Omega-3 Polyunsaturated Fatty Acids for the Treatment of IgA Nephropathy
Abstract
:1. Introduction
2. Significance of Therapeutic Intervention in IgAN
3. History of Treatment of IgAN with ω-3 PUFAs
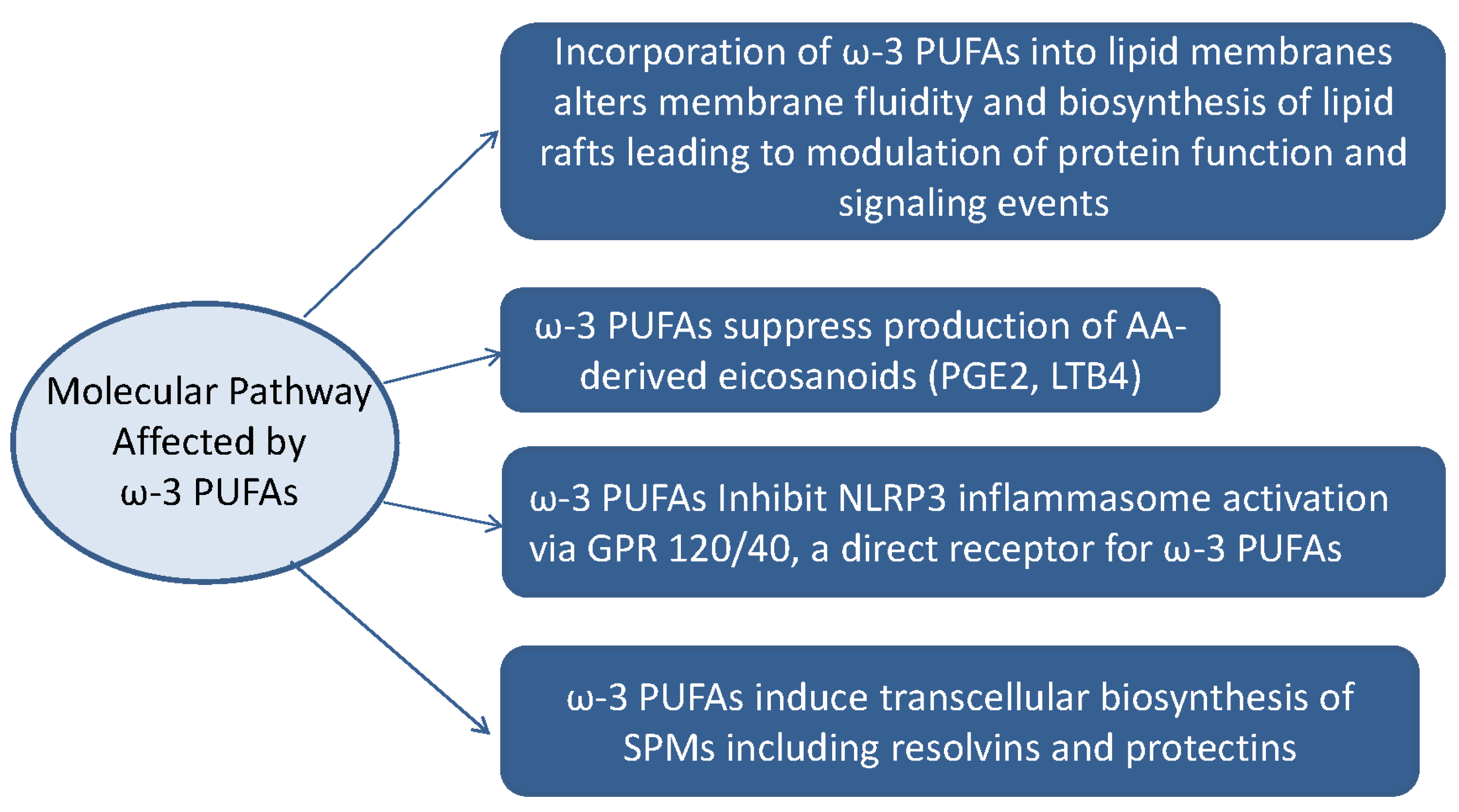
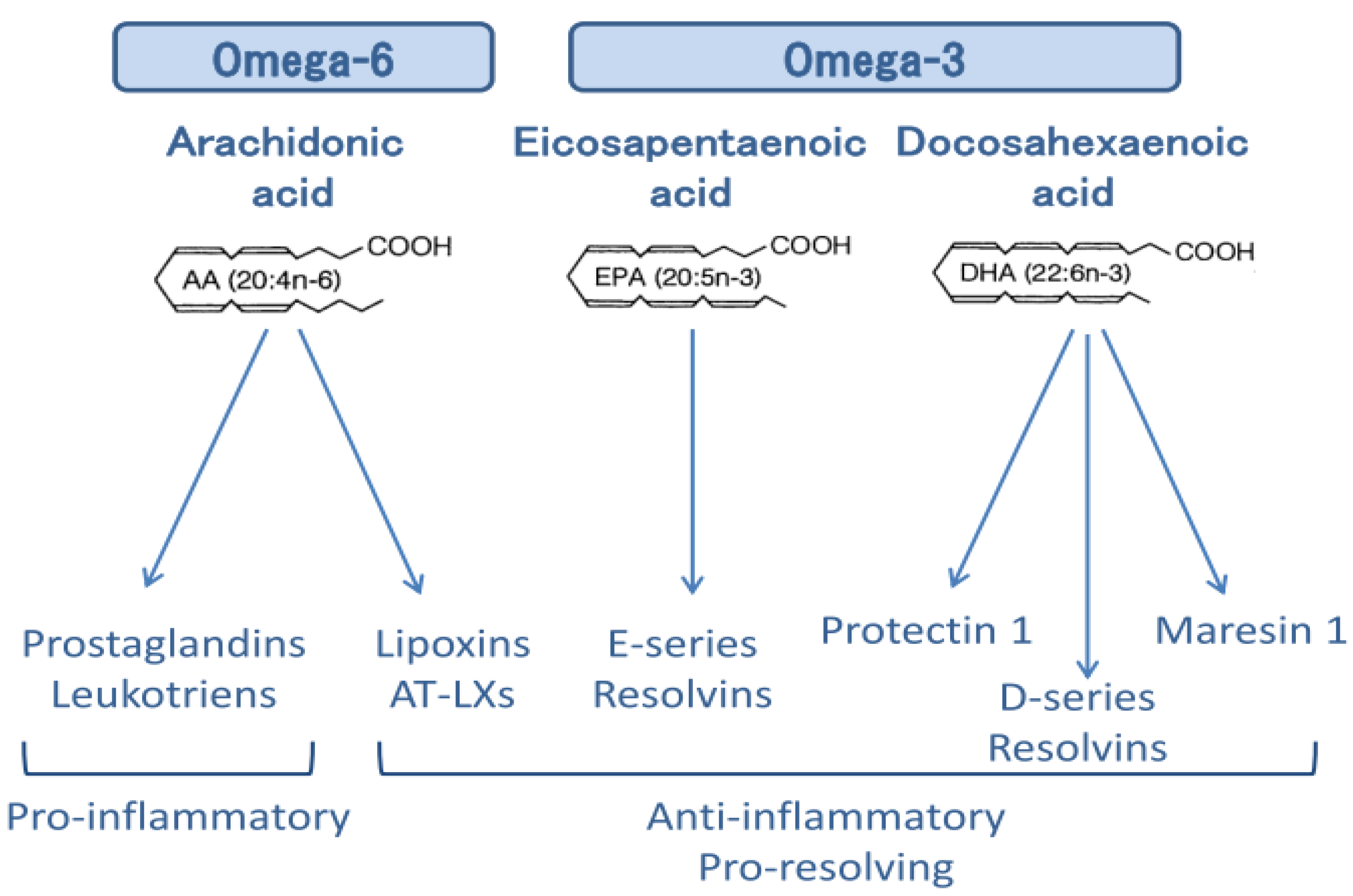
4. Anti-Inflammatory Actions of ω-3 PUFAs
5. Combination Therapy of Aspirin and EPA
6. Conclusions
Conflicts of Interest
References
- Chan, J.C.M.; Trachtman, H. Modulating the Progression in IgA Nephropathy. Nephron Clin. Pract. 2006, 104, C61–C68. [Google Scholar] [CrossRef] [PubMed]
- Berthoux, F.; Mohey, H.; Laurent, B.; Mariat, C.; Afiani, A.; Thibaudin, L. Predicting the risk for dialysis or death in IgA nephropathy. J. Am. Soc. Nephrol. 2011, 22, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Barbour, S.J.; Reich, H.N. Risk stratification of patients with IgA nephropathy. Am. J. Kidney Dis. 2012, 59, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, E.; Zamora, I.; Ballarín, J.A.; Arce, Y.; Jiménez, S.; Quereda, C.; Olea, T.; Martínez-Ara, J.; Segarra, A.; Bernis, C.; et al. Long-term outcomes of IgA nephropathy presenting with minimal or no proteinuria. J. Am. Soc. Nephrol. 2012, 23, 1753–1760. [Google Scholar] [CrossRef] [PubMed]
- Knoop, T.; Vikse, B.E.; Svarstad, E.; Leh, S.; Reisæter, A.V.; Bjørneklett, R. Mortality in patients with IgA nephropathy. Am. J. Kidney Dis. 2013, 62, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Hotta, O.; Miyazaki, M.; Furuta, T.; Tomioka, S.; Chiba, S.; Horigome, I.; Abe, K.; Taguma, Y. Tonsillectomy and steroid pulse therapy significantly impact on clinical remission in patients with IgA nephropathy. Am. J. Kidney Dis. 2001, 38, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, H.; Fujimoto, S.; Hara, S.; Sato, Y.; Yamada, K.; Kitamura, K. Effect of tonsillectomy plus steroid pulse therapy on clinical remission of IgA nephropathy: A controlled study. Clin. J. Am. Soc. Nephrol. 2008, 3, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Rauen, T.; Eitner, F.; Fitzner, C.; Sommerer, C.; Zeier, M.; Otte, B.; Panzer, U.; Peters, H.; Benck, U.; Mertens, P.R.; et al. Intensive Supportive Care plus Immunosuppression in IgA Nephropathy. N. Engl. J. Med. 2015, 373, 2225–2236. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Sell, J.E.; Pestka, J.J. Quantitative assessment of mesangial immunoglobulin A (IgA) accumulation, elevated circulating IgA immune complexes, and hematuria during vomitoxin-induced IgA nephropathy. Fundam. Appl. Toxicol. 1991, 17, 197–207. [Google Scholar] [CrossRef]
- Pestka, J.J.; Zhou, H.R. Interleukin-6-deficient mice refractory to IgA dysregulation but not anorexia induction by vomitoxin (deoxynivalenol) ingestion. Food Chem. Toxicol. 2000, 38, 565–575. [Google Scholar] [CrossRef]
- Hamazaki, T.; Tateno, S.; Shishido, H. Eicosapentaenoic acid and IgA nephropathy. Lancet 1984, 1, 11017–11018. [Google Scholar] [CrossRef]
- Alexopoulos, E.; Stangou, M.; Pantzaki, A.; Kirmizis, D.; Memmos, D. Treatment of severe IgA nephropathy with omega-3 fatty acids: the effect of a “very low dose” regimen. Ren. Fail. 2004, 26, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Sulikowsa, B.; Niewegłowski, T.; Manitius, J.; Lysiak-Szydłowska, W.; Rutkowski, B. Effect of 12-month therapy with omega-3 polyunsaturated acids on glomerular filtration response to dopamine in IgA nephropathy. Am. J. Nephrol. 2004, 24, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Donadio, J.V., Jr.; Bergstralh, E.J.; Offord, K.P.; Spencer, D.C.; Holley, K.E. A controlled trial of fish oil in IgA nephropathy. Mayo Nephrology Collaborative Group. N. Engl. J. Med. 1994, 331, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Donadio, J.V., Jr.; Grande, J.P.; Bergstralh, E.J.; Dart, R.A.; Larson, T.S.; Spencer, D.C. The long-term outcome of patients with IgA nephropathy treated with fish oil in a controlled trial. Mayo Nephrology Collaborative Group. J. Am. Soc. Nephrol. 1999, 10, 1772–1777. [Google Scholar] [PubMed]
- Donadio, J.V., Jr.; Larson, T.S.; Bergstralh, E.J.; Grande, J.P. A randomized trial of high-dose compared with low-dose omega-3 fatty acids in severe IgA nephropathy. J. Am. Soc. Nephrol. 2001, 12, 791–799. [Google Scholar] [PubMed]
- Ferraro, P.M.; Ferraccioli, G.F.; Gambaro, G.; Fulignati, P.; Costanzi, S. Combined treatment with renin-angiotensin system blockers and polyunsaturated fatty acids in proteinuric IgA nephropathy: A randomized controlled trial. Nephrol. Dial. Transplant. 2009, 24, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, T.; Iwasaki, C.; Tanaka, K.; Ochi, A.; Shimizu, A.; Shiohira, S.; Itabashi, M.; Takei, T.; Uchida, K.; Tsuchiya, K.; et al. Effects of combination therapy with renin-angiotensin system inhibitors and eicosapentaenoic acid on IgA nephropathy. Intern. Med. 2013, 52, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M.; Origasa, H.; Matsuzaki, M.; Matsuzawa, Y.; Saito, Y.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H.; et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet 2007, 369, 1090–1098. [Google Scholar] [CrossRef]
- Bennett, W.M.; Walker, R.G.; Kincaid-Smith, P. Treatment of IgA nephropathy with eicosapentanoic acid (EPA): A two-year prospective trial. Clin. Nephrol. 1989, 31, 128–131. [Google Scholar] [PubMed]
- Pettersson, E.E.; Rekola, S.; Berglund, L.; Sundqvist, K.G.; Angelin, B.; Diczfalusy, U.; Björkhem, I.; Bergström, J. Treatment of IgA nephropathy with omega-3-polyunsaturated fatty acids: A prospective, double-blind, randomized study. Clin. Nephrol. 1994, 41, 183–190. [Google Scholar] [PubMed]
- Hogg, R.J.; Lee, J.; Nardelli, N.; Julian, B.A.; Cattran, D.; Waldo, B.; Wyatt, R.; Jennette, J.C.; Sibley, R.; Hyland, K.; et al. Clinical trial to evaluate omega-3 fatty acids and alternate day prednisone in patients with IgA nephropathy: Report from the Southwest Pediatric Nephrology Study Group. Clin. J. Am. Soc. Nephrol. 2006, 1, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Terano, T.; Salmon, J.A.; Higgs, G.A.; Moncada, S. Eicosapentaenoic acid as a modulator of inflammation. Effect on prostaglandin and leukotriene synthesis. Biochem. Pharmacol. 1986, 35, 779–785. [Google Scholar] [CrossRef]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Jiang, W.; Spinetti, T.; Tardivel, A.; Castillo, R.; Bourquin, C.; Guarda, G.; Tian, Z.; Tschopp, J.; Zhou, R. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity 2013, 38, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Chen, Z.; Bhat, O.M.; Zhang, Q.; Abais-Battad, J.M.; Conley, S.M.; Ritter, J.K.; Li, P.L. NLRP3 inflammasome is reported as a novel target for docosahexaenoic acid metabolites to abrogate glomerular injury. J. Lipid Res. 2017, 58, 1080–1090. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Hamberg, M.; Samuelsson, B. Lipoxins: Novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proc. Natl. Acad. Sci. USA 1984, 81, 5335–5339. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Clish, C.B.; Brannon, J.; Colgan, S.P.; Chiang, N.; Gronert, K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J. Exp. Med. 2000, 192, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Hong, S.; Gronert, K.; Colgan, S.P.; Devchand, P.R.; Mirick, G.; Moussignac, R.L. Resolvins: A family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med. 2002, 196, 1025–1037. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Marcheselli, V.L.; Serhan, C.N.; Bazan, N.G. Neuroprotectin D1: A docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc. Natl. Acad. Sci. USA 2004, 101, 8491–8496. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Yang, R.; Martinod, K.; Kasuga, K.; Pillai, P.S.; Porter, T.F.; Oh, S.F.; Spite, M. Maresins: Novel macrophage mediators with potent antiinflammatory and proresolving actions. J. Exp. Med. 2009, 206, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Spite, M.; Serhan, C.N. Novel lipid mediators promote resolution of acute inflammation: Impact of aspirin and statins. Circ. Res. 2010, 107, 1170–1184. [Google Scholar] [CrossRef] [PubMed]
- Hirahashi, J.; Hanafusa, N.; Wada, T.; Arita, M.; Hishikawa, K.; Hayashi, M.; Nangaku, M. Aspirin and Eicosapentaenoic Acid May Arrest Progressive IgA Nephropathy: A Potential Alternative to Immunosuppression. Intern. Med. 2015, 54, 2377–2382. [Google Scholar] [CrossRef] [PubMed]
- Hirahashi, J.; Jo, A.; Ueda, K.; Tojo, A.; Fujita, T. Successful treatment of antineutrophil cytoplasmic antibody-associated vasculitis with eicosapentaenoic acid. Ann. Intern. Med. 2012, 156, 755–756. [Google Scholar] [CrossRef] [PubMed]
- Hirahashi, J.; Kawahata, K.; Arita, M.; Iwamoto, R.; Hishikawa, K.; Honda, M.; Hamasaki, Y.; Tanaka, M.; Okubo, K.; Kurosawa, M.; et al. Immunomodulation with eicosapentaenoic acid supports the treatment of autoimmune small-vessel vasculitis. Sci. Rep. 2014, 4, 6406. [Google Scholar] [CrossRef] [PubMed]
- Vane, J.R.; Botting, R.M. The mechanism of action of aspirin. Thromb. Res. 2003, 110, 255–258. [Google Scholar] [CrossRef]
- Arita, M.; Bianchini, F.; Aliberti, J.; Sher, A.; Chiang, N.; Hong, S.; Yang, R.; Petasis, N.A.; Serhan, C.N. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J. Exp. Med. 2005, 201, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Block, R.C.; Dier, U.; Calderonartero, P.; Shearer, G.C.; Kakinami, L.; Larson, M.K.; Harris, W.S.; Georas, S.; Mousa, S.A. The Effects of EPA+DHA and Aspirin on Inflammatory Cytokines and Angiogenesis Factors. World J. Cardiovasc. Dis. 2012, 2, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Lev, E.I.; Solodky, A.; Harel, N.; Mager, A.; Brosh, D.; Assali, A.; Roller, M.; Battler, A.; Kleiman, N.S.; Kornowski, R. Treatment of aspirin-resistant patients with omega-3 fatty acids versus aspirin dose escalation. J. Am. Coll. Cardiol. 2010, 55, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, Y.; Ye, Y.; Lin, Y.; Freeberg, S.Y.; Nishi, S.P.; Martinez, J.D.; Huang, M.H.; Uretsky, B.F.; Perez-Polo, J.R. Augmentation of myocardial production of 15-epi-lipoxin-a4 by pioglitazone and atorvastatin in the rat. Circulation 2006, 114, 929–935. [Google Scholar] [CrossRef] [PubMed]
| First Author, Reference | Year | Intervention | Population | Duration of Follow-Up, Years | Main Findings |
|---|---|---|---|---|---|
| Hamazaki [11] | 1984 | EPA (1.6 g) + DHA (1.0 g) | 10 | 1 | EPA might be a safe and useful agent to stop the progression of IgAN |
| Control: EPA/DHA (−) | 10 | ||||
| Alexopoulos [12] | 2004 | EPA (0.9 g) + DHA (0.6 g) | 14 | 4 | A “very low dose” of omega-3 PUFAs is effective in slowing renal progression in high-risk patients with IgAN |
| Control: EPA/DHA (−) | 14 | ||||
| Sulikowsa [13] | 2004 | EPA (0.8 g) + DHA (0.5 g) | 20 | 1 | Omega-3 PUFAs supplementation is associated with the improvement of both renal vascular function and tubule function |
| Donaldio [14,15] | 1994 | EPA (1.9 g) + DHA (1.5 g) | 55 | 6.4 | Early and prolonged treatment with fish oil slows renal progression for high-risk patients with IgA nephropathy |
| 1999 | Control: placebo | 15 | 6.8 | ||
| Donaldio [16] | 2001 | High dose EPA (3.8 g) + DHA (2.9 g) Low dose EPA (1.9 g) + DHA (1.5 g) | 36 | 2 | Low-dose and high-dose omega-3 fatty acids were similar in slowing the rate of renal function loss in high-risk patients with IgAN |
| 37 | |||||
| Ferraro [17] | 2009 | EPA + DHA (2.6 g)+ RASB | 15 | 0.5 | Omega-3 PUFAs associated with RASB reduced proteinuria in patients with IgAN more than RASB alone |
| Control: RASB | 15 | ||||
| Moriyama [18] | 2013 | RASB + EPA (0.9–1.8 g) | 18 | 1 | EPA accelerates the effects of RASB and thus decreases the proteinuria observed in patients with IgAN |
| Control:RASB + dilazep | 20 | ||||
| Bennett [19] | 1989 | EPA (10 g) | 17 | 2 | EPA does not alter the course of established mesangial IgA nephropathy |
| Control: no treatment | 20 | ||||
| Pettersson [20] | 1994 | EPA (3.3 g) + DHA (1.8 g) | 15 | 0.5 | By 0.5 year, omega-3 PUFAs supplements resulted in a slight but significant reduction in GFR |
| Control: EPA/DHA (−) | 17 | ||||
| Hogg [21] | 2006 | EPA (1.9 g) + DHA (1.5 g) | 32 | 2 | The effect of omega-3 PUFAs on proteinuria in patients with IgAN is dosage-dependent |
| placebo | 31 |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirahashi, J. Omega-3 Polyunsaturated Fatty Acids for the Treatment of IgA Nephropathy. J. Clin. Med. 2017, 6, 70. https://doi.org/10.3390/jcm6070070
Hirahashi J. Omega-3 Polyunsaturated Fatty Acids for the Treatment of IgA Nephropathy. Journal of Clinical Medicine. 2017; 6(7):70. https://doi.org/10.3390/jcm6070070
Chicago/Turabian StyleHirahashi, Junichi. 2017. "Omega-3 Polyunsaturated Fatty Acids for the Treatment of IgA Nephropathy" Journal of Clinical Medicine 6, no. 7: 70. https://doi.org/10.3390/jcm6070070





