The Value of Coenzyme Q10 Determination in Mitochondrial Patients
Abstract
:1. Introduction
2. Diagnostic Issues of CoQ Deficiency Syndromes
3. CoQ Determination in Biological Samples. What Can We Expect?
3.1. Blood Plasma
3.2. Blood Cells
3.3. Muscle
3.4. Fibroblasts
3.5. Urine
3.6. Other Biological Samples
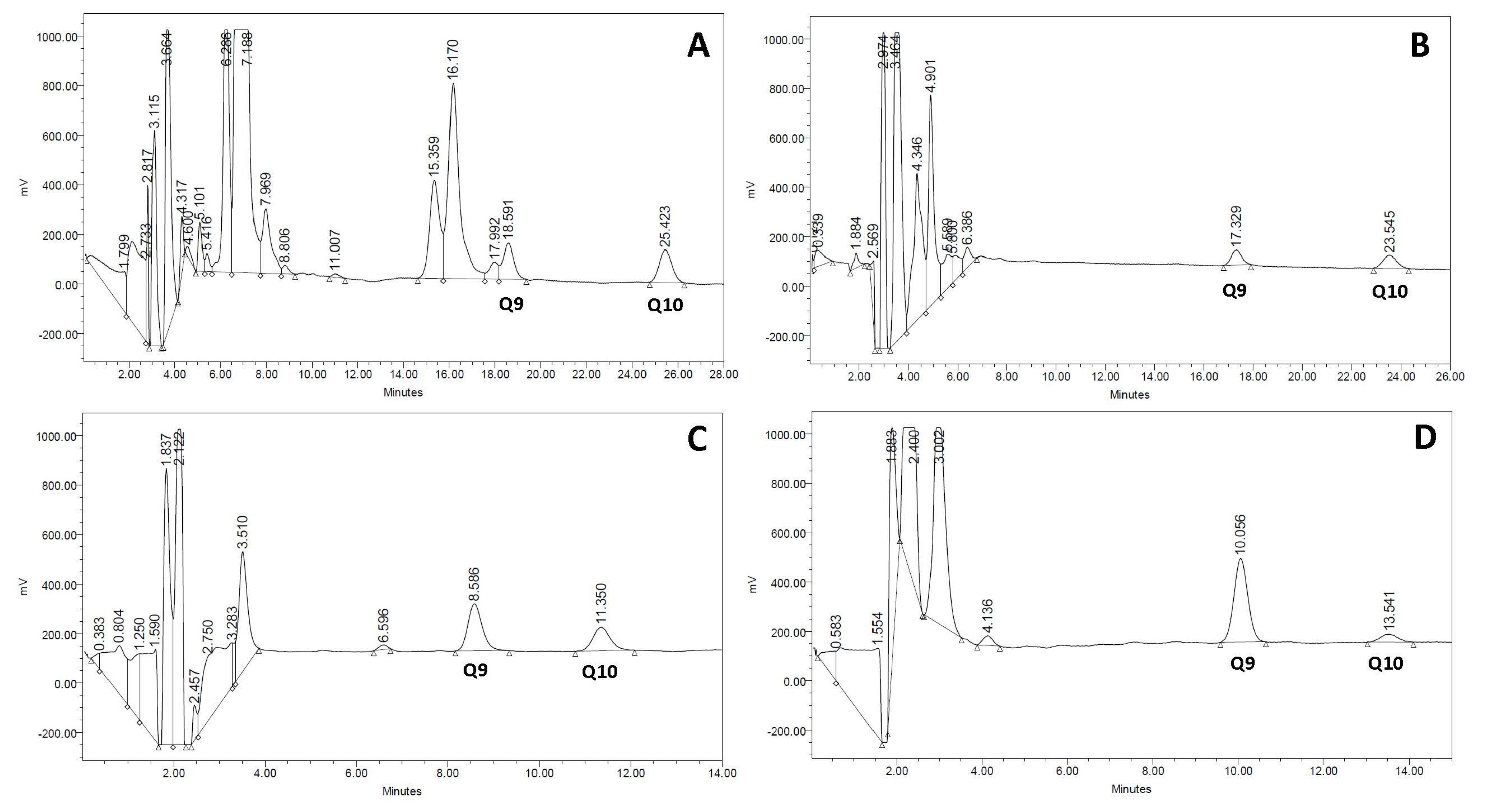
4. CoQ Quantification: Technical Aspects
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Genova, M.L.; Lenaz, G. New developments on the functions of coenzyme Q in mitochondria. Biofactors 2011, 37, 330–354. [Google Scholar] [CrossRef] [PubMed]
- Emmanuele, V.; López, L.C.; Berardo, A.; Naini, A.; Tadesse, S.; Wen, B.; D’Agostino, E.; Solomon, M.; DiMauro, S.; Quinzii, C.; et al. Heterogeneity of coenzyme Q10 deficiency: Patient study and literature review. Arch. Neurol. 2012, 69, 978–983. [Google Scholar] [CrossRef] [PubMed]
- Yubero, D.; Montero, R.; Martín, M.A.; Montoya, J.; Ribes, A.; Grazina, M.; Trevisson, E.; Rodriguez-Aguilera, J.C.; Hargreaves, I.P.; Salviati, L.; et al. CoQ deficiency study group. Secondary coenzyme Q10 deficiencies in oxidative phosphorylation (OXPHOS) and non-OXPHOS disorders. Mitochondrion 2016, 30, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Desbats, M.A.; Lunardi, G.; Doimo, M.; Trevisson, E.; Salviati, L. Genetic bases and clinical manifestations of coenzyme Q10 (CoQ 10) deficiency. J. Inherit. Metab. Dis. 2015, 38, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Miles, M.O.; Putnam, P.E.; Miles, L.; Tang, P.H.; De Grauw, A.J.; Wong, B.L.; Horn, P.S.; Foote, H.L.; Rothenberg, M.E. Acquired coenzyme Q10 deficiency in children with recurrent food intolerance and allergies. Mitochondrion 2011, 11, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Sacconi, S.; Trevisson, E.; Salviati, L.; Aymé, S.; Rigal, O.; Redondo, A.G.; Mancuso, M.; Siciliano, G.; Tonin, P.; Angelini, C.; et al. Coenzyme Q10 is frequently reduced in muscle of patients with mitochondrial myopathy. Neuromuscul. Disord. 2010, 20, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Montero, R.; Grazina, M.; López-Gallardo, E.; Montoya, J.; Briones, P.; Navarro-Sastre, A.; Land, J.M.; Hargreaves, I.P.; Artuch, R.; Coenzyme Q10 Deficiency Study Group. Coenzyme Q10 deficiency in mitochondrial DNA depletion syndromes. Mitochondrion 2013, 13, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Miles, M.V.; Miles, L.; Tang, P.H.; Horn, P.S.; Steele, P.E.; DeGrauw, A.J.; Wong, B.L.; Bove, K.E. Systematic evaluation of muscle coenzyme Q10 content in children with mitochondrial respiratory chain enzyme deficiencies. Mitochondrion 2008, 8, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Yubero, D.; Adin, A.; Montero, R.; Jou, C.; Jiménez-Mallebrera, C.; García-Cazorla, A.; Nascimento, A.; O’Callaghan, M.M.; Montoya, J.; Gort, L.; et al. A statistical algorithm showing coenzyme Q10 and citrate synthase as biomarkers for mitochondrial respiratory chain enzyme activities. Sci. Rep. 2016, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Montini, G.; Malaventura, C.; Salviati, L. Early coenzyme Q10 supplementation in primary coenzyme Q10 deficiency. N. Engl. J. Med. 2008, 358, 2849–2850. [Google Scholar] [CrossRef] [PubMed]
- Yubero, D.; Montero, R.; Armstrong, J.; Espinós, C.; Palau, F.; Santos-Ocaña, C.; Salviati, L.; Navas, P.; Artuch, R. Molecular diagnosis of coenzyme Q10 deficiency. Expert Rev. Mol. Diagn. 2015, 15, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Leshinsky-Silver, E.; Levine, A.; Nissenkorn, A.; Barash, V.; Perach, M.; Buzhaker, E.; Shahmurov, M.; Polak-Charcon, S.; Lev, D.; Lerman-Sagie, T. Neonatal liver failure and Leigh syndrome possibly due to CoQ-responsive OXPHOS deficiency. Mol. Genet. Metab. 2003, 79, 288–293. [Google Scholar] [CrossRef]
- Lerman-Sagie, T.; Rustin, P.; Lev, D.; Yanoov, M.; Leshinsky-Silver, E.; Sagie, A.; Ben-Gal, T.; Munnich, A. Dramatic improvement in mitochondrial cardiomyopathy following treatment with idebenone. J. Inherit. Metab. Dis. 2001, 24, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Fragaki, K.; Cano, A.; Benoist, J.F.; Rigal, O.; Chaussenot, A.; Rouzier, C.; Bannwarth, S.; Caruba, C.; Chabrol, B.; Paguis-Flucklinger, V. Fatal heart failure associated with CoQ10 and multiple OXPHOS deficiency in a child with propionic acidaemia. Mitochondrion 2011, 11, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Rustin, P.; Munnich, A.; Rotig, A. Mitochondrial respiratory chain dysfunction caused by coenzyme Q deficiency. Methods Enzymol. 2004, 382, 81–88. [Google Scholar] [PubMed]
- Quinzii, C.M.; Kattah, A.G.; Naini, A.; Akman, H.O.; Mootha, V.K.; DiMauro, S.; Hirano, M. Coenzyme Q deficiency and cerebellar ataxia associated with an aprataxin mutation. Neurology 2005, 64, 539–541. [Google Scholar] [CrossRef] [PubMed]
- Fragaki, K.; Chaussenot, A.; Benoist, J.F.; Ait-El-Mkadem, S.; Bannwarth, S.; Rouzier, C.; Cochaud, C.; Paquis-Flucklinger, V. Coenzyme Q10 defects may be associated with a deficiency of Q10-independent mitochondrial respiratory chain complexes. Biol. Res. 2016, 49, 4. [Google Scholar] [CrossRef] [PubMed]
- Turkowicz, M.J.; Karpińska, J. Analytical problems with the determination of coenzyme Q10 in biological samples. Biofactors 2013, 39, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Ogasahara, S.; Engel, A.G.; Frens, D.; Mack, D. Muscle coenzyme Q deficiency in familial mitochondrial encephalomyopathy. Proc. Natl. Acad. Sci. USA 1989, 86, 2379–2382. [Google Scholar] [CrossRef] [PubMed]
- Duncan, A.J.; Heales, S.J.; Mills, K.; Eaton, S.; Land, J.M.; Hargreaves, I.P. Determination of coenzyme Q10 status in blood mononuclear cells, skeletal muscle, and plasma by HPLC with di-propoxy-coenzyme Q10 as an internal standard. Clin. Chem. 2005, 51, 2380–2382. [Google Scholar] [CrossRef] [PubMed]
- Palamakula, A.; Soliman, M.; Khan, M.M. Regional permeability of coenzyme Q10 in isolated rat gastrointestinal tracts. Pharmazie 2005, 60, 212–214. [Google Scholar] [PubMed]
- Miles, M.V. The uptake and distribution of coenzyme Q10. Mitochondrion 2007, 7, S72–S77. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Bysted, A.; Holmer, G. Coenzyme Q10 in the diet–daily intake and relative bioavailability. Mol. Aspects Med. 1997, 18, S251–S254. [Google Scholar] [CrossRef]
- Salviati, L.; Sacconi, S.; Murer, L.; Zacchello, G.; Franceschini, L.; Laverda, A.M.; Basso, G.; Quinzii, C.; Angelini, C.; Hirano, M.; et al. Infantile encephalomyopathy and nephropathy with CoQ10 deficiency: A CoQ10-responsive condition. Neurology 2005, 65, 606–608. [Google Scholar] [CrossRef] [PubMed]
- Artuch, R.; Vilaseca, M.A.; Moreno, J.; Lambruschini, N.; Cambra, F.J.; Campistol, J. Decreased serum ubiquinone-10 concentrations in phenylketonuria. Am. J. Clin. Nutr. 1999, 70, 892–895. [Google Scholar] [PubMed]
- Hübner, C.; Hoffmann, G.F.; Charpentier, C.; Gibson, K.M.; Finckh, B.; Puhl, H.; Lehr, H.A.; Kohlschütter, A. Decreased plasma ubiquinone-10 concentration in patients with mevalonate kinase deficiency. Pediatr. Res. 1993, 34, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Delgadillo, V.; O’Callaghan, M.M.; Artuch, R.; Montero, R.; Pineda, M. Genistein supplementation in patients affected by Sanfilippo disease. J. Inherit. Metab. Dis. 2011, 34, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Yanjanin, N.M.; Bianconi, S.; Pavan, W.J.; Porter, F.D. Oxidative stress in Niemann-Pick disease, type C. Mol. Genet. Metab. 2010, 101, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.M.; Korlipara, L.V.; Hart, P.E.; Bradley, J.L.; Schapira, A.H. Coenzyme Q10 and vitamin E deficiency in Friedreich’s ataxia: Predictor of efficacy of vitamin E and coenzyme Q10 therapy. Eur. J. Neurol. 2008, 15, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Haas, D.; Niklowitz, P.; Hoffmann, G.F.; Andler, W.; Menke, T. Plasma and thrombocyte levels of coenzyme Q10 in children with Smith-Lemli-Opitz syndrome (SLOS) and the influence of HMG-CoA reductase inhibitors. Biofactors 2008, 32, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Bhagavan, H.N.; Chopra, R.K. Plasma coenzyme Q10 response to oral ingestion of coenzyme Q10 formulations. Mitochondrion 2007, 7, S78–88. [Google Scholar] [CrossRef] [PubMed]
- Turunen, M.; Olson, J.; Dallner, G. Metabolism and function of coenzyme Q. Biochim. Biophys. Acta 2004, 1660, 171–199. [Google Scholar] [CrossRef] [PubMed]
- Arias, A.; García-Villoria, J.; Rojo, A.; Buján, N.; Briones, P.; Ribes, A. Analysis of coenzyme Q(10) in lymphocytes by HPLC-MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 908, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Barshop, B.A.; Gangoiti, J.A. Analysis of coenzyme Q in human blood and tissues. Mitochondrion 2007, 7, S89–S93. [Google Scholar] [CrossRef] [PubMed]
- Crane, F.L. Discovery of ubiquinone (coenzyme Q) and an overview of function. Mitochondrion 2007, 7, S2–S7. [Google Scholar] [CrossRef] [PubMed]
- Niklowitz, P.; Menke, T.; Andler, W.; Okun, J.G. Simultaneous analysis of coenzyme Q10 in plasma, erythrocytes and platelets: Comparison of the antioxidant level in blood cells and their environment in healthy children and after oral supplementation in adults. Clin. Chim. Acta 2004, 342, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Miles, M.V.; Tang, P.H.; Miles, L.; Steele, P.E.; Moye, M.J.; Horn, P.S. Validation and application of an HPLC-EC method for analysis of coenzyme Q10 in blood platelets. Biomed. Chromatogr. 2008, 22, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- Galinier, A.; Carrière, A.; Fernandez, Y.; Bessac, A.M.; Caspar-Bauguil, S.; Periquet, B.; Comtat, M.; Thouvenot, J.P.; Casteilla, L. Biological validation of coenzyme Q redox state by HPLC-EC measurement: Relationship between coenzyme Q redox state and coenzyme Q content in rat tissues. FEBS Lett. 2004, 578, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Trevisson, E.; DiMauro, S.; Navas, P.; Salviati, L. Coenzyme Q deficiency in muscle. Curr. Opin. Neurol. 2011, 24, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Montero, R.; Sánchez-Alcázar, J.A.; Briones, P.; Hernández, A.R.; Cordero, M.D.; Trevisson, E.; Salviati, L.; Pineda, M.; García-Cazorla, A.; Navas, P.; et al. Analysis of coenzyme Q10 in muscle and fibroblasts for the diagnosis of CoQ10 deficiency syndromes. Clin. Biochem. 2008, 41, 697–700. [Google Scholar] [CrossRef] [PubMed]
- Buján, N.; Arias, A.; Montero, R.; García-Villoria, J.; Lissens, W.; Seneca, S.; Espinós, C.; Navas, P.; De Meirleir, L.; Artuch, R.; et al. Characterization of CoQ10 biosynthesis in fibroblasts of patients with primary and secondary CoQ10deficiency. J. Inherit. Metab. Dis. 2014, 37, 53–62. [Google Scholar] [CrossRef] [PubMed]
- López, L.C.; Quinzii, C.M.; Area, E.; Naini, A.; Rahman, S.; Schuelke, M.; Salviati, L.; Dimauro, S.; Hirano, M. Treatment of CoQ(10) deficient fibroblasts with ubiquinone, CoQ analogs, and vitamin C: Time-and compound-dependent effects. PLoS ONE 2010, 5, e11897. [Google Scholar] [CrossRef] [PubMed]
- Duncan, A.J.; Bitner-Glindzicz, M.; Meunier, B.; Costello, H.; Hargreaves, I.P.; López, L.C.; Hirano, M.; Quinzii, C.M.; Sadowski, M.I.; Hardy, J.; et al. A nonsense mutation in COQ9 causes autosomal-recessive neonatal-onset primary coenzyme Q10 deficiency: A potentially treatable form of mitochondrial disease. Am. J. Hum. Genet. 2009, 84, 558–566. [Google Scholar] [CrossRef] [PubMed]
- López, L.C.; Luna-Sánchez, M.; García-Corzo, L.; Quinzii, C.M.; Hirano, M. Pathomechanisms in coenzyme q10-deficient human fibroblasts. Mol. Syndromol. 2014, 5, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Quinzii, C.; Naini, A.; Salviati, L.; Trevisson, E.; Navas, P.; Dimauro, S.; Hirano, M. A mutation in para-hydroxybenzoate-polyprenyl transferase (COQ2) causes primary coenzyme Q10 deficiency. Am. J. Hum. Genet. 2006, 78, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Lagier-Tourenne, C.; Tazir, M.; Lopez, L.C.; Quinzii, C.M.; Assoum, M.; Drouot, N.; Busso, C.; Makri, S.; Ali-Pacha, L.; Benhassine, T.; et al. ADCK3, an ancestral kinase, is mutated in a form of recessive ataxia associated with coenzyme Q10 deficiency. Am. J. Hum. Genet. 2008, 82, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Geromel, V.; Kadhom, N.; Cebalos-Picot, I.; Ouari, O.; Polidori, A.; Munnich, A.; Rötig, A.; Rustin, P. Superoxide-induced massive apoptosis in cultured skin fibroblasts harboring the neurogenic ataxia retinitis pigmentosa (NARP) mutation in the ATPase-6 gene of the mitochondrial DNA. Hum. Mol. Genet. 2001, 10, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Quinzii, C.M.; López, L.C.; Gilkerson, R.W.; Dorado, B.; Coku, J.; Naini, A.B.; Lagier-Tourenne, C.; Schuelke, M.; Salviati, L.; Carrozzo, R.; et al. Reactive oxygen species, oxidative stress, and cell death correlate with level of CoQ10 deficiency. FASEB J. 2010, 24, 3733–3743. [Google Scholar] [CrossRef] [PubMed]
- López-Martín, J.M.; Salviati, L.; Trevisson, E.; Montini, G.; DiMauro, S.; Quinzii, C.; Hirano, M.; Rodriguez-Hernandez, A.; Cordero, M.D.; Sánchez-Alcázar, J.A.; et al. Missense mutation of the COQ2 gene causes defects of bioenergetics and de novo pyrimidine synthesis. Hum. Mol. Genet. 2007, 16, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Hernández, A.; Cordero, M.D.; Salviati, L.; Artuch, R.; Pineda, M.; Briones, P.; Gómez Izquierdo, L.; Cotán, D.; Navas, P.; Sánchez-Alcázar, J.A. Coenzyme Q deficiency triggers mitochondria degradation by mitophagy. Autophagy 2009, 5, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Cotan, D.; Cordero, M.D.; Garrido-Maraver, J.; Oropesa-Avila, M.; Rodriquez-Hernandez, A.; Gomez Izquierdo, L.; De la Mata, M.; De Miquel, M.; Lorite, J.B.; Infante, E.R.; et al. Secondary coenzyme Q10 deficiency triggers mitocondria degradation by mitophagy in MELAS fibroblasts. FABEB J. 2011, 25, 2669–2687. [Google Scholar]
- Fragaki, K.; Ait-El-Mkadem, S.; Chaussenot, A.; Gire, C.; Menqual, R.; Bonesso, L.; Beneteau, M.; Ricci, J.E.; Desquiret-Dumas, V.; Procaccio, V.; et al. Refractory epilepsy and mitocondrial dysfunction due to GM3 synthase deficiency. Eur. J. Hum. Genet. 2013, 21, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Heeringa, S.F.; Chernin, G.; Chaki, M.; Zhou, W.; Sloan, A.J.; Ji, Z.; Xie, L.X.; Salviati, L.; Hurd, T.W.; Vega-Warner, V.; et al. COQ6 mutations in human patients produce nephrotic syndrome with sensorineural deafness. J. Clin. Investig. 2011, 121, 2013–2024. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Gee, H.Y.; Woerner, S.; Xie, L.X.; Vega-Warner, V.; Lovric, S.; Fang, H.; Song, X.; Cattran, D.C.; Avila-Casado, C.; et al. ADCK4 mutations promote steroid-resistant nephrotic syndrome through CoQ10 biosynthesis disruption. J. Clin. Investig. 2013, 123, 5179–5189. [Google Scholar] [CrossRef] [PubMed]
- Yubero, D.; Montero, R.; Ramos, M.; Neergheen, V.; Navas, P.; Artuch, R.; Hargreaves, I. Determination of urinary coenzyme Q10 by HPLC with electrochemical detection: Reference values for a paediatric population. Biofactors 2015, 41, 424–230. [Google Scholar] [CrossRef] [PubMed]
- Emma, F.; Montini, G.; Parikh, S.M.; Salviati, L. Mitochondrial dysfunction in inherited renal disease and acute kidney injury. Nat. Rev. Nephrol. 2016, 12, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Diomedi-Camassei, F.; Di Giandomenico, S.; Santorelli, F.M.; Cardi, G.; Piemonte, F.; Montini, G.; Ghiggeri, G.M.; Murer, L.; Barisoni, L.; Pastore, A.; et al. COQ2 nephropathy: A newly described inherited mitochondriopathy with primary renal involvement. J. Am. Soc. Nephrol. 2007, 18, 2773–2780. [Google Scholar] [CrossRef] [PubMed]
- Sheeran, F.L.; Pepe, S. Posttranslational modifications and dysfunction of mitochondrial enzymes in human heart failure. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E449–E460. [Google Scholar] [CrossRef] [PubMed]
- Duberley, K.E.; Hargreaves, I.P.; Chaiwatanasirikul, K.A.; Heales, S.J.; Land, J.M.; Rahman, S.; Mills, K.; Eaton, S. Coenzyme Q10 quantification in muscle, fibroblasts and cerebrospinal fluid by liquid chromatography/tandem mass spectrometry using a novel deuterated internal standard. Rapid Commun. Mass Spectrom. 2013, 27, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Martinefski, M.; Samassa, P.; Lucangioli, S.; Tripodi, V. A novel non-invasive sampling method using buccal mucosa cells for determination of coenzyme Q10. Anal. Bioanal. Chem. 2015, 407, 5529–5533. [Google Scholar] [CrossRef] [PubMed]
| Tissue | Advantages | Limitations |
|---|---|---|
| Plasma | Minimally invasive Identification of secondary CoQ deficiencies CoQ treatment monitoring | Low diagnostic yield for CoQ deficiency in mitochondrial disorders CoQ values modified by external sources |
| Leukocytes Platelets | Minimally invasive Correlation with CoQ tissue levels CoQ treatment monitoring | Fresh preparation Time-consuming Few reported experiences in mitochondrial disorders. |
| Muscle | Good diagnostic yield for CoQ deficiency Other mitochondrial studies can be performed | Invasive No treatment monitoring |
| Fibroblasts | Good diagnostic yield for some CoQ deficiencies Functional studies can be performed (CoQ biosynthesis) Unlimited biological material for further studies | False negative results in some cases |
| Urine | Non-invasive Easily detectable CoQ values Treatment monitoring purposes | Correlation with kidney CoQ status remains to be established |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yubero, D.; Allen, G.; Artuch, R.; Montero, R. The Value of Coenzyme Q10 Determination in Mitochondrial Patients. J. Clin. Med. 2017, 6, 37. https://doi.org/10.3390/jcm6040037
Yubero D, Allen G, Artuch R, Montero R. The Value of Coenzyme Q10 Determination in Mitochondrial Patients. Journal of Clinical Medicine. 2017; 6(4):37. https://doi.org/10.3390/jcm6040037
Chicago/Turabian StyleYubero, Delia, George Allen, Rafael Artuch, and Raquel Montero. 2017. "The Value of Coenzyme Q10 Determination in Mitochondrial Patients" Journal of Clinical Medicine 6, no. 4: 37. https://doi.org/10.3390/jcm6040037





