The Effect of Music on Exercise Intensity among Children with Autism Spectrum Disorder: A Pilot Study
Abstract
:1. Introduction
1.1. Impact of Exercise on Well-Being
1.2. Exercise Intensity
1.3. Music and Exercise
1.4. The Present Study
2. Methods
2.1. Participants
2.2. Procedures
2.3. Measures
2.3.1. Demographics
2.3.2. Maladaptive Behaviors
2.3.3. Adaptive Behavior
2.3.4. Autism Symptom Severity
2.3.5. Exercise Intensity
2.4. Analytic Plan
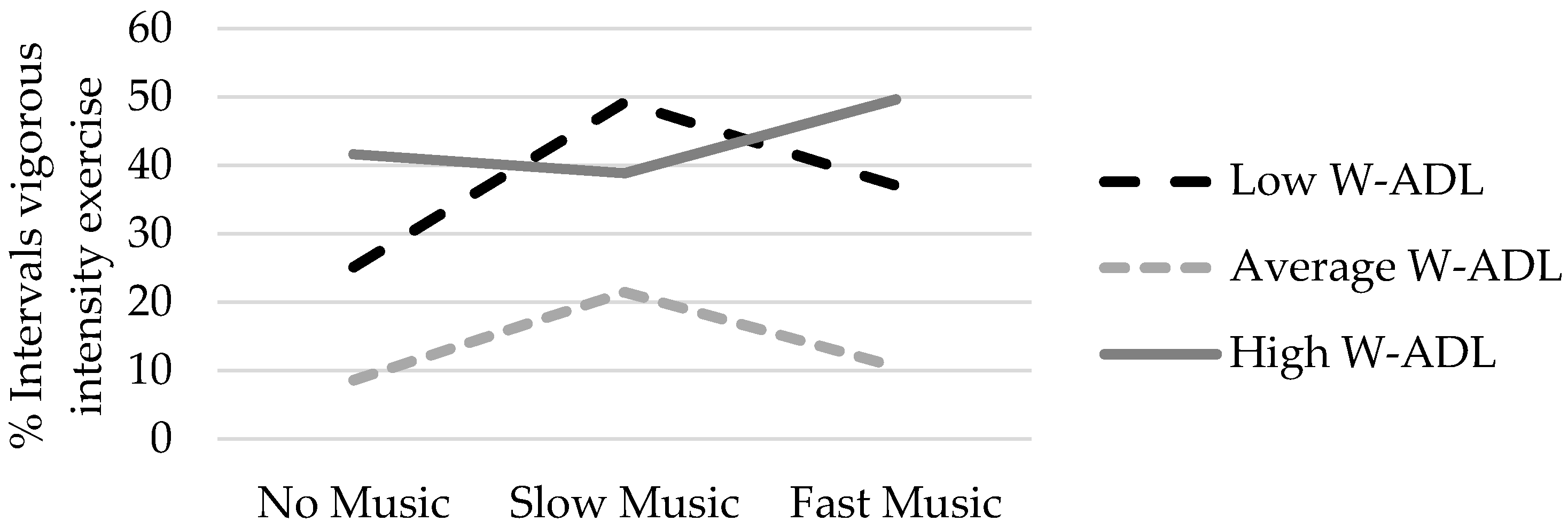
3. Results
3.1. Preliminary Analyses
3.2. Effect of Music Condition on Exercise Intensity
3.3. Student Characteristics
4. Discussion
Limitations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Centers for Disease Control and Prevention. Prevalence of autism spectrum disorders among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014, 61, 1–21. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5); American Psychiatric Association Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Matson, J.L.; Shoemaker, M. Intellectual disability and its relationship to autism spectrum disorders. J. Res. Dev. Disabil. 2009, 30, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.M.; Pescatello, L.S.; Bhat, A.N. Current perspectives on physical activity and exercise recommendations for children and adolescents with autism spectrum disorders. J. Am. Phys. Ther. Assoc. 2014, 94, 875–889. [Google Scholar] [CrossRef] [PubMed]
- Ming, X.; Brimacombe, M.; Wagner, G.C. Prevalence of motor impairment in autism spectrum disorders. Brain Dev. 2007, 29, 565–570. [Google Scholar] [CrossRef] [PubMed]
- DeLorey, T.M.; Handforth, A.; Anagnostaras, S.G.; Homanics, G.E.; Minassian, B.A.; Asatourian, A.; Fanselow, M.S.; Delgado-Escueta, A.; Ellison, G.D.; Olsen, R.W. Mice lacking the beta3 subunit of the GABAA receptor have the epilepsy phenotype and many of the behavioral characteristics of Angelman syndrome. J. Neurosci. 1998, 18, 8505–8514. [Google Scholar] [PubMed]
- Gillberg, C.; Coleman, M. The Biology of the Autistic Syndromes; MacKeith Press: London, UK, 2000. [Google Scholar]
- Nelson, K.B.; Grether, J.K.; Croen, L.A.; Dambrosia, J.M.; Dickens, B.F.; Jelliffe, L.L.; Hansen, R.L.; Phillips, T.M. Neuropeptides and neurotrophins in neonatal blood of children with autism or mental retardation. Ann. Neurol. 2001, 49, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Whitaker-Azmitia, P.M. Serotonin and brain development: Role in human developmental diseases. Brian Res. Bull. 2001, 56, 479–485. [Google Scholar] [CrossRef]
- Green, D.; Charman, T.; Pickles, A.; Chandler, S.; Loucas, T.; Simonoff, E.; Baird, G. Impairment in movement skills of children with autism spectrum disorders. Dev. Med. Child Neurol. 2008, 51, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.Y. Objectively measured physical activity between children with autism spectrum disorders and children without disabilities during inclusive recess settings in Taiwan. J. Autism Dev. Disord. 2007, 38, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans. 2008. Available online: http://www.health.gov/paguidelines (accessed on 12 January 2018).
- Rotheram-Fuller, E.; Kasari, C.; Chamberlain, B.; Locke, J. Social involvement of children with autism spectrum disorders in elementary school classrooms. J. Child Psychol. Psychiatry 2010, 51, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- King, G.; Law, M.; King, S.; Rosenbaum, P.; Kertoy, M.K.; Young, N.L. A conceptual model of the factors affecting the recreation and leisure participation of children with disabilities. Phys. Occup. Ther. Pediatr. 2003, 23, 63–90. [Google Scholar] [CrossRef] [PubMed]
- Lobstein, T.; Baur, L.; Uauy, R. Obesity in children and young people: A crisis in public health. J. Obes. Rev. 2004, 5, 4–85. [Google Scholar] [CrossRef] [PubMed]
- Fleshner, F. Physical activity and stress resistance: Sympathetic nervous system adaptations prevent stress-induced immunosuppression. Exerc. Sport Sci. Rev. 2005, 33, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Hillier, A.; Murphy, D.; Ferrara, C. A pilot study: Short-term reduction in salivary cortisol following low level physical exercise and relaxation among adolescents and young adults on the autism spectrum. Stress Health 2011, 27, 395–402. [Google Scholar] [CrossRef]
- Brand, G.; Jossen, S.; Holsboer-Trachsler, E.; Puhse, U.; Gerber, M. Impact of aerobic exercise on sleep and motor skills in children with autism spectrum disorders-a pilot study. J. Neuropsychiatr. Dis. Treat. 2015, 11, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- Petrus, C.; Adamson, S.R.; Block, L.; Einarson, S.J.; Sharifnejad, M.; Harris, S.R. Effects of exercise interventions on stereotypic behaviours in children with autism spectrum disorder. Physiother. Can. 2008, 60, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Ming, X.; Brimacombe, M.; Chaaban, J.; Zimmerman-Bier, B.; Wagner, G.C. Autism spectrum disorders; Concurrent clinical disorders. J. Child Neurol. 2008, 23, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.M.; Malow, B.A. Sleep and autism spectrum disorders. J. Pediatr. Clin. N. Am. 2011, 58, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Schreck, K.A.; Mulick, J.A.; Smith, A.F. Sleep problems as possible predictors of intensified symptoms of autism. Res. Dev. Disabil. 2004, 25, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, P.A.; Franzini, L.R.; Karen, R.L. The salutary effects of light calisthenics and relaxation training on self-stimulation in the developmentally disabled. J. Behav. Resid. Treat. 1992, 7, 15–22. [Google Scholar] [CrossRef]
- Lang, R.; Koegel, L.K.; Ashbaugh, K.; Regester, A.; Ence, W.; Smth, W. Physical exercise and individuals with autism spectrum disorders: A systematic review. J. Res. Autism Spectr. Disord. 2010, 4, 565–576. [Google Scholar] [CrossRef]
- Van Remoortel, H.; Camillo, C.A.; Langer, D.; Hornix, M.; Demeyer, H.; Burtin, C.; Decramer, M.; Gosselink, R.; Janssens, W.; Troosters, T. Moderate intense physical activity depends on selected Metabolic Equivalent of Task (MET) cut-off and type of data analysis. PLoS ONE 2013, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kern, L.; Koegel, R.L.; Dunlap, G. The influence of vigorous versus mild exercise on autistic stereotyped behaviors. J. Autism Dev. Disord. 1984, 14, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Levinson, L.J.; Reid, G. The effects of exercise intensity on the stereotypic behaviors of individuals with autism. J. Adapt. Phys. Act. Q. 1993, 10, 255–268. [Google Scholar] [CrossRef]
- Wigram, T.; Gold, C. Music therapy in the assessment and treatment of autistic spectrum disorder: Clinical application and research evidence. J. Child Care Health, Dev. 2005, 32, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Orr, T.J.; Myles, B.S.; Carlson, J.K. The impact of rhythmic entrainment on a person with autism. Focus Autism Other Dev. Disabil. 1998, 13, 163–166. [Google Scholar] [CrossRef]
- Kim, J.; Wigram, T.; Gold, C. The effects of improvisational music therapy on joint attention behaviors in autistic children: A randomized controlled study. J. Autism Dev. Disord. 2008, 38, 1758. [Google Scholar] [CrossRef] [PubMed]
- Berger, D.S. Pilot study investigating the efficacy of tempo-specific rhythm interventions in music-based treatment addressing hyper-arousal, anxiety, system pacing, and redirection of fight-or-flight fear behaviors in children with autism spectrum disorder (ASD). J. Biomusic. Eng. 2011, 2, 1–15. [Google Scholar] [CrossRef]
- Elliot, D.; Carr, S.; Orne, D. The effect of motivational music on sub-maximal exercise. Eur. J. Sport Sci. 2007, 5, 97–106. [Google Scholar] [CrossRef]
- Edworthy, J.; Waring, H. The effects of music tempo and loudness level on treadmill exercise. J. Ergon. 2007, 49, 1597–1610. [Google Scholar] [CrossRef] [PubMed]
- Becker, N.; Brett, S.; Chambliss, C.; Crowers, K.; Haring, P.; Marsh, C.; Montemayor, R. Mellow and frenetic antecedent music during athletic performance of children, adults, and seniors. J. Percept. Motor Skills 1994, 79, 1043–1046. [Google Scholar] [CrossRef] [PubMed]
- Christensen, D.L. Prevalence and characteristics of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2012. MMWR Surveill Summ. 2016, 65, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Bruininks, R.H.; Woodcock, R.W.; Weatherman, R.F.; Hill, B.K. Scales Independent Behavior-Revised; Riverside: Itasca, IL, USA, 1996. [Google Scholar]
- Maenner, M.J.; Smith, L.E.; Hong, J.; Makuch, R.; Greenberg, J.S.; Mailick, M.R. Evaluation of activities of daily living scale for adolescents and adults with developmental disabilities. Disabil. Health J. 2013, 6, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Auyeung, B.; Baron-Cohen, S.; Wheelwright, S.; Allison, C. The autism spectrum quotient: Children’s version (AQ-Child9). J. Autism Dev. Disord. 2007, 38, 1230–1240. [Google Scholar] [CrossRef] [PubMed]
- Eston, R.G.; Rowlands, A.V.; Ingledew, D.K. Validity of heart rate, pedometry, and accelerometry for predicting the energy cost of children’s activities. J. Appl. Physiol. 1998, 84, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Kalas, A. Joint attention responses of children with autism spectrum disorder to simple versus complex music. J. Music Ther. 2012, 49, 430–452. [Google Scholar] [CrossRef] [PubMed]
- Hillier, A.; Kopec, J.; Poto, N.; Tivarus, M.; Beversdorf, D.Q. Increased physiological responsiveness to preferred music among young adults with autism spectrum disorders. Psychol. Music 2016, 43, 481–492. [Google Scholar] [CrossRef]
- Alderman, B.L.; Beighle, A.; Pangrazi, R.P. Enhancing motivation in physical education. J. Phys. Educ. Recreat. Dance 2006, 77, 41–51. [Google Scholar] [CrossRef]
- Raitakari, O.T.; Porkka, K.V.; Räsänen, L.; Viikari, J.S. Relations of life-style with lipids, blood pressure and insulin in adolescents and young adults: The cardiovascular risk in young finns study. Atherosclerosis 1994, 111, 237–246. [Google Scholar] [CrossRef]
| Variable | M | (SD) | Min | Max |
|---|---|---|---|---|
| Age (years) | 9.31 | (2.25) | 5.00 | 13.00 |
| Body mass index (kg/m2) | 19.58 | (5.84) | 14.60 | 33.00 |
| Adaptive behavior (W-ADL) | 13.83 | (5.63) | 4.00 | 22.00 |
| Maladaptive behaviors (SIB-R) | 119.08 | (10.90) | 97.00 | 133.00 |
| Autism symptom severity (AQ-Child) | 86.62 | (12.35) | 59.00 | 105.00 |
| Variable | No Music | Slow Music (60–80 bpm) | Fast Music (144–160 bpm) | Repeated Measures ANOVA | |||
|---|---|---|---|---|---|---|---|
| M | (SD) | M | (SD) | M | (SD) | F | |
| Average METs b | |||||||
| Structured exercise | 7.66 | (1.91) | 8.47 a | (1.35) | 6.56 a | (1.92) | 5.66 * |
| Unstructured exercise | 4.66 a | (1.25) | 5.42 a | (1.51) | 4.74 | (1.39) | 4.14 * |
| % Time vigorous intensity | |||||||
| Structured exercise | 74.69% | (32.30) | 81.73% | (18.34) | 59.02% | (34.43) | 3.06 † |
| Unstructured exercise | 20.09% a | (18.83) | 31.92% a | (24.05) | 25.72% | (25.54) | 3.82 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woodman, A.C.; Breviglia, E.; Mori, Y.; Golden, R.; Maina, J.; Wisniewski, H. The Effect of Music on Exercise Intensity among Children with Autism Spectrum Disorder: A Pilot Study. J. Clin. Med. 2018, 7, 38. https://doi.org/10.3390/jcm7030038
Woodman AC, Breviglia E, Mori Y, Golden R, Maina J, Wisniewski H. The Effect of Music on Exercise Intensity among Children with Autism Spectrum Disorder: A Pilot Study. Journal of Clinical Medicine. 2018; 7(3):38. https://doi.org/10.3390/jcm7030038
Chicago/Turabian StyleWoodman, Ashley C., Emily Breviglia, Yumiko Mori, Rebecca Golden, John Maina, and Hannah Wisniewski. 2018. "The Effect of Music on Exercise Intensity among Children with Autism Spectrum Disorder: A Pilot Study" Journal of Clinical Medicine 7, no. 3: 38. https://doi.org/10.3390/jcm7030038




