Novel Pharmacological Activity of Artesunate and Artemisinin: Their Potential as Anti-Tubercular Agents
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Animals
2.3. Preparation of Anti-MTB Drugs
2.4. Preparation and Culture Conditions of M. tuberculosis
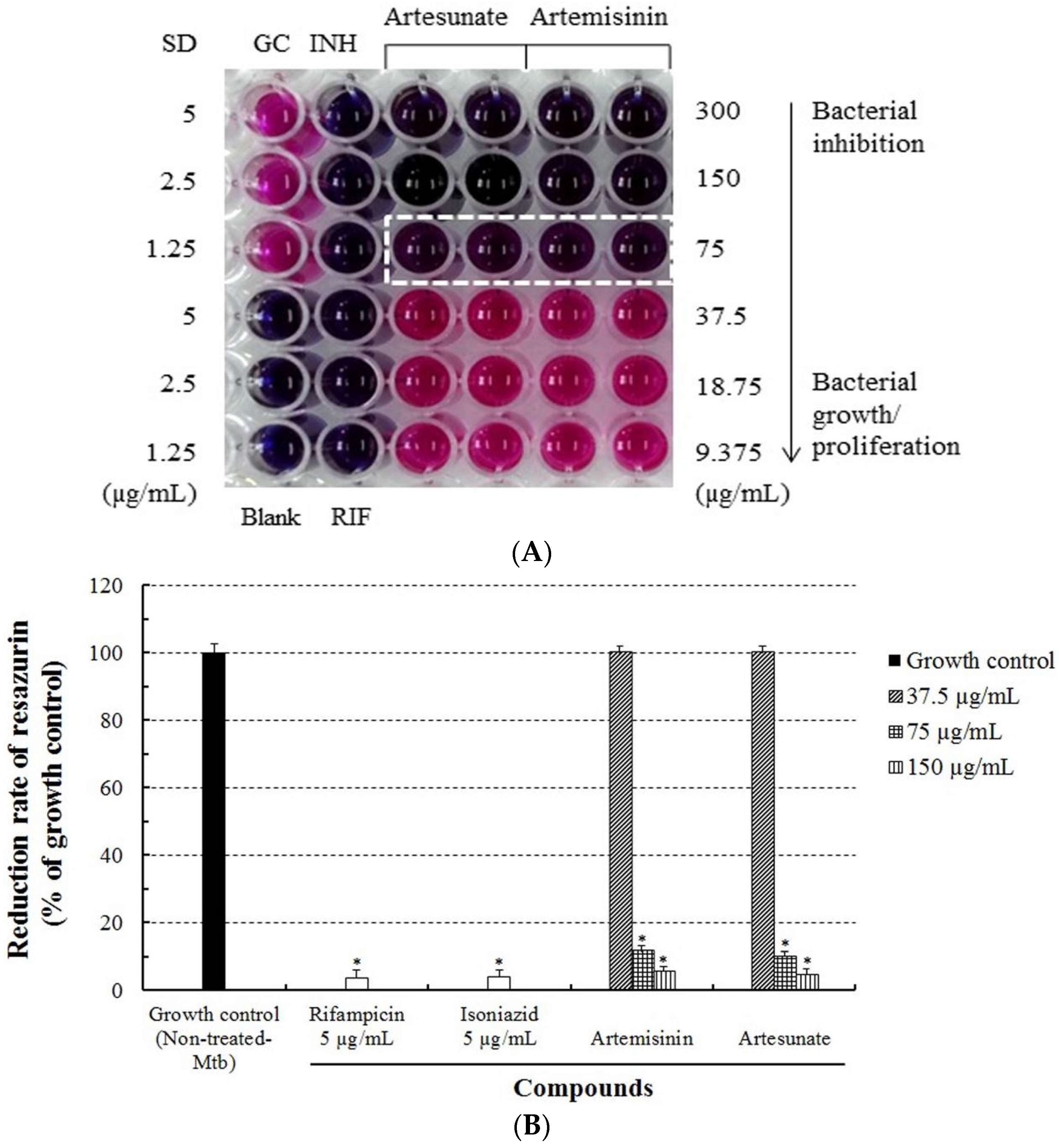
2.5. Determination of Drug Susceptibility of M. tuberculosis
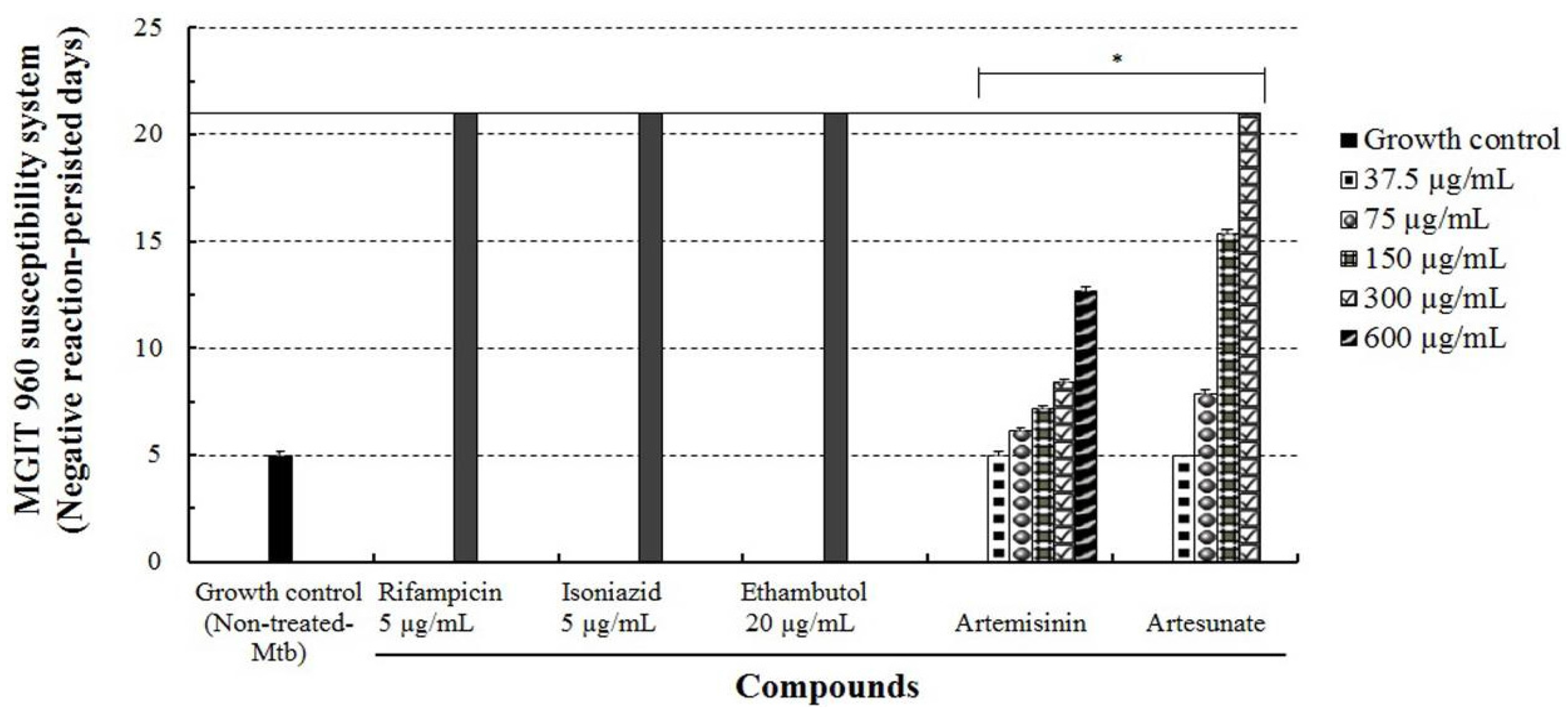
2.6. Evaluation of Drug Susceptibility of M. tuberculosis by MGIT 960 System Assay
2.7. Drug Susceptibility Test of M. tuberculosis by Ogawa Slant Medium Assay
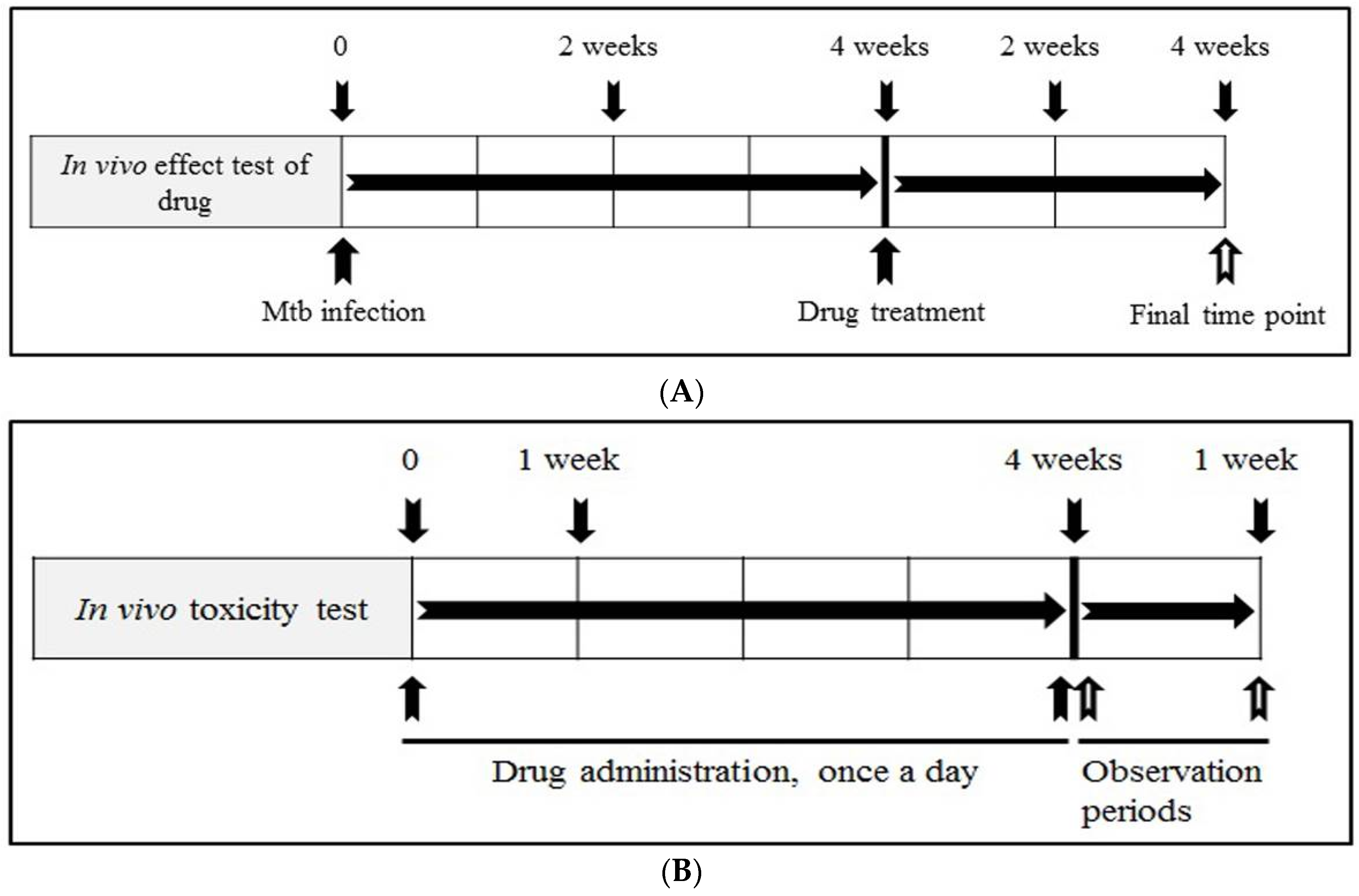
2.8. Evaluation of In Vivo Anti-Mtb Effects of Artesunate and Artemisinin
2.9. Measurement of Body Weight and In Vivo Toxicity Test
2.10. Statistical Analysis
3. Results
3.1. Evaluation of Anti-Mtb Activity of Artesunate and Artemisinin against M. tuberculosis
3.2. Anti-Mtb Effects of Artesunate and Artemisinin against the Growth/Proliferation of M. tuberculosis
3.3. Determination of Anti-Mtb Effect of Artesunate by the Ogawa Slant Medium Assay
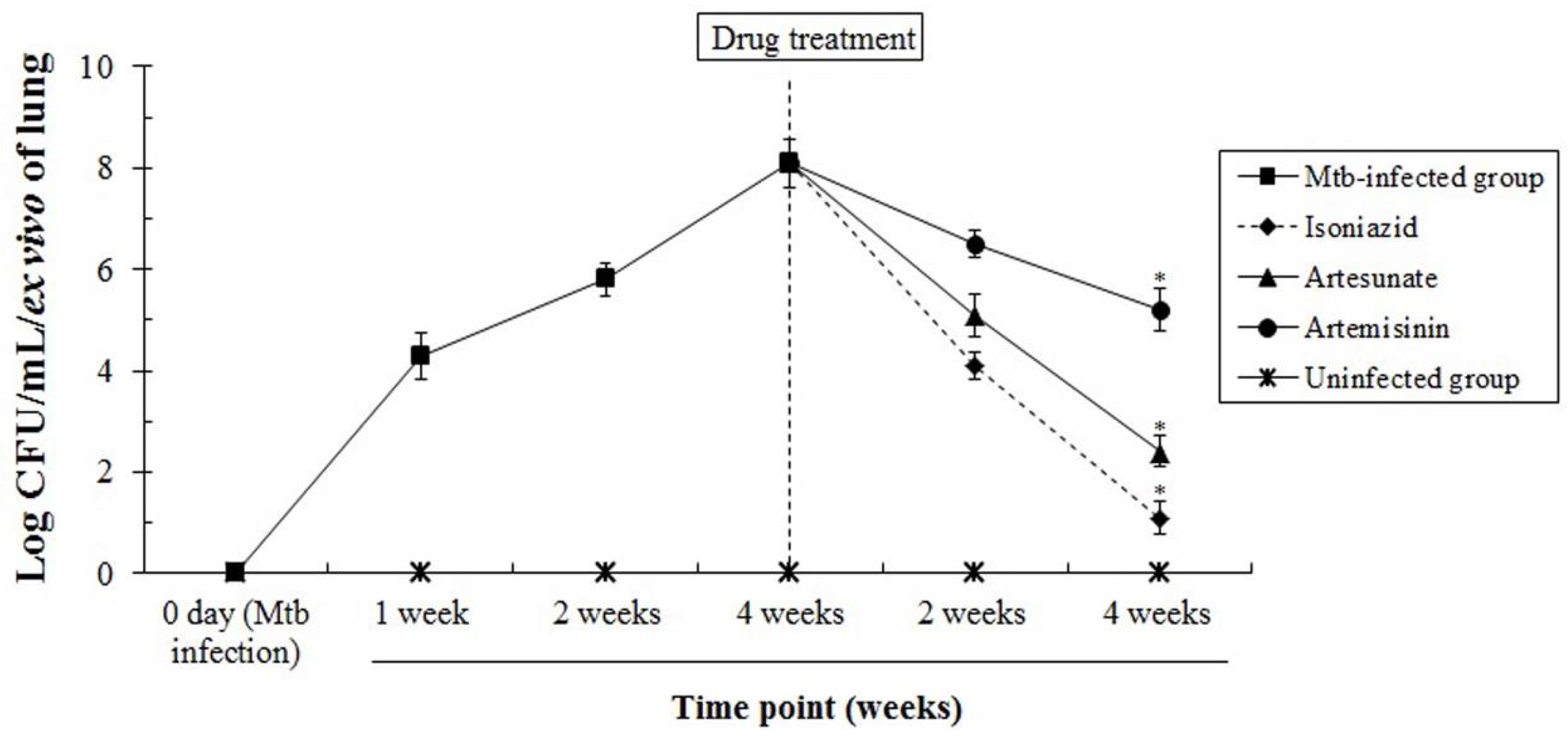
3.4. The In Vivo Anti-Mtb Effects of Artesunate and Artemisinin against M. tuberculosis
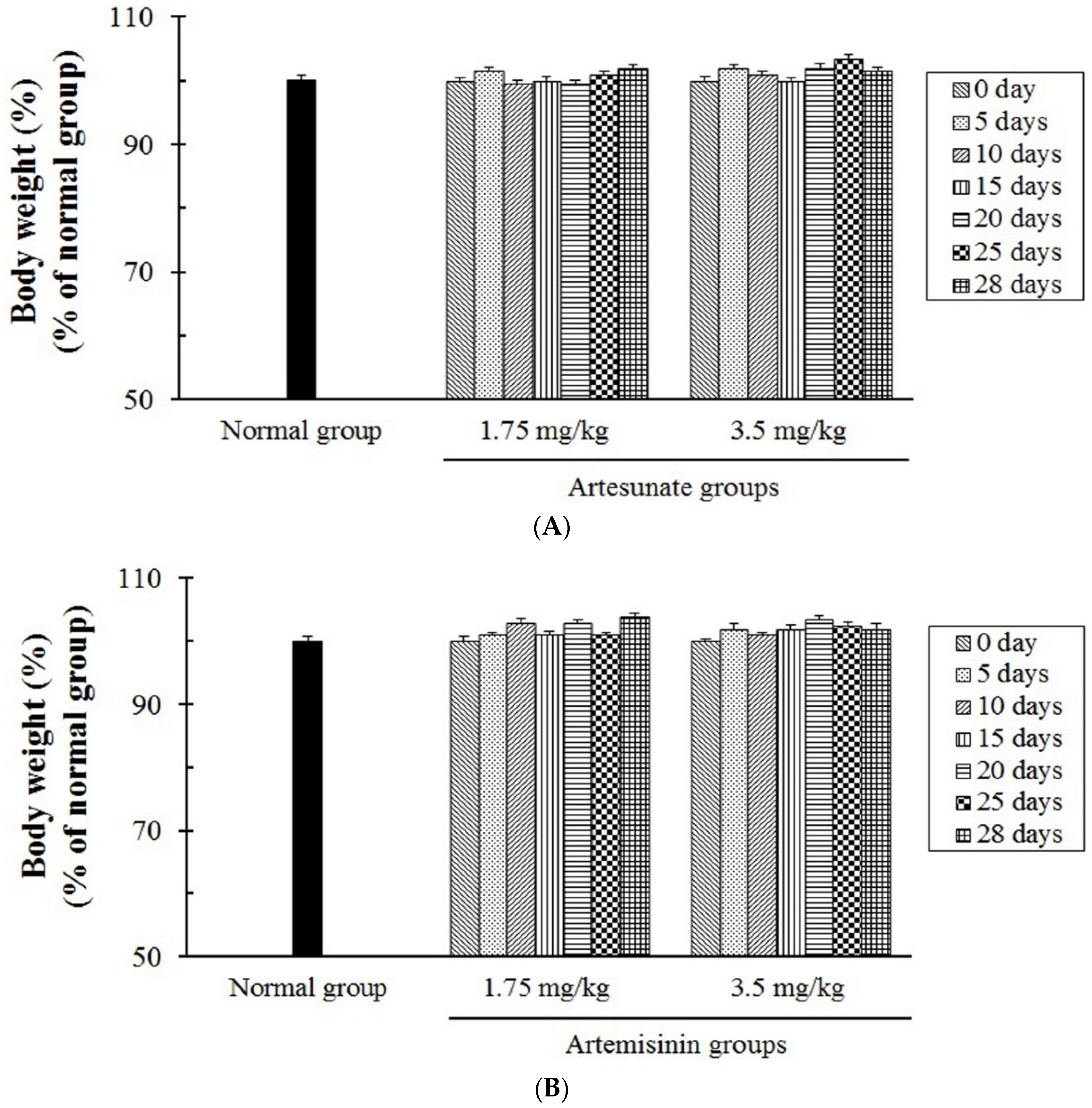
3.5. The In Vivo Toxicity Tests of Artesunate and Artemisinin
4. Discussion
Acknowledgments
Conflicts of Interest
Abbreviations
| Mtb | Mycobacterium tuberculosis |
| TB | Tuberculosis |
| MDR-TB | Multidrug-resistant TB |
| XDR-TB | Extensively drug-resistant TB |
| REMA | Resazurin microtiter assay |
| DMSO | Dimethylsulfoxide |
| OADC | Oleic acid/albumin/dextrose/catalase |
| MIC | Minimum inhibitory concentration |
References
- Global Tuberculosis Report 2014; WHO Report; World Health Organization Press: Geneva, Switzerland, 2014.
- Global Tuberculosis Report 2015; WHO Report; World Health Organization Press: Geneva, Switzerland, 2015.
- Lee, M.; Lee, J.; Carroll, M.W.; Choi, H.; Min, S.; Song, T.; Via, L.E.; Goldfeder, L.C.; Kang, E.; Jin, B.; et al. Linezolid for treatment of chronic extensively drug-resistant tuberculosis. N. Engl. J. Med. 2012, 367, 1508–1518. [Google Scholar] [CrossRef] [PubMed]
- Koul, A.; Arnoult, E.; Lounis, N.; Guillemont, J.; Andries, K. The challenge of new drug discovery for tuberculosis. Nature 2011, 469, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Hartkoorn, R.C.; Sala, C.; Neres, J.; Pojer, F.; Magnet, S.; Mukherjee, R.; Uplekar, S.; Boy-Röttger, S.; Altmann, K.H.; Cole, S.T. Towards a new tuberculosis drug: Pyridomycin—Nature’s isoniazid. EMBO Mol. Med. 2012, 4, 1032–1042. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.; Kumar, P.; Parashar, V.; Vilchèze, C.; Veyron-Churlet, R.; Freundlich, J.S.; Barnes, S.W.; Walker, J.R.; Szymonifka, M.J.; Marchiano, E.; et al. Antituberculosis thiophenes define a requirement for Pks13 in mycolic acid biosynthesis. Nat. Chem. Biol. 2013, 9, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Pethe, K.; Bifani, P.; Jang, J.; Kang, S.; Park, S.; Ahn, S.; Jiricek, J.; Jung, J.; Jeon, H.K.; Cechetto, J.; et al. Discovery of Q203, a potent clinical candidate for the treatment of tuberculosis. Nat. Med. 2013, 19, 1157–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Tao, L.; Xu, H. Chinese herbal medicines as a source of molecules with anti-enterovirus 71 activity. Chin. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.H.; Jiang, M.H.; Chu, J.P. Antiparasitic effects of Zingiber officinale (Ginger) extract against Toxoplasma gondii. J. Appl. Biomed. 2013, 11, 15–26. [Google Scholar] [CrossRef]
- Choi, W.H.; Chu, J.P.; Jiang, M.H.; Baek, S.H.; Park, H.D. Effects of Fraction obtained from Korean Corni Fructus extracts causing anti-proliferation and p53-dependent apoptosis in A549 lung cancer cells. Nutr. Cancer. 2011, 63, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Chen, K.P. Anti-HBV agents derived from botanical origin. Fitoterapia 2013, 84, 140–157. [Google Scholar] [CrossRef] [PubMed]
- Nguta, J.M.; Appiah-Opong, R.; Nyarko, A.K.; Yeboah-Manu, D.; Addo, P.G.; Otchere, I.; Kissi-Twum, A. Antimycobacterial and cytotoxic activity of selected medicinal plant extracts. J. Ethnopharmacol. 2016, 182, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.H.; Ye, F.W.; Shen, Y.M. Siderochelins with anti-mycobacterial activity from Amycolatopsis sp. LZ149. Chin. J. Nat. Med. 2015, 13, 69–72. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiang, X.; Gao, F.; Song, J.; Sun, J.; Wang, L.; Sun, X.; Lu, Z.; Zhang, H. Identification of plant-derived natural products as potential inhibitors of the Mycobacterium tuberculosis proteasome. BMC Complement. Altern. Med. 2014, 14, 400. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Hussain, S.; Verma, R.; Sharma, P. Anti-Mycobacterial screening of five Indian medicinal plants and partial purification of active extracts of Cassia sophera and Urtica dioica. Asian Pac. J. Trop. Med. 2013, 6, 366–371. [Google Scholar] [CrossRef]
- Choi, W.H.; Lee, I.A. The anti-tubercular activity of Melia azedarach L. and Lobelia chinensis Lour. and their potential as effective anti-Mycobacterium tuberculosis candidate agents. Asian Pac. J. Trop. Biomed. 2016, 6, 830–835. [Google Scholar] [CrossRef]
- Blanchard, J. Molecular mechanisms of drug resistance in Mycobacterium tuberculosis. Annu. Rev. Biochem. 1996, 65, 215–239. [Google Scholar] [CrossRef] [PubMed]
- Casal, M.; Guitierrrez, J.; Gonzalez, J.; Ruiz, P. In vitro Susceptibility of Mycobacterium tuberculosis to a new macrolide antibiotics: R28965. Tubercle 1987, 68, 141–143. [Google Scholar] [CrossRef]
- Brown, B.A.; Wallace, R.J.; Onyi, G.O.; De Rosas, V.; Wallace, R.J., 3rd. Activity of four macrolides, including clarithromycin, against Mycobacterium fortuitum, Mycobacterium chelonae and M. Chelonae like organisms. Antimicrob. Agents Chemther. 1992, 36, 180–184. [Google Scholar] [CrossRef]
- David, H.L.; Takayama, K.; Goldman, D.S. Susceptibility of mycobacterial D-alnyl-D-alanine synthetase to D-cycloserine. Am. Rev. Respir. Dis. 1969, 100, 579–581. [Google Scholar] [PubMed]
- Efferth, T.; Marschall, M.; Wang, X.; Huong, S.M.; Hauber, I.; Olbrich, A.; Kronschnabl, M.; Stamminger, T.; Huang, E.S. Antiviral activity of artesunate towards wild-type, recombinant, and ganciclovir-resistant human cytomegaloviruses. J. Mol. Med. 2002, 80, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Romero, M.R.; Wolf, D.G.; Stamminger, T.; Marin, J.J.; Marschall, M. The antiviral activities of artemisinin and artesunate. Clin. Infect. Dis. 2008, 47, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Milbradt, J.; Auerochs, S.; Korn, K.; Marschall, M. Sensitivity of human herpes virus 6 and other human herpes viruses to the broad-spectrum anti-infective drug artesunate. J. Clin. Virol. 2009, 46, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Wang, Y.; Kokudo, N.; Fang, D.; Tang, W. Traditional Chinese medicine and related active compounds against hepatitis B virus infection. Biosci. Trends 2010, 4, 39–47. [Google Scholar] [PubMed]
- Jones-Brando, L.; D’Angelo, J.; Posner, G.H.; Yolken, R. In vitro inhibition of Toxoplasma gondii by four new derivatives of artemisinin. Antimicrob. Agents Chemother. 2006, 50, 4206–4208. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, J.G.; Bordon, C.; Posner, G.H.; Yolken, R.; Jones-Brando, L. Artemisinin derivatives inhibit Toxoplasma gondii in vitro at multiple steps in the lytic cycle. J. Antimicrob. Chemother. 2009, 63, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Hencken, C.P.; Jones-Brando, L.; Bordon, C.; Stohler, R.; Mott, B.T.; Yolken, R.; Posner, G.H.; Woodard, L.E. Thiazole, oxadiazole, and carboxamide derivatives of artemisinin are highly selective and potent inhibitors of Toxoplasma gondii. J. Med. Chem. 2010, 53, 3594–3601. [Google Scholar] [CrossRef] [PubMed]
- Keiser, J.; Morson, G. Fasciola hepatica: Tegumental alterations in adult flukes following in vitro and in vivo administration of artesunate and artemether. Exp. Parasitol. 2008, 118, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Keiser, J.; Rinaldi, L.; Veneziano, V.; Mezzino, L.; Tanner, M.; Utzinger, J.; Cringoli, G. Efficacy and safety of artemether against a natural Fasciola hepatica infection in sheep. Parasitol. Res. 2008, 103, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Galal, A.M.; Ross, S.A.; Jacob, M.; ElSohly, M.A. Antifungal activity of artemisinin derivatives. J. Nat. Prod. 2005, 68, 1274–1276. [Google Scholar] [CrossRef] [PubMed]
- Gautam, P.; Upadhyay, S.K.; Hassan, W.; Madan, T.; Sirdeshmukh, R.; Sundaram, C.S.; Gade, W.N.; Basir, S.F.; Singh, Y.; Sarma, P.U. Transcriptomic and proteomic profile of Aspergillus fumigatus on exposure to artemisinin. Mycopathologia 2011, 172, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Cuzzocrea, S.; Saadat, F.; Di Paola, R.; Mirshafiey, A. Artemether: A new therapeutic strategy in experimental rheumatoid arthritis. Immunopharmacol. Immunotoxicol. 2005, 27, 615–630. [Google Scholar] [CrossRef] [PubMed]
- Mirshafiey, A.; Saadat, F.; Attar, M.; Di Paola, R.; Sedaghat, R.; Cuzzocrea, S. Design of a new line in treatment of experimental rheumatoid arthritis by artesunate. Immunopharmacol. Immunotoxicol. 2006, 28, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, S.; Wang, Y.; Zhou, C.; Chen, G.; Shen, W.; Li, C.; Lin, W.; Lin, S.; Huang, H.; et al. Inhibitory effect of the antimalarial agent artesunate on collagen-induced arthritis in rats through nuclear factor kappa B and mitogen-activated protein kinase signaling pathway. Transl. Res. 2013, 161, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Maibach, H.I. Topical application of artesunate on guinea pig allergic contact dermatitis. Contact Dermat. 1994, 30, 280–282. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chen, H.; Liu, X.G. Immunoregulatory effect of artesunate on allergic contact dermatitis and its mechanism. Yao Xue Xue Bao 2012, 47, 884–889. (In Chinese) [Google Scholar] [PubMed]
- Cheng, C.; Ng, D.S.; Chan, T.K.; Guan, S.P.; Ho, W.E.; Koh, A.H.; Bian, J.S.; Lau, H.Y.; Wong, W.S. Anti-allergic action of anti-malarial drug artesunate in experimental mast cell-mediated anaphylactic models. Allergy 2013, 68, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, H.; Zheng, J.; Cheng, J.; Liu, W.; Ding, G.; Wang, L.; Luo, P.; Lu, Y.; Cao, H.; et al. The antimalarial artemisinin synergizes with antibiotics to protect against lethal live Escherichia coli challenge by decreasing proinflammatory cytokine release. Antimicrob. Agents Chemother. 2006, 50, 2420–2427. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Li, B.; Zheng, X.; Liu, X.; Cen, Y.; Li, J.; Pan, X.; Cao, H.; Zheng, J.; Zhou, H. Artesunate in combination with oxacillin protect sepsis model mice challenged with lethal live methicillin-resistant Staphylococcus aureus (MRSA) via its inhibition on proinflammatory cytokines release and enhancement on antibacterial activity of oxacillin. Int. Immunopharmacol. 2011, 11, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Dunstan, H.; Sauerbrey, A.; Miyachi, H.; Chitambar, C.R. The anti-malarial artesunate is also active against cancer. Int. J. Oncol. 2001, 18, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Sauerbrey, A.; Olbrich, A.; Gebhart, E.; Rauch, P.; Weber, H.O.; Hengstler, J.G.; Halatsch, M.E.; Volm, M.; Tew, K.D.; et al. Molecular modes of action of artesunate in tumor cell lines. Mol. Pharmacol. 2003, 64, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Giaisi, M.; Merling, A.; Krammer, P.H.; Li-Weber, M. Artesunate induces ROS-mediated apoptosis in doxorubicin-resistant T leukemia cells. PLoS ONE 2007, 2, e693. [Google Scholar] [CrossRef] [PubMed]
- Steinbruck, L.; Pereira, G.; Efferth, T. Effects of artesunate on cytokinesis and G2/M cell cycle progression of tumour cells and budding yeast. Cancer Genom. Proteom. 2010, 7, 337–346. [Google Scholar] [PubMed]
- Ho, W.E.; Xu, Y.J.; Xu, F.; Cheng, C.; Peh, H.Y.; Huang, S.M.; Tannenbaum, S.R.; Ong, C.N.; Wong, W.S.F. Anti-Malarial drug artesunate restores metabolic changes in experimental allergic asthma. Metabolomics 2015, 11, 380–390. [Google Scholar] [CrossRef]
- Zhou, F.W.; Lei, H.S.; Fan, L.; Jiang, L.; Liu, J.; Peng, X.M.; Xu, X.R.; Chen, L.; Zhou, C.H.; Zou, Y.Y.; et al. Design, synthesis, and biological evaluation of dihydroartemisinin-fluoroquinolone conjugates as a novel type of potential antitubercular agents. Bioorg. Med. Chem. Lett. 2014, 24, 1912–1917. [Google Scholar] [CrossRef] [PubMed]

| The Tested Compounds | Structure | Mol. Weight (g/M) | Concentrations (µg/mL) 1 | ||||
|---|---|---|---|---|---|---|---|
| 300 | 150 | 75 | 37.5 | 18.75 | |||
| Artesunate |  | 384.42 | + | + | + | − | − |
| Artemisinin |  | 282.33 | + | + | + | − | − |
| Different Assays 1 | Incubation Days | MIC (µg/mL) of the Compounds against Mtb H37Rv Growth | |
|---|---|---|---|
| Artesunate | Artemisinin | ||
| REMA | 5 | 75 | 75 |
| MGIT 960 system | 21 | 300 | >600 |
| Ogawa slant medium | 21 | 300 | >600 |
| The Tested Compounds | Side Effects of Rats Treated with Artesunate and Artemisinin, Respectively | ||
|---|---|---|---|
| The Loss of Body Weight | Vomiting | Diarrhea | |
| Artesunate | − | − | − |
| Artemisinin | − | − | − |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, W.H. Novel Pharmacological Activity of Artesunate and Artemisinin: Their Potential as Anti-Tubercular Agents. J. Clin. Med. 2017, 6, 30. https://doi.org/10.3390/jcm6030030
Choi WH. Novel Pharmacological Activity of Artesunate and Artemisinin: Their Potential as Anti-Tubercular Agents. Journal of Clinical Medicine. 2017; 6(3):30. https://doi.org/10.3390/jcm6030030
Chicago/Turabian StyleChoi, Won Hyung. 2017. "Novel Pharmacological Activity of Artesunate and Artemisinin: Their Potential as Anti-Tubercular Agents" Journal of Clinical Medicine 6, no. 3: 30. https://doi.org/10.3390/jcm6030030





