Controversies and Advances in Gestational Diabetes—An Update in the Era of Continuous Glucose Monitoring
Abstract
:1. Introduction
2. Classification and Diagnosis
2.1. Controversies on the IADPSG Diagnostic Criteria
2.2. Controversies in Early Pregnancy and the Role of HbA1c in Pregnancy
3. Current Glucose Treatment Targets in Diabetic Pregnancies
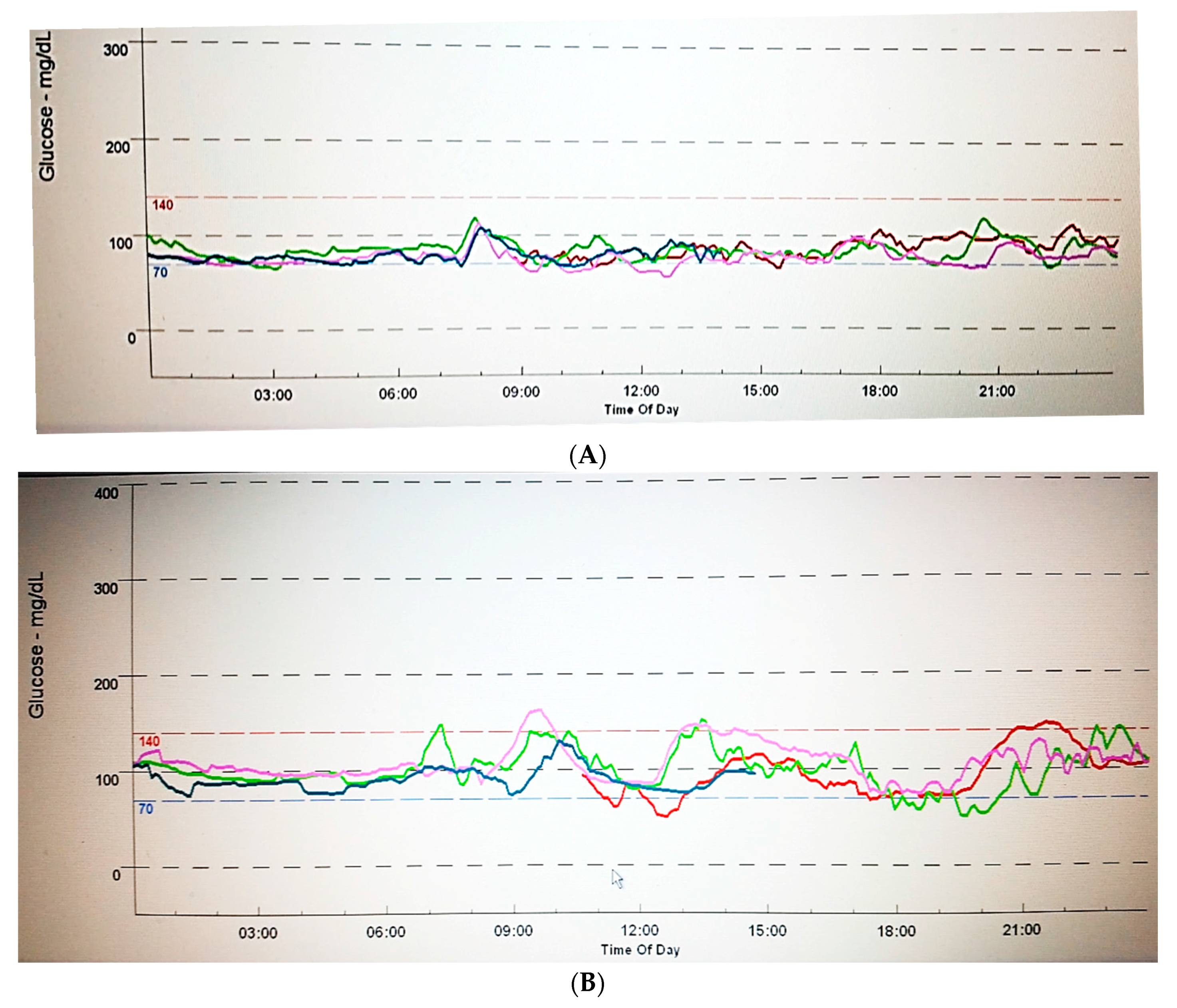
4. Comprehensive Glucose Management: Beyond Glucose Time Point Measures
5. Comprehensive Pregnancy Management beyond Glucose
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Marathe, P.H.; Gao, H.X.; Close, K.L. American Diabetes Association standards of medical care in diabetes 2017. J. Diabetes 2017, 9, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Barbour, L.A. Changing perspectives in pre-existing diabetes and obesity in pregnancy: Maternal and infant short- and long-term outcomes. Curr. Opin. Endocrinol. Diabetes Obes. 2014, 21, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Metzger, B.E.; Lowe, L.P.; Dyer, A.R.; Trimble, E.R.; Chaovarindr, U.; Coustan, D.R.; Hadden, D.R.; McCance, D.R.; Hod, M.; McIntyre, H.D. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. Engl. 2008, 358, 1991–2002. [Google Scholar] [CrossRef]
- Catalano, P.M.; Hauguel-De Mouzon, S. Is it time to revisit the Pedersen hypothesis in the face of the obesity epidemic? Am. J. Obstet. Gynecol. 2011, 204, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Weile, L.K.; Kahn, J.G.; Marseille, E.; Jensen, D.M.; Damm, P.; Lohse, N. Global cost-effectiveness of GDM screening and management: Current knowledge and future needs. Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 206–224. [Google Scholar] [CrossRef] [PubMed]
- Metzger, B.E.; Gabbe, S.G.; Persson, B.; Buchanan, T.A.; Catalano, P.A.; Damm, P.; Dyer, A.R.; Leiva, A.; De Hod, M.; Kitzmiler, J.L.; et al. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Blumer, I.; Hadar, E.; Hadden, D.R.; Jovanovič, L.; Mestman, J.H.; Murad, M.H.; Yogev, Y. Diabetes and pregnancy: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2013, 98, 4227–4249. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy; WHO: Geneva, Switzerland, 2013; pp. 1–62. [Google Scholar]
- Committee on Obstetric Practice. Practice bulletin No. 137: Gestational diabetes mellitus. Obstet. Gynecol. 2013, 122, 406–416. [Google Scholar]
- Caughey, A.B. Practice bulletin No. 180: Gestational diabetes mellitus. Obstet. Gynecol. 2017, 130, 17e–31e. [Google Scholar]
- Carpenter, M.W.; Coustan, D.R. Criteria for screening tests for gestational diabetes. Am. J. Obstet. Gynecol. 1982, 144, 768–773. [Google Scholar] [CrossRef]
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979, 28, 1039–1057. [Google Scholar]
- Roeckner, J.T.; Sanchez-Ramos, L.; Jijon-Knupp, R.; Kaunitz, A.M. Single abnormal value on 3-h oral glucose tolerance test during pregnancy is associated with adverse maternal and neonatal outcomes: A systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2016, 215, 287–297. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Health. National Institutes of Health Consensus Development Conference statement: Diagnosing gestational diabetes mellitus. Obstet. Gynecol. 2013, 122, 358–369. [Google Scholar]
- Meltzer, S.J.; Snyder, J.; Penrod, J.R.; Nudi, M.; Morin, L. Gestational diabetes mellitus screening and diagnosis: A prospective randomised controlled trial comparing costs of one-step and two-step methods. BJOG Int. J. Obstet. Gynaecol. 2010, 117, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Mission, J.F.; Ohno, M.S.; Cheng, Y.W.; Caughey, A.B. Gestational diabetes screening with the new IADPSG guidelines: A cost-effectiveness analysis. Am. J. Obstet. Gynecol. 2012, 207, 326. [Google Scholar] [CrossRef] [PubMed]
- Yumei, W.; Huixia, Y.; Weiwei, Z.; Hongyun, Y.; Haixia, L.; Jie, Y.; Cuilin, Z. International Association of Diabetes and Pregnancy Study Group criteria is suitable for gestational diabetes mellitus diagnosis: Further evidence from China. Chin. Med. J. Engl. 2014, 127, 3553–3556. [Google Scholar]
- Mcintyre, H.D.; Sacks, D.A.; Barbour, L.A.; Feig, D.S.; Catalano, P.M.; Damm, P. Issues with the diagnosis and classification of hyperglycemia in early pregnancy. Diabetes Care 2016, 39, 53–54. [Google Scholar] [CrossRef] [PubMed]
- Corrado, F.; D’Anna, R.; Cannata, M.L.; Interdonato, M.L.; Pintaudi, B.; Di Benedetto, A. Correspondence between first-trimester fasting glycaemia, and oral glucose tolerance test in gestational diabetes diagnosis. Diabetes Metab. 2012, 38, 458–461. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.W.; Yang, H.X.; Wei, Y.M.; Yan, J.; Wang, Z.L.; Li, X.L.; Wu, H.R.; Li, N.; Zhang, M.H.; Liu, X.H.; et al. Evaluation of the value of fasting plasma glucose in the first prenatal visit to diagnose gestational diabetes mellitus in China. Diabetes Care 2013, 36, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.C.E.; Rowan, J.; Florkowski, C.M. Is there a role for HbA1c in pregnancy? Curr. Diab. Rep. 2016, 16, 5. [Google Scholar] [CrossRef] [PubMed]
- Cosson, E.; Carbillon, L.; Valensi, P. High fasting plasma glucose during early pregnancy: A review about early gestational diabetes mellitus. J. Diabetes Res. 2017, 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.C.E.; Moore, M.P.; Gullam, J.E.; Mohamed, K.; Rowan, J. An early pregnancy HbA1c ≥5.9% (41 mmol/mol) is optimal for detecting diabetes and identifies women at increased risk of adverse pregnancy outcomes. Diabetes Care 2014, 37, 2953–2959. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, T.L.; Friedman, J.E.; Van Pelt, R.E.; Barbour, L.A. Patterns of glycemia in normal pregnancy: Should the current therapeutic targets be challenged? Diabetes Care 2011, 34, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Harmon, K.A.; Gerard, L.; Jensen, D.R.; Kealey, E.H.; Hernandez, T.L.; Reece, M.S.; Barbour, L.A.; Bessesen, D.H. Continuous glucose profiles in obese and normal-weight pregnant women on a controlled diet: Metabolic determinants of fetal growth. Diabetes Care 2011, 34, 2198–2204. [Google Scholar] [CrossRef] [PubMed]
- Langer, O.; Levy, J.; Brustman, L.; Anyaegbunam, A.; Merkatz, R.; Divon, M. Glycemic control in gestational diabetes mellitus-how tight is tight enough: Small for gestational age versus large for gestational age? Am. J. Obstet. Gynecol. 1989, 161, 646–653. [Google Scholar] [CrossRef]
- Prutsky, G.J.; Domecq, J.P.; Wang, Z.; Carranza Leon, B.G.; Elraiyah, T.; Nabhan, M.; Sundaresh, V.; Vella, A.; Montori, V.M.; Murad, M.H. Glucose targets in pregnant women with diabetes: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2013, 98, 4319–4324. [Google Scholar] [CrossRef] [PubMed]
- Murphy, H.R.; Rayman, G.; Lewis, K.; Kelly, S.; Johal, B.; Duffield, K.; Fowler, D.; Campbell, P.J.; Temple, R.C. Effectiveness of continuous glucose monitoring in pregnant women with diabetes: Randomised clinical trial. BMJ 2008, 337, a1680. [Google Scholar] [CrossRef] [PubMed]
- Secher, A.; Ringholm, L.; Andersen, H.; Damm, P.; Mathiesen, E. The effect of real-time continuous glucose monitoring in pregnant women with diabetes. Diabetes Care 2013, 36, 1877–1883. [Google Scholar] [CrossRef] [PubMed]
- Mazze, R.; Yogev, Y.; Langer, O. Measuring glucose exposure and variability using continuous glucose monitoring in normal and abnormal glucose metabolism in pregnancy. J. Matern. Fetal. Neonatal Med. 2012, 25, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Taslimi, M.M.; Navabi, K.; Acosta, R.; Helmer, A.; El-Sayed, Y.Y. Concealed maternal blood glucose excursions correlate with birth weight centile. J. Diabetes Sci. Technol. 2008, 2, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Siegmund, T.; Rad, N.T.; Ritterath, C.; Siebert, G.; Henrich, W.; Buhling, K.J. Longitudinal changes in the continuous glucose profile measured by the CGMS in healthy pregnant women and determination of cut-off values. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 139, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Carreiro, M.P.; Lauria, M.W.; Naves, G.N.T.; Miranda, P.A.C.; Leite, R.B.; Rajão, K.M.A.B.; De Aguiar, R.A.L.P.; Nogueira, A.I.; Ribeiro-Oliveira, A. Seventy two-hour glucose monitoring profiles in mild gestational diabetes mellitus: Differences from healthy pregnancies and influence of diet counseling. Eur. J. Endocrinol. 2016, 175, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Moy, F.M.; Ray, A.; Buckley, B.S.; West, H.M. Techniques of monitoring blood glucose during pregnancy for women with pre-existing diabetes. Cochrane Database Syst. Rev. 2017, 11, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Lv, L.; Liang, Z.; Wang, Y.; Wen, J.; Lin, X.; Zhou, Y.; Mai, C.; Niu, J. Continuous glucose monitoring effects on maternal glycemic control and pregnancy outcomes in patients with gestational diabetes mellitus: A prospective cohort study. J. Clin. Endocrinol. Metab. 2014, 99, 4674–4682. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, T.L.; Barbour, L.A. A standard approach to continuous glucose monitor data in pregnancy for the study of fetal growth and infant outcomes. Diabetes Technol. Ther. 2013, 15, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Law, G.R.; Ellison, G.T.H.; Secher, A.L.; Damm, P.; Mathiesen, E.R.; Temple, R.; Murphy, H.R.; Scott, E.M. Analysis of continuous glucose monitoring in pregnant women with diabetes: Distinct temporal patterns of glucose associated with large-for-gestational-age infants. Diabetes Care 2015, 38, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Mcgrath, R.T.; Glastras, S.J.; Seeho, S.K.; Scott, E.S.; Fulcher, G.R. Association between glycemic variability, neonates in women with type 1 diabetes. Diabetes Care 2017, 40, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Dalfrà, M.G.; Chilelli, N.C.; Di Cianni, G.; Mello, G.; Lencioni, C.; Biagioni, S.; Scalese, M.; Sartore, G.; Lapolla, A. Glucose fluctuations during gestation: An additional tool for monitoring pregnancy complicated by diabetes. Int. J. Endocrinol. 2013, 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Ihnat, M.A. “Glycaemic variability”: A new therapeutic challenge in diabetes and the critical care setting. Diabet. Med. 2010, 27, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-M.; Zhao, L.-H.; Su, J.-B.; Qiao, H.-F.; Wang, X.-H.; Xu, F.; Chen, T.; Chen, J.-F.; Wu, G.; Wang, X.-Q. Glycemic variability in normal glucose tolerance women with the previous gestational diabetes mellitus. Diabetol. Metab. Syndr. 2015, 7, 82. [Google Scholar] [CrossRef] [PubMed]
- Freire, C.M.; Barbosa, F.B.; de Almeida, M.C.C.; Miranda, P.A.; Barbosa, M.; Nogueira, A.; Guimarães, M.; Nunes, M.D.C.P.; Ribeiro-Oliveira, A. Previous gestational diabetes is independently associated with increased carotid intima-media thickness, similarly to metabolic syndrome—A case control study. Cardiovasc. Diabetol. 2012, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Varner, M.W.; Rice, M.M.; Landon, M.B.; Casey, B.M.; Reddy, U.M.; Wapner, R.J.; Rouse, D.J.; Tita, A.T.N.; Thorp, J.M.; Chien, E.K.; et al. Pregnancies after the diagnosis of mild gestational diabetes mellitus and risk of cardiometabolic disorders. Obstet. Gynecol. 2017, 129, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Madhu, S.V.; Muduli, S.K.; Avasthi, R. Abnormal glycemic profiles by CGMS in obese first-degree relatives of type 2 diabetes mellitus patients. Diabetes Technol. Ther. 2013, 15, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Tao, M.; Zhou, J.; Zhu, J.; Lu, W.; Jia, W. Continuous glucose monitoring reveals abnormal features of postprandial glycemic excursions in women with polycystic ovarian syndrome. Postgrad. Med. 2011, 123, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Lowe, L.P.; Metzger, B.E.; Lowe, W.L.; Dyer, A.R.; McDade, T.W.; McIntyre, H.D. Inflammatory mediators and glucose in pregnancy: Results from a subset of the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study. J. Clin. Endocrinol. Metab. 2010, 95, 5427–5434. [Google Scholar] [CrossRef] [PubMed]
- Son, G.H.; Kwon, J.Y.; Kim, Y.H.; Park, Y.W. Maternal serum triglycerides as predictive factors for large-for-gestational age newborns in women with gestational diabetes mellitus. Acta Obstet. Gynecol. Scand. 2010, 89, 700–704. [Google Scholar] [CrossRef] [PubMed]
- The National Institute for Health and Care Excellence. NICE Medtech Innovation Briefing FreeStyle Libre for Glucose Monitoring; The National Institute for Health and Care Excellence: London, UK, 2017; pp. 1–18. [Google Scholar]
- Scholtens, D.M.; Bain, J.R.; Reisetter, A.C.; Muehlbauer, M.J.; Nodzenski, M.; Stevens, R.D.; Ilkayeva, O.; Lowe, L.P.; Metzger, B.E.; Newgard, C.B.; et al. Metabolic networks and metabolites underlie associations between maternal glucose during pregnancy and newborn size at birth. Diabetes 2016, 65, 2039–2050. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.M.; McIntyre, H.D.; Cruickshank, J.K.; McCance, D.R.; Dyer, A.R.; Metzger, B.E.; Lowe, L.P.; Trimble, E.R.; Coustan, D.R.; Hadden, D.R.; et al. The hyperglycemia and adverse pregnancy outcome study: Associations of GDM and obesity with pregnancy outcomes. Diabetes Care 2012, 35, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Dandona, P.; Aljada, A.; Bandyopadhyay, A. Inflammation : The link between insulin resistance, obesity and diabetes. Trends Immunol. 2016, 25, 4–7. [Google Scholar] [CrossRef]
- Schmidt, M.I.; Duncan, B.B.; Sharrett, A.R.; Lindberg, G.; Savage, P.J.; Offenbacher, S.; Azambuja, M.I.; Tracy, R.P.; Heiss, G. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): A cohort study. Lancet (London, England) 1999, 353, 1649–1652. [Google Scholar] [CrossRef]
- Richardson, A.C.; Carpenter, M.W. Inflammatory mediators in gestational diabetes mellitus. Obstet. Gynecol. Clin. N. Am. 2007, 34, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Heitritter, S.M.; Solomon, C.G.; Mitchell, G.F.; Skali-Ounis, N.; Seely, E.W. Subclinical inflammation and vascular dysfunction in women with previous gestational diabetes mellitus. J. Clin. Endocrinol. Metab. 2005, 90, 3983–3988. [Google Scholar] [CrossRef] [PubMed]
- Dandona, P.; Mohanty, P.; Ghanim, H.; Aljada, A.; Browne, R.; Hamouda, W.; Prabhala, A.; Afzal, A.; Garg, R. The suppressive effect of dietary restriction and weight loss in the obese on the generation of reactive oxygen species by leukocytes, lipid peroxidation, and protein carbonylation. J. Clin. Endocrinol. Metab. 2001, 86, 355–362. [Google Scholar] [PubMed]
- Saad, M.J.A.; Santos, A.; Prada, P.O. Linking gut microbiota and inflammation to obesity and insulin resistance. Physiology (Bethesda) 2016, 31, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Isolauri, E.; Laitinen, K.; Salminen, S. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am. J. Clin. Nutr. 2008, 88, 894–899. [Google Scholar] [PubMed]
- Catalano, P.M. Trying to understand gestational diabetes. Diabet. Med. 2014, 31, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Lekva, T.; Paasche Roland, M.C.; Michelsen, A.E.; Friis, C.M.; Aukrust, P.; Bollerslev, J.; Henriksen, T.; Ueland, T. Large reduction in adiponectin during pregnancy is associated with large for gestational age newborns. J. Clin. Endocrinol. Metab. 2017, 102, 552–2559. [Google Scholar] [CrossRef] [PubMed]
- Barrett, H.L.; Dekker Nitert, M.; McIntyre, H.D.; Callaway, L.K. Normalizing metabolism in diabetic pregnancy: Is it time to target lipids? Diabetes Care 2014, 37, 1484–1493. [Google Scholar] [CrossRef] [PubMed]
- Han, E.S.; Krauss, R.M.; Xu, F.; Sridhar, S.B.; Ferrara, A.; Quesenberry, C.P.; Hedderson, M.M. Prepregnancy adverse lipid profile and subsequent risk of gestational diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 2721–2727. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Koulman, A.; Petry, C.J.; Jenkins, B.; Matthews, L.; Hughes, I.A.; Acerini, C.L.; Ong, K.K.; Dunger, D.B. An unbiased lipidomics approach identifies early second trimester lipids predictive of maternal glycemic traits and gestational diabetes mellitus. Diabetes Care 2016, 39, 2232–2239. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, A.I.; Souza Santos, R.A.; Simões e Silva, A.C.; Cabral, A.C.V.; Vieira, R.L.P.; Drumond, T.C.; de Campos Machado, L.J.; Freire, C.M.V.; Ribeiro-Oliveira, A. The pregnancy-induced increase of plasma angiotensin-(1-7) is blunted in gestational diabetes. Regul. Pept. 2007, 141, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Langer, O.; Yogev, Y.; Xenakis, E.M.J.; Brustman, L. Overweight and obese in gestational diabetes: The impact on pregnancy outcome. Am. J. Obstet. Gynecol. 2005, 192, 1768–1776. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.F.; Abell, S.K.; Ranasinha, S.; Misso, M.; Boyle, J.A.; Black, M.H.; Li, N.; Hu, G.; Corrado, F.; Rode, L.; et al. Association of gestational weight gain with maternal and infant outcomes: A systematic review and meta-analysis. JAMA 2017, 317, 2207–2225. [Google Scholar] [CrossRef] [PubMed]
- Joy, S.; Roman, A.; Istwan, N.; Rhea, D.; Desch, C.; Stanziano, G.; Saltzman, D. The effect of maternal obesity on pregnancy outcomes of women with gestational diabetes controlled with diet only, glyburide, or insulin. Am. J. Perinatol. 2012, 29, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E.; Natali, A.; Muscelli, E.; Nilsson, P.M.; Golay, A.; Laakso, M.; Beck-Nielsen, H.; Mari, A. Natural history and physiological determinants of changes in glucose tolerance in a non-diabetic population: The RISC Study. Diabetologia 2011, 54, 1507–1516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunford, A.R.; Sangster, J.M. Maternal and paternal periconceptional nutrition as an indicator of offspring metabolic syndrome risk in later life through epigenetic imprinting: A systematic review. Diabetes Metab. Syndr. 2017, 11 (Suppl. 2), 655S–662S. [Google Scholar] [CrossRef] [PubMed]
| Plasma or Serum Level Carpenter and Coustan | Plasma Level NDDG | |
|---|---|---|
| Fasting blood glucose | ≥5.3 mmol/L (95 mg/dL) | ≥5.8 mmol/L (105 mg/dL) |
| One hour | ≥10.0 mmol/L (180 mg/dL) | ≥10.6 mmol/L (190 mg/dL) |
| Two hours | ≥8.6 mmol/L (155 mg/dL) | ≥9.2 mmol/L (165 mg/dL) |
| Three hours | ≥7.8 mmol/L (140 mg/dL) | ≥8.0 mmol/L (145 mg/dL) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carreiro, M.P.; Nogueira, A.I.; Ribeiro-Oliveira, A. Controversies and Advances in Gestational Diabetes—An Update in the Era of Continuous Glucose Monitoring. J. Clin. Med. 2018, 7, 11. https://doi.org/10.3390/jcm7020011
Carreiro MP, Nogueira AI, Ribeiro-Oliveira A. Controversies and Advances in Gestational Diabetes—An Update in the Era of Continuous Glucose Monitoring. Journal of Clinical Medicine. 2018; 7(2):11. https://doi.org/10.3390/jcm7020011
Chicago/Turabian StyleCarreiro, Marina P., Anelise I. Nogueira, and Antonio Ribeiro-Oliveira. 2018. "Controversies and Advances in Gestational Diabetes—An Update in the Era of Continuous Glucose Monitoring" Journal of Clinical Medicine 7, no. 2: 11. https://doi.org/10.3390/jcm7020011




