Red Blood Cell Transfusion Need for Elective Primary Posterior Lumbar Fusion in A High-Volume Center for Spine Surgery
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design, Setting, and Selection of Participants
2.2. Data Collection, Processing, and Outcomes
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Martin, B.I.; Deyo, R.A.; Mirza, S.K.; Turner, J.A.; Comstock, B.A.; Hollingworth, W.; Sullivan, S.D. Expenditures and health status among adults with back and neck problems. JAMA 2008, 299, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, C.L.; Macwan, K.; Sundararajan, K.; Rampersaud, Y.R. Perioperative outcomes and adverse events of minimally invasive versus open posterior lumbar fusion: Meta-analysis and systematic review. J. Neurosurg. Spine 2016, 24, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Willner, D.; Spennati, V.; Stohl, S.; Tosti, G.; Aloisio, S.; Bilotta, F. Spine surgery and blood loss: Systematic review of clinical evidence. Anesth. Analg. 2016, 123, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Basques, B.A.; Anandasivam, N.S.; Webb, M.L.; Samuel, A.M.; Lukasiewicz, A.M.; Bohl, D.D.; Grauer, J.N. Risk factors for blood transfusion with primary posterior lumbar fusion. Spine 2015, 40, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.S.; Burke, J.P.; Dolan, R.T.; Fitzpatrick, P.; O’Byrne, J.M.; McCormack, D.; Synnott, K.; Poynton, A.R. Risk analysis of blood transfusion requirements in emergency and elective spinal surgery. Eur. Spine J. 2011, 20, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Seicean, A.; Alan, N.; Seicean, S.; Neuhauser, D.; Weil, R.J. The effect of blood transfusion on short-term, perioperative outcomes in elective spine surgery. J. Clin. Neurosci. 2014, 21, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Elgafy, H.; Bransford, R.J.; McGuire, R.A.; Dettori, J.R.; Fischer, D. Blood loss in major spine surgery: Are there effective measures to decrease massive hemorrhage in major spine fusion surgery? Spine 2010, 35, S47–S56. [Google Scholar] [CrossRef] [PubMed]
- Rankin, D.; Zuleta-Alarcon, A.; Soghomonyan, S.; Abdel-Rasoul, M.; Castellon-Larios, K.; Bergese, S.D. Massive blood loss in elective spinal and orthopedic surgery: Retrospective review of intraoperative transfusion strategy. J. Clin. Anesth. 2017, 37, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Sun, T.W.; Luo, G.; Zhang, C. Efficacy of antifibrinolytic agents on surgical bleeding and transfusion requirements in spine surgery: A meta-analysis. Eur. Spine J. 2017, 26, 140–154. [Google Scholar] [CrossRef] [PubMed]
- Segal, J.B.; Guallar, E.; Powe, N.R. Autologous blood transfusion in the United States: Clinical and nonclinical determinants of use. Transfusion 2001, 41, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, H.; Yoneoka, D. Trends in the utilization of blood transfusions in spinal fusion in the United States from 2000 to 2009. Spine 2014, 39, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Aoude, A.; Nooh, A.; Fortin, M.; Aldebeyan, S.; Jarzem, P.; Ouellet, J.; Weber, M.H. Incidence, Predictors, and postoperative complications of blood transfusion in thoracic and lumbar fusion surgery: An analysis of 13,695 patients from the American College of Surgeons National Surgical Quality Improvement Program Database. Glob. Spine J. 2016, 6, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Shander, A.; Hofmann, A.; Ozawa, S.; Theusinger, O.M.; Gombotz, H.; Spahn, D.R. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion 2010, 50, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Carabini, L.M.; Zeeni, C.; Moreland, N.C.; Gould, R.W.; Avram, M.J.; Hemmer, L.B.; Bebawy, J.F.; Sugrue, P.A.; Koski, T.R.; Koht, A.; et al. Development and validation of a generalizable model for predicting major transfusion during spine fusion surgery. J. Neurosurg. Anesthesiol. 2014, 26, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Owens, R.K., 2nd; Crawford, C.H., 3rd; Djurasovic, M.; Canan, C.E.; Burke, L.O.; Bratcher, K.R.; McCarthy, K.J.; Carreon, L.Y. Predictive factors for the use of autologous cell saver transfusion in lumbar spinal surgery. Spine 2013, 38, E217–E222. [Google Scholar] [CrossRef] [PubMed]
- Berenholtz, S.M.; Pronovost, P.J.; Mullany, D.; Garrett, E.; Ness, P.M.; Dorman, T.; Klag, M.J. Predictors of transfusion for spinal surgery in Maryland, 1997 to 2000. Transfusion 2002, 42, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Holman, P.J.; Suki, D.; McCutcheon, I.; Wolinsky, J.P.; Rhines, L.D.; Gokaslan, Z.L. Surgical management of metastatic disease of the lumbar spine: Experience with 139 patients. J. Neurosurg. Spine 2005, 2, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Carling, M.S.; Zarhoud, J.; Jeppsson, A.; Eriksson, B.I.; Brisby, H. Preoperative plasma fibrinogen concentration, factor XIII activity, perioperative bleeding, and transfusions in elective orthopaedic surgery: A prospective observational study. Thromb. Res. 2016, 139, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, H.; Yoneoka, D. Predictors of allogeneic blood transfusion in spinal fusion in the United States, 2004–2009. Spine 2014, 39, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Morcos, M.W.; Jiang, F.; McIntosh, G.; Johnson, M.; Christie, S.; Wai, E.; Ouellet, J.; Bailey, C.; Ahn, H.; Paquet, J.; et al. Predictors of blood transfusion in posterior lumbar spinal fusion: A Canadian Spine Outcome and Research Network (CSORN) study. Spine 2018, 43, E25–E39. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Zaw, A.S.; Khine, H.E.; Maharajan, K.; Wai, K.L.; Tan, B.; Mastura, S.; Goy, R. Blood loss and transfusion requirements in metastatic spinal tumor surgery: Evaluation of influencing factors. Ann. Surg. Oncol. 2016, 23, 2079–2086. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Cammisa, F.P., Jr.; Sandhu, H.S.; Girardi, F.P.; Khan, S.N. Factors predicting hospital stay, operative time, blood loss, and transfusion in patients undergoing revision posterior lumbar spine decompression, fusion, and segmental instrumentation. Spine 2002, 27, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Torres-Claramunt, R.; Ramírez, M.; López-Soques, M.; Saló, G.; Molina-Ros, A.; Lladó, A.; Cáceres, E. Predictors of blood transfusion in patients undergoing elective surgery for degenerative conditions of the spine. Arch. Orthop. Trauma Surg. 2012, 132, 1393–1398. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, G.A.; Santrach, P.J.; Oliver, W.C., Jr.; Horlocker, T.T.; Shaughnessy, W.J.; Cabanela, M.E.; Bryant, S. The predictors of red cell transfusions in total hip arthroplasties. Transfusion 1996, 36, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, B.; Merckx, P.; Paugam-Burtz, C.; Dauzac, C.; Agostini, M.M.; Guigui, P.; Mantz, J. Individual probability of allogeneic erythrocyte transfusion in elective spine surgery: The predictive model of transfusion in spine surgery. Anesthesiology 2009, 110, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- McCunniff, P.T.; Young, E.S.; Ahmadinia, K.; Kusin, D.J.; Ahn, U.M.; Ahn, N.U. Chronic antiplatelet use associated with increased blood loss in lumbar spinal surgery despite adherence to protocols. Orthopedics 2016, 39, e695–e700. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Kwon, K.Y.; Woo, J.H. Comparison of blood loss according to use of aspirin in lumbar fusion patients. Eur. Spine J. 2014, 23, 1777–1782. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.B.; Cho, K.J.; Moon, K.H.; Jung, J.H.; Jung, S.J. Does low-dose aspirin increase blood loss after spinal fusion surgery? Spine J. 2011, 11, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Glance, L.G.; Dick, A.W.; Mukamel, D.B.; Fleming, F.J.; Zollo, R.A.; Wissler, R.; Salloum, R.; Meredith, U.W.; Osler, T.M. Association between intraoperative blood transfusion and mortality and morbidity in patients undergoing noncardiac surgery. Anesthesiology 2011, 114, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Sanoufa, M.; Smisson, W.; Floyd, H.; Robinson, J.S. The effect of anaemia on hospital length of stay in lumbar decompression and fusion procedures. J. Perioper. Pract. 2015, 25, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Musallam, K.M.; Tamim, H.M.; Richards, T.; Spahn, D.R.; Rosendaal, F.R.; Habbal, A.; Khreiss, M.; Dahdaleh, F.S.; Khavandi, K.; Sfeir, P.M.; et al. Preoperative anaemia and postoperative outcomes in non-cardiac surgery: A retrospective cohort study. Lancet 2011, 378, 1396–1407. [Google Scholar] [CrossRef]
- Baron, D.M.; Hochrieser, H.; Posch, M.; Metnitz, B.; Rhodes, A.; Moreno, R.P.; Pearse, R.M.; Metnitz, P. Preoperative anaemia is associated with poor clinical outcome in non-cardiac surgery patients. Br. J. Anaesth. 2014, 113, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Li, Z.; Sheng, H.; Tan, M.; Yang, F.; Liang, L.; Zhao, J. Intraoperative blood loss, postoperative drainage, and recovery in patients undergoing lumbar spinal surgery. BMC Surg. 2015, 15, 76. [Google Scholar] [CrossRef] [PubMed]
- Kotze, A.; Carter, L.A.; Scally, A.J. Effect of a patient blood management programme on preoperative anaemia, transfusion rate, and outcome after primary hip or knee arthroplasty: A quality improvement cycle. Br. J. Anaesth. 2012, 108, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Ng, O.; Keeler, B.D.; Mishra, A.; Simpson, A.; Neal, K.; Brookes, M.J.; Acheson, A.G. Iron therapy for pre-operative anaemia. Cochrane Database Syst. Rev. 2015, 22, CD011588. [Google Scholar]
- Theusinger, O.M.; Kind, S.L.; Seifert, B.; Borgeat, L.; Gerber, C.; Spahn, D.R. Patient blood management in orthopaedic surgery: A four-year follow-up of transfusion requirements and blood loss from 2008 to 2011 at the Balgrist University Hospital in Zurich, Switzerland. Blood Transfus. 2014, 12, 195–203. [Google Scholar] [PubMed]
- Yagi, M.; Hasegawa, J.; Nagoshi, N.; Iizuka, S.; Kaneko, S.; Fukuda, K.; Takemitsu, M.; Shioda, M.; Machida, M. Does the intraoperative tranexamic acid decrease operative blood loss during posterior spinal fusion for treatment of adolescent idiopathic scoliosis? Spine 2012, 37, E1336–E1342. [Google Scholar] [CrossRef] [PubMed]
- Kushioka, J.; Yamashita, T.; Okuda, S.; Maeno, T.; Matsumoto, T.; Yamasaki, R.; Iwasaki, M. High-dose tranexamic acid reduces intraoperative and postoperative blood loss in posterior lumbar interbody fusion. J. Neurosurg. Spine 2017, 26, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, K.; Li, F.N.; Huang, X.; Li, Q.; Chen, Z.; Tang, Y.B.; Shen, H.X.; Song, Q.X. Effectiveness of tranexamic acid in reducing blood loss in spinal surgery: A meta-analysis. BMC Musculoskelet. Disord. 2014, 15, 448. [Google Scholar] [CrossRef] [PubMed]
- Zeeni, C.; Carabini, L.M.; Gould, R.W.; Bebawy, J.F.; Hemmer, L.B.; Moreland, N.C.; Koski, T.R.; Koht, A.; Schafer, M.F.; Ondra, S.L.; et al. The implementation and efficacy of the northwestern high risk spine protocol. World Neurosurg. 2014, 26, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, V.A.; Davenport, D.L.; Saha, S.P.; Austin, P.C.; Zwischenberger, J.B. Surgical outcomes and transfusion of minimal amounts of blood in the operating room. Arch. Surg. 2012, 147, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Mehra, T.; Seifert, B.; Bravo-Reiter, S.; Wanner, G.; Dutkowski, P.; Holubec, T.; Moos, R.M.; Volbracht, J.; Manz, M.G.; Spahn, D.R. Implementation of a patient blood management monitoring and feedback program significantly reduces transfusions and costs. Transfusion 2015, 55, 2807–2815. [Google Scholar] [CrossRef] [PubMed]
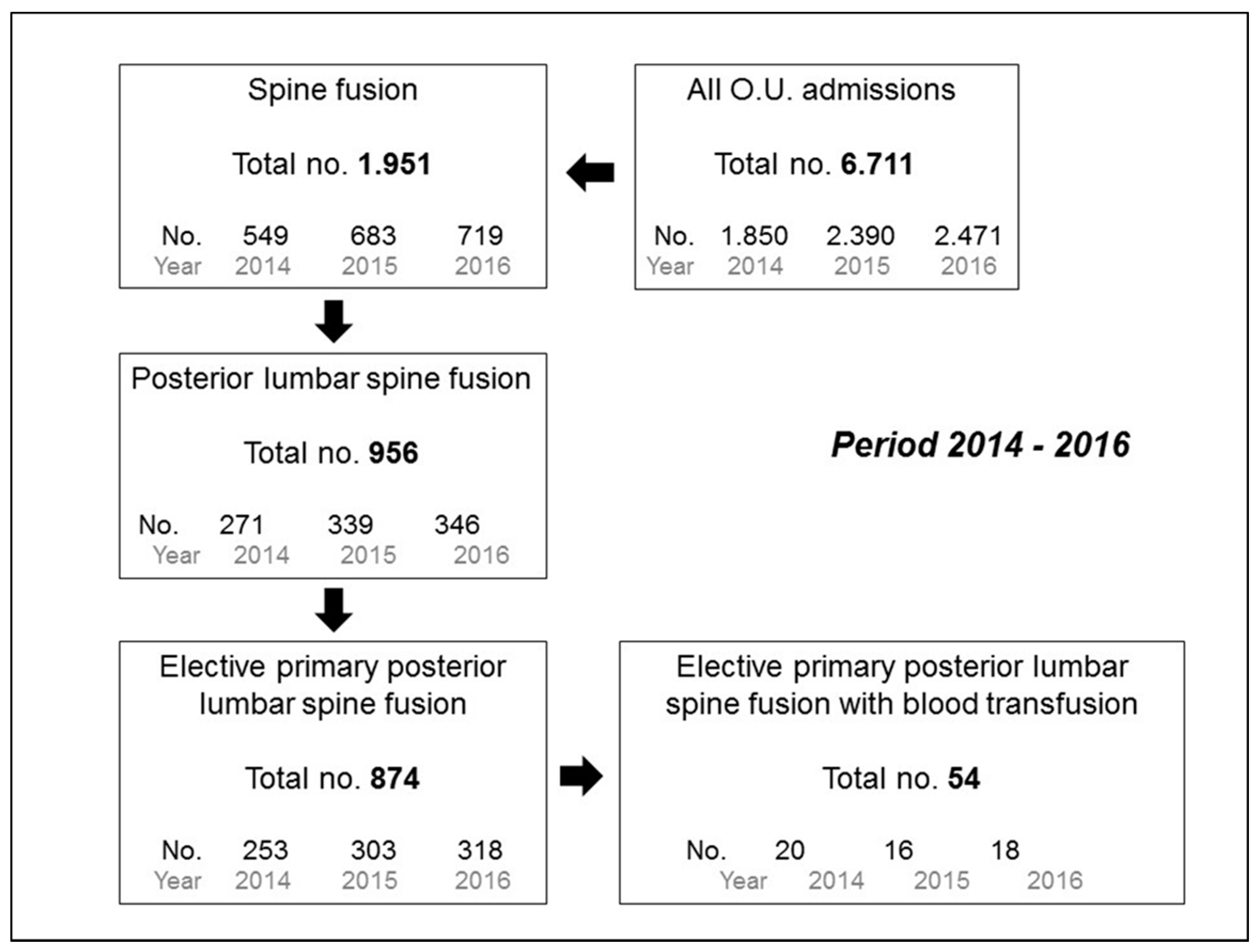
| Population, n | 874 |
|---|---|
| Age, year | 66 (56–73) |
| Male, n (%) | 333 (38) |
| BMI, kg/m2 | 26 (23–29) |
| Comorbities, n (%) | |
| Ischemic cardiomyopathy | 42 (5) |
| Arterial hypertension | 421 (49) |
| Diabetes | 82 (10) |
| Use of anticoagulant/antiplatelet drugs | 138 (16) |
| ASA, class | 2 (1–2) |
| HB pre-surgery, g/dL | 14 (13.1–14.9) |
| HCT pre-surgery, % | 42 (40–45) |
| INR pre-surgery, ratio | 1 (0.96–1.04) |
| PTT pre-surgery, ratio | 1.01 (0.95–1.07) |
| Platelets, n*103/mm3 | 232 (198–270) |
| Operative time, min | 117 (96–140) |
| HB on day 0 after surgery, g/dL | 11.6 (10.7–12.7) * |
| HB on day 1 after surgery, g/dL | 11.4 (10.4–12.4) *§ |
| HB on day 2 after surgery, g/dL | 10.3 (9.4–11.2) *§ |
| HB on day 3 after surgery, g/dL | 9.2 (8.6–10.1) *§ |
| Transfused patients, n (%) | 54 (6) |
| LOS, day | 4 (4–5) |
| Transfused (n = 54) | Not Transfused (n = 820) | p Value | |
|---|---|---|---|
| Male, n (%) | 9 (17) | 324 (40) | 0.00 |
| Age, year | 71 (63–74) | 66 (55–73) | <0.01 |
| BMI, kg/m2 | 25 (23–28) | 26 (23–29) | 0.22 |
| Comorbities, n (%) | |||
| Ischemic cardiomyopathy | 4 (8) | 38 (5) | 0.34 |
| Arterial hypertension | 29 (56) | 392 (49) | 0.34 |
| Diabetes | 11 (20) | 71 (9) | 0.01 |
| Use of anticoagulant/antiplatelet drugs | 14 (26) | 124 (16) | 0.04 |
| ASA, class | 2 (2–3) | 2 (1–2) | 0.00 |
| HB pre-surgery, g/dL | 12.7 (11.3–13.3) | 14 (13.2–14.9) | <0.01 |
| HCT pre-surgery, % | 38 (35–40) | 43 (40–45) | <0.01 |
| INR pre-surgery, ratio | 1 (0.95–1.04) | 1 (0.96–1.04) | 0.98 |
| PTT pre-surgery, ratio | 0.99 (0.94–1.06) | 1.01 (0.95–1.07) | 0.43 |
| Platelets, n*103/mm3 | 235 (203–266) | 232 (198–270) | 0.88 |
| Operative time, min | 133 (114–151) | 117 (95–139) | 0.00 |
| HB on day 0 after surgery, g/dL | 9.9 (9.2–10.8) * | 12.1 (11.2–13.1) * | <0.01 |
| Hb on day 1 after surgery, g/dL | 9.1 (8.8–9.9) *§ | 11.5 (10.6–12.4) *§ | <0.01 |
| HB on day 2 after surgery, g/dL | 8.3 (7.8–9.1) *§ | 10.4 (9.6–11.4) *§ | <0.01 |
| HB on day 3 after surgery, g/dL | 8.5 (7.9–9.6) *# | 9.4 (8.7–10.3) *§ | <0.01 |
| HB at transfusion, g/dL | 7.8 (7.4–7.9) * | N.A. | N.A. |
| RBC units transfused, n | 2 (2–2) | N.A. | N.A. |
| LOS, day | 6 (5–7) | 4 (4–5) | <0.01 |
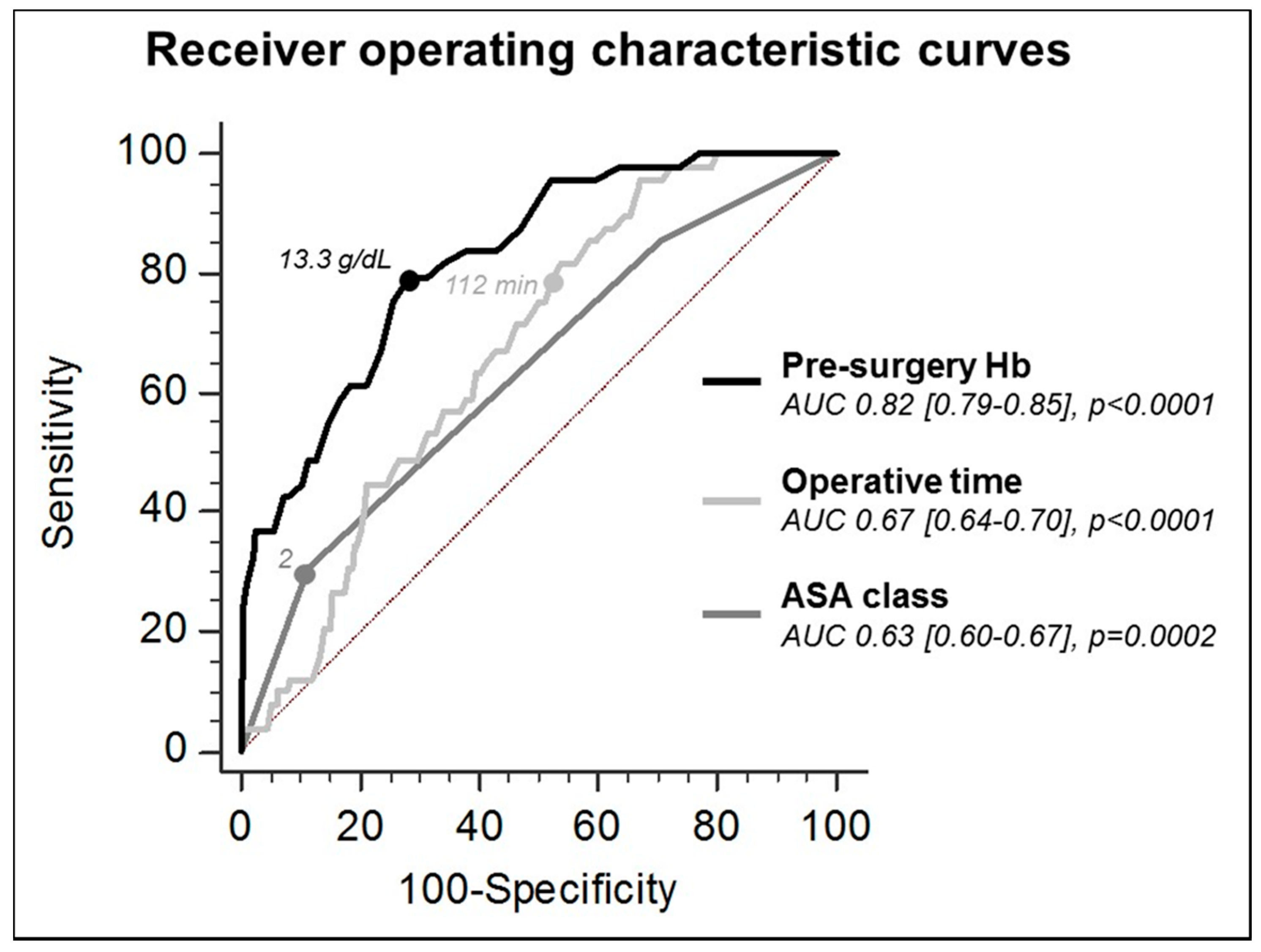
| Univariate | Multivariable | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Gender, female | 3.266 | 1.575–6.772 | 0.01 | 1.342 | 0.554–3.249 | 0.52 |
| Age | 1.037 | 1.009–1.066 | 0.01 | 1.022 | 0.986–1.059 | 0.24 |
| HB pre-surgery | 2.937 | 2.231–3.865 | <0.01 | 2.838 | 2.108–3.820 | <0.01 |
| ASA class | 2.431 | 1.527–3.870 | 0.00 | 1.773 | 1.032–3.046 | 0.04 |
| Presence of diabetes | 2.627 | 1.297–5.319 | 0.01 | 1.625 | 0.660–3.999 | 0.29 |
| Use of anticoag/platelets | 1.908 | 1.032–3.54 | 0.05 | 1.713 | 0.782–3.754 | 0.18 |
| Operative time | 1.014 | 1.007–1.021 | 0.00 | 1.016 | 1.007–1.024 | 0.00 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ristagno, G.; Beluffi, S.; Tanzi, D.; Belloli, F.; Carmagnini, P.; Croci, M.; D’Aviri, G.; Menasce, G.; Pastore, J.C.; Pellanda, A.; et al. Red Blood Cell Transfusion Need for Elective Primary Posterior Lumbar Fusion in A High-Volume Center for Spine Surgery. J. Clin. Med. 2018, 7, 19. https://doi.org/10.3390/jcm7020019
Ristagno G, Beluffi S, Tanzi D, Belloli F, Carmagnini P, Croci M, D’Aviri G, Menasce G, Pastore JC, Pellanda A, et al. Red Blood Cell Transfusion Need for Elective Primary Posterior Lumbar Fusion in A High-Volume Center for Spine Surgery. Journal of Clinical Medicine. 2018; 7(2):19. https://doi.org/10.3390/jcm7020019
Chicago/Turabian StyleRistagno, Giuseppe, Simonetta Beluffi, Dario Tanzi, Federica Belloli, Paola Carmagnini, Massimo Croci, Giuseppe D’Aviri, Guido Menasce, Juan C. Pastore, Armando Pellanda, and et al. 2018. "Red Blood Cell Transfusion Need for Elective Primary Posterior Lumbar Fusion in A High-Volume Center for Spine Surgery" Journal of Clinical Medicine 7, no. 2: 19. https://doi.org/10.3390/jcm7020019




