via mitogen-activated protein kinase and phosphatidylinositol kinase. Circ. Res. 2003, 93, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Kupatt, C.; Horstkotte, J.; Vlastos, G.A.; Pfosser, A.; Lebherz, C.; Semisch, M.; Thalgott, M.; Büttner, K.; Browarzyk, C.; Mages, J.; et al. Embryonic endothelial progenitor cells expressing a broad range of proangiogenic and remodeling factors enhance vascularization and tissue recovery in acute and chronic ischemia. FASEB J. 2005, 19, 1576–1578. [Google Scholar] [CrossRef] [PubMed]
- Casals, G.; Ros, J.; Sionis, A.; Davidson, M.M.; Morales-Ruiz, M.; Jiménez, W. Hypoxia induces B-type natriuretic peptide release in cell lines derived from human cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H550–H555. [Google Scholar] [CrossRef] [PubMed]
- Chesney, J.A.; Mitchell, R.A. 25 Years On: A Retrospective on Migration Inhibitory Factor in Tumor Angiogenesis. Mol. Med. 2015, 21, S19–S24. [Google Scholar] [CrossRef] [PubMed]
- Zwiech, R. Macrophage migration inhibitory factor urinary excretion revisited—MIF a potent predictor of the immunosuppressive treatment outcomes in patients with proliferative primary glomerulonephritis. BMC Immunol. 2015, 16, 47. [Google Scholar] [CrossRef] [PubMed]
| Parameters | HTX | NICM | ICM | p-Value | p-Value |
|---|---|---|---|---|---|
| (HTX vs. ICM) | (ICM vs. NICM) | ||||
| n | 10 | 10 | 10 | ||
| Sex (men) | 7 | 8 | 7 | 1 | 1 |
| Age (years) | 59 ± 5 | 60 ±14 | 56 ± 8 | 0.3712 | 0.4953 |
| NYHA class | 1 ± 0 | 3 ± 1 | 4 ± 0.5 | <0.0001 | 0.0081 |
| LV-EF (%) | 57 ± 6 | 28 ± 4 | 22 ± 6 | <0.0001 | 0.1212 |
| Creatinine (mg/dL) | 2.3 ± 1.5 | 1.1 ± 0.4 | 1.3 ± 0.3 | 0.0324 | 0.2066 |
| White blood cell count (×1000/µL) | 6 ± 3 | 8 ± 3 | 8 ± 3 | 0.0938 | 0.7805 |
| CRP (mg/dL) | 1.4 ± 0.6 | 2.2 ± 1 | 5 ± 3.7 | 0.3288 | 0.5048 |
| Medication (%) | |||||
| Beta blockers | 60 | 90 | 100 | 0.0821 | 0.5908 |
| Diuretics | 80 | 100 | 100 | 0.3292 | 1 |
| ACEI/ARB | 80 | 100 | 70 | 0.8752 | 0.1712 |
| Histopathological Criteria | HTX | NICM |
|---|---|---|
| Cardiac fibrosis, % | 100 | 100 |
| Cardiac hypertrophy, % | 70 | 80 |
| Positive virus serology, % | 40 | 40 |
| Grade of rejection, % 0R | 100 | na |
| Dallas criteria, % negative | na | 100 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pohl, J.; Hendgen-Cotta, U.B.; Stock, P.; Luedike, P.; Baba, H.A.; Kamler, M.; Rassaf, T. Myocardial Expression of Macrophage Migration Inhibitory Factor in Patients with Heart Failure. J. Clin. Med. 2017, 6, 95. https://doi.org/10.3390/jcm6100095
Pohl J, Hendgen-Cotta UB, Stock P, Luedike P, Baba HA, Kamler M, Rassaf T. Myocardial Expression of Macrophage Migration Inhibitory Factor in Patients with Heart Failure. Journal of Clinical Medicine. 2017; 6(10):95. https://doi.org/10.3390/jcm6100095
Chicago/Turabian StylePohl, Julia, Ulrike B. Hendgen-Cotta, Pia Stock, Peter Luedike, Hideo Andreas Baba, Markus Kamler, and Tienush Rassaf. 2017. "Myocardial Expression of Macrophage Migration Inhibitory Factor in Patients with Heart Failure" Journal of Clinical Medicine 6, no. 10: 95. https://doi.org/10.3390/jcm6100095






