C-Reactive Protein to Albumin Ratio Predicts 30-Day and 1-Year Mortality in Postoperative Patients after Admission to the Intensive Care Unit
Abstract
:1. Introduction
2. Materials and Methods
3. Results
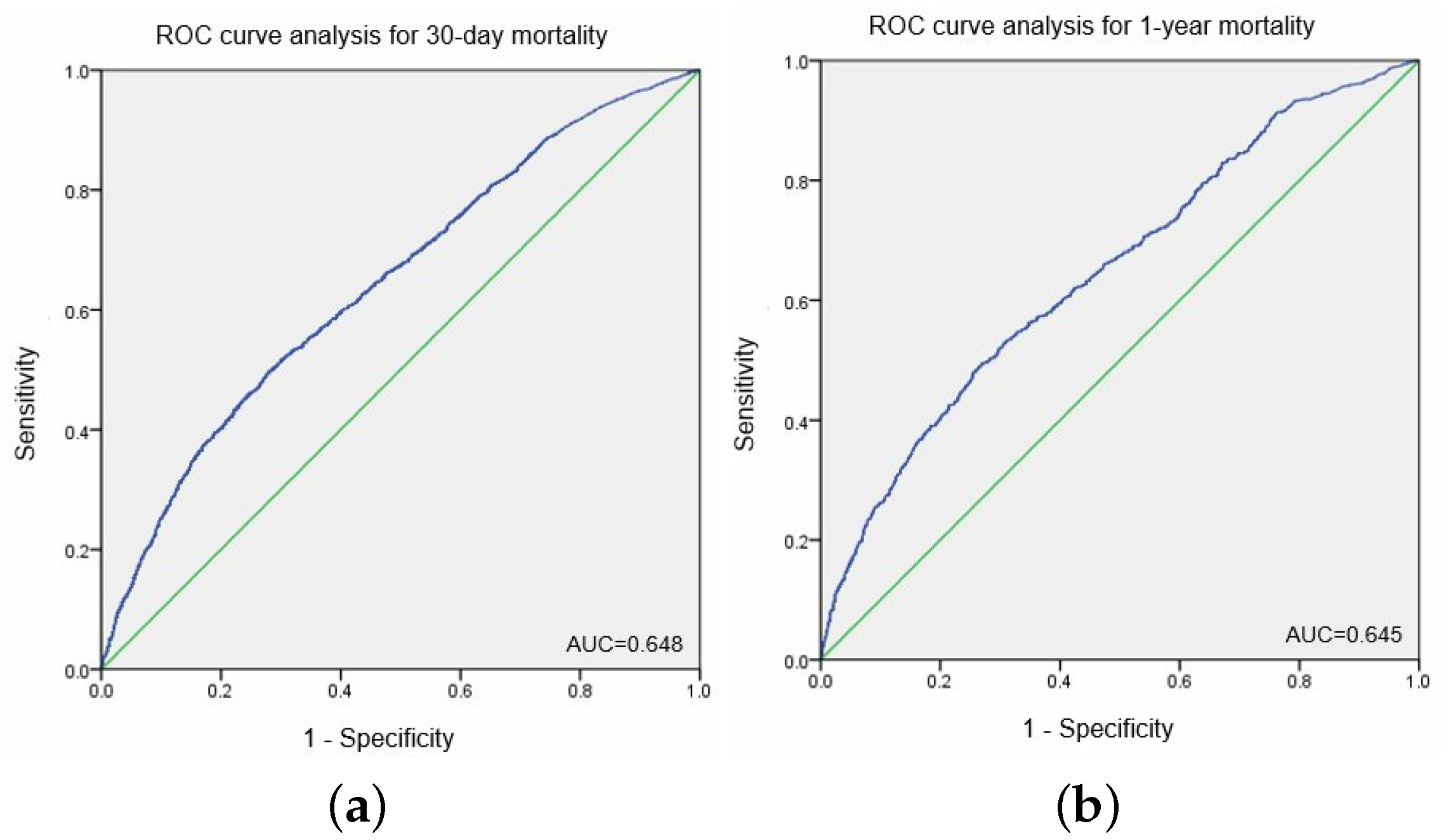
3.1. Cut-off Point of CRP/ALB Ratio for Postoperative 30-Day and 1-Year Mortality after ICU Admission
3.2. Cox Proportional Hazard Model in Relation to 30-Day and 1-Year Mortality in Postoperative Patients after Admission to the ICU
3.3. Subgroup Analysis According to Type of Surgery, Diagnosis of Cancer, and ICU Admission after Emergency Surgery for 1-Year and 30-Day Mortality
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Devran, O.; Karakurt, Z.; Adiguzel, N.; Gungor, G.; Mocin, O.Y.; Balci, M.K.; Celik, E.; Salturk, C.; Takir, H.B.; Kargin, F.; et al. C-reactive protein as a predictor of mortality in patients affected with severe sepsis in intensive care unit. Multidiscip. Respir. Med. 2012, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Quispe, E.A.; Li, X.M.; Yi, H. Comparison and relationship of thyroid hormones, il-6, il-10 and albumin as mortality predictors in case-mix critically ill patients. Cytokine 2016, 81, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Povoa, P. C-reactive protein: A valuable marker of sepsis. Intensive Care Med. 2002, 28, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Carriere, I.; Dupuy, A.M.; Lacroux, A.; Cristol, J.P.; Delcourt, C. Pathologies Oculaires Liees a l’Age Study Group. Biomarkers of inflammation and malnutrition associated with early death in healthy elderly people. J. Am. Geriatr. Soc. 2008, 56, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Dominguez de Villota, E.; Mosquera, J.M.; Rubio, J.J.; Galdos, P.; Diez Balda, V.; de la Serna, J.L.; Tomas, M.I. Association of a low serum albumin with infection and increased mortality in critically ill patients. Intensive Care Med. 1980, 7, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Ahn, J.Y.; Song, J.E.; Choi, H.; Ann, H.W.; Kim, J.K.; Kim, J.H.; Jeon, Y.D.; Kim, S.B.; Jeong, S.J.; et al. The C-reactive protein/albumin ratio as an independent predictor of mortality in patients with severe sepsis or septic shock treated with early goal-directed therapy. PLoS ONE 2015, 10, e0132109. [Google Scholar] [CrossRef] [PubMed]
- Ranzani, O.T.; Zampieri, F.G.; Forte, D.N.; Azevedo, L.C.; Park, M. C-reactive protein/albumin ratio predicts 90-day mortality of septic patients. PLoS ONE 2013, 8, e59321. [Google Scholar] [CrossRef] [PubMed]
- Siekmann, W.; Eintrei, C.; Magnuson, A.; Sjolander, A.; Matthiessen, P.; Myrelid, P.; Gupta, A. Surgical and not analgesic technique affects postoperative inflammation following colorectal cancer surgery: A prospective, randomized study. Colorectal. Dis. 2017, 19, O186–O195. [Google Scholar] [CrossRef] [PubMed]
- Arima, K.; Yamashita, Y.I.; Hashimoto, D.; Nakagawa, S.; Umezaki, N.; Yamao, T.; Tsukamoto, M.; Kitano, Y.; Yamamura, K.; Miyata, T.; et al. Clinical usefulness of postoperative C-reactive protein/albumin ratio in pancreatic ductal adenocarcinoma. Am. J. Surg. 2017. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Ge, X.; Liu, Z.; Du, S.; Ai, S.; Guan, W. Postoperative C-reactive protein/albumin ratio as a novel predictor for short-term complications following gastrectomy of gastric cancer. World J. Surg. Oncol. 2017, 15, 191. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Tian, G.W.; Wang, Y.; Zhang, H.; Wang, Z.H.; Li, G. Prognostic role of the pretreatment C-reactive protein/albumin ratio in solid cancers: A meta-analysis. Sci. Rep. 2017, 7, 41298. [Google Scholar] [CrossRef] [PubMed]
- Joen, J.S.; Ji, S.M. Diagnostic value of procalcitonin and CRP in critically ill patients admitted with suspected sepsis. J. Dent. Anesth. Pain Med. 2015, 15, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Huang, C.; Zhou, H.; Li, C.; Fan, L.; Chen, J.; Zhang, G.; Liu, Y.; Cui, Z.; Qi, D.; et al. Association between serum C-reactive protein value and prognosis of patients with non-small cell lung cancer: A meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 10633–10639. [Google Scholar] [PubMed]
- Munteanu, A.; Munteanu, D.; Tigan, S.; Bartos, A.; Iancu, C. How do surgical stress and low perioperative serum protein and albumin impact upon short term morbidity and mortality in gastric cancer surgery? Clujul. Med. 2017, 90, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, A.; Ohnishi, S.; Nakao, S.; Iwashita, Y.; Hashimoto, N.; Ishida, K.; Kondo, Y.; Ishitsuka, Y.; Irie, T. Factors affecting serum albumin in the perioperative period of colorectal surgery: A retrospective study. BMC Res. Notes 2015, 8, 638. [Google Scholar] [CrossRef] [PubMed]
- Shiota, G.; Umeki, K.; Okano, J.; Kawasaki, H. Hepatocyte growth factor and acute phase proteins in patients with chronic liver diseases. J. Med. 1995, 26, 295–308. [Google Scholar] [PubMed]
- Nakamura, N.; Hatano, E.; Iguchi, K.; Seo, S.; Taura, K.; Uemoto, S. Posthepatectomy liver failure affects long-term function after resection for hepatocellular carcinoma. World J. Surg. 2016, 40, 929–936. [Google Scholar] [CrossRef] [PubMed]

| Total (n = 11,832) | Number (Percent) | Median (IQR) |
|---|---|---|
| Gender, male | 6979 (59.0%) | - |
| Age (year) | - | 66 (53–74) |
| Body mass index (kg m–2) | - | 23.3 (20.9–25.7) |
| Operative characteristics | ||
| - Operation time (h) | - | 3.4 (1.7–5.0) |
| - Emergency operation | 1290 (10.9%) | - |
| - Surgery type | ||
| - Cardiovascular or thoracic surgery | 4642 (39.2%) | - |
| - General surgery | 2663 (22.5%) | - |
| - Neuro or spine surgery | 2134 (18.0%) | - |
| - Neuro or spine surgery | 1873 (15.8%) | - |
| - Orthopedic, OBGY, urologic surgery | 520 (4.4%) | - |
| Duration in ICU (h) | - | 23.0 (18.0–51.0) |
| Duration in hospital (day) | - | 16.0 (11.0–30.0) |
| Preoperative morbidity | ||
| - APACHE II | - | 22.0 (15.0–28.0) |
| - ASA classification | ||
| - I | 1372 (11.6%) | - |
| - II | 4723 (39.9%) | - |
| - III | 4863 (41.1%) | - |
| - IV + V + VI | 874 (7.4%) | - |
| - History of hypertension | 2588 (21.9%) | - |
| - History of ischemic heart disease | 1891 (16.0%) | - |
| - History of diabetes mellitus | 1227 (10.4%) | - |
| - Diagnosis of cancer | 3875 (32.8%) | - |
| Preoperative laboratory test results within one month | ||
| - Blood urea nitrogen (mg dL–1) | - | 14 (11–20) |
| - Creatinine (mg dL–1) | - | 0.8 (0.6–1.1) |
| - Aspartate aminotransferase (IU L–1) | - | 26 (19–44) |
| - Alanine aminotransferase (IU L–1) | - | 18 (11–30) |
| - Hemoglobin (g dL–1) | - | 11.2 (9.9–12.7) |
| - Platelet (103 μL–1) | - | 183 (133–242) |
| - White blood cell (103 μL–1) | - | 10.4 (7.5–14.1) |
| - Serum sodium (mmol L–1) | - | 139 (136–141) |
| - Serum potassium (mmol L–1) | - | 4.0 (3.7–4.3) |
| Laboratory results within 24 h after ICU admission time | ||
| - C-reactive protein (mg L–1) | - | 40.2 (6.0–88.4) |
| - Albumin (g L–1) | - | 32 (27–37) |
| - C-reactive protein/albumin ratio | - | 1.2 (0.1–4.8) |
| 1-year mortality after ICU admission | - | 2246 (19.0%) |
| 30-day mortality after ICU admission | - | 745 (6.3%) |
| Variable | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | |
| Gender: male (vs. female) | 1.05 (0.90–1.21) | 0.556 | - | - |
| Age | 1.02 (1.02–1.03) | <0.001 | 1.02 (1.01–1.02) | <0.001 |
| Body mass index (kg m–2) | 0.94 (0.92–0.95) | <0.001 | 0.97 (0.95–0.98) | <0.001 |
| - <18.5 vs. 18.5–25.0 | 1.61 (1.30–2.00) | <0.001 | 1.29 (1.03–1.61) | 0.024 |
| - >25 vs. 18.5–25.0 | 0.72 (0.60–0.86) | <0.001 | 0.84 (0.70–1.00) | 0.050 |
| Operation time (h) | 0.96 (0.93–1.00) | 0.007 | 0.96 (0.93–0.98) | 0.003 |
| Emergency operation | 1.86 (1.55–2.25) | <0.001 | 1.32 (1.08–1.62) | 0.007 |
| APACHE II | 1.10 (1.09–1.11) | <0.001 | - | - |
| ASA classification | ||||
| - I | 1 | - | 1 | - |
| - II | 0.87 (0.66–1.16) | 0.345 | 0.86 (0.64–1.15) | 0.309 |
| - III | 1.58 (1.21–2.06) | 0.001 | 1.12 (0.85–1.47) | 0.439 |
| - IV + V + VI | 3.50 (2.60–4.71) | <0.001 | 2.03 (1.50–2.76) | <0.001 |
| Blood urea nitrogen (mg dL–1) (>20 vs. ≤20) | 3.66 (3.17–4.22) | <0.001 | 1.55 (1.29–1.87) | <0.001 |
| CRP (mg L–1) (>10 vs. ≤10) | 2.19 (1.80–2.66) | <0.001 | - | - |
| Creatinine (mg dL–1) (>1.3 vs. ≤1.3) | 3.94 (3.40–4.56) | <0.001 | 1.72 (1.43–2.08) | <0.001 |
| AST (IU L–1) (>40 vs. ≤40) | 2.59 (2.25–3.00) | <0.001 | 1.56 (1.30–1.88) | <0.001 |
| ALT (IU L–1) (>40 vs. ≤40) | 2.25 (1.93–2.63) | <0.001 | 1.38 (1.13–1.68) | 0.001 |
| Hemoglobin (g dL–1) (<7 vs. ≥7) | 4.62 (3.28–6.52) | <0.001 | 1.96 (1.33–2.89) | 0.001 |
| Platelet count (103 uL–1) (<100 vs. ≥100) | 3.15 (2.69–3.69) | <0.001 | 2.10 (1.76–2.49) | <0.001 |
| White blood cell (103 uL–1) | ||||
| - <4 vs. 4–10 | 2.93 (2.23–3.85) | <0.001 | 1.80 (1.35–2.41) | <0.001 |
| - >10 vs. 4–10 | 1.21 (1.04–1.41) | 0.016 | 1.13 (0.96–1.33) | 0.132 |
| Serum sodium (mmol L–1) | ||||
| - <135 vs. 135–145 | 2.58 (2.18–3.05) | <0.001 | 1.67 (1.39–2.00) | <0.001 |
| - >145 vs. 135–145 | 4.55 (3.69–5.61) | <0.001 | 2.54 (2.02–3.19) | <0.001 |
| Serum potassium (mEq L–1) | ||||
| - <3.5 vs. 3.5–5 | 1.80 (1.49–2.18) | <0.001 | 1.33 (1.09–1.63) | 0.005 |
| - >5 vs. 3.5–5 | 4.34 (3.44–5.47) | <0.001 | 2.22 (1.73–2.85) | <0.001 |
| History of hypertension | 1.54 (1.26–1.87) | <0.001 | 1.15 (0.90–1.46) | 0.258 |
| History of diabetes mellitus | 1.47 (1.12–1.93) | 0.006 | 1.24 (0.90–1.70) | 0.184 |
| History of ischemic heart disease | 1.58 (1.25–1.99) | <0.001 | 1.57 (1.20–2.04) | 0.001 |
| Diagnosis of cancer | 1.00 (0.86–1.17) | 0.972 | - | - |
| CRP/albumin (dichotomized) | ||||
| - ≤1.75 | 1 | - | 1 | - |
| - >1.75 | 2.14 (1.85–2.47) | <0.001 | 1.30 (1.11–1.53) | 0.001 |
| CRP/albumin (continuous) * | 1.08 (1.01–1.08) | <0.001 | 1.07 (1.05–1.09) | <0.001 |
| Variable | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | |
| Gender: male (vs. female) | 1.23 (1.13–1.34) | <0.001 | 1.07 (0.98–1.15) | 0.153 |
| Age | 1.03 (1.02–1.03) | <0.001 | 1.02 (1.02–1.03) | <0.001 |
| Body mass index (kg m–2) | ||||
| - <18.5 vs. 18.5–25.0 | 2.10 (1.87–2.35) | <0.001 | 1.74 (1.54–1.95) | <0.001 |
| - >25 vs. 18.5–25.0 | 0.60 (0.54–0.67) | <0.001 | 0.71 (0.64–0.79) | <0.001 |
| Operation time (h) | 0.99 (0.97–1.01) | 0.232 | - | - |
| Emergency operation | 1.33 (1.18–1.51) | <0.001 | 1.16 (1.13–1.21) | 0.009 |
| APACHE II | 1.05 (1.04–1.06) | <0.001 | - | - |
| ASA classification | ||||
| - I | 1 | - | 1 | - |
| - II | 1.08 (0.93–1.27) | 0.324 | 0.94 (0.80–1.10) | 0.437 |
| - III | 1.58 (1.36–1.84) | <0.001 | 1.26 (1.07–1.47) | 0.005 |
| - IV + V + VI | 2.50 (2.08–3.00) | <0.001 | 1.86 (1.54–2.25) | <0.001 |
| Blood urea nitrogen (mg dL–1) (>20 vs. ≤20) | 2.75 (2.53–2.99) | <0.001 | 1.62 (1.45–1.79) | <0.001 |
| CRP (mg L–1) (>10 vs. ≤10) | 2.00 (1.80–2.23) | <0.001 | - | - |
| Creatinine (mg dL–1) (>1.3 vs. ≤1.3) | 2.57 (2.35–2.81) | <0.001 | 1.34 (1.19–1.49) | <0.001 |
| AST (IU L–1) (>40 vs. ≤40) | 1.57 (1.44–1.71) | <0.001 | 1.18 (1.05–1.31) | 0.005 |
| ALT (IU L–1) (>40 vs. ≤40) | 1.58 (1.43–1.74) | <0.001 | 1.17 (1.04–1.33) | 0.012 |
| Hemoglobin (g dL–1) (<7 vs. ≥7) | 2.74 (2.11–3.57) | <0.001 | 1.56 (1.17–2.07) | 0.002 |
| Platelet count (103 uL–1) (<100 vs. ≥100) | 1.87 (1.68–2.08) | <0.001 | 1.53 (1.37–1.71) | <0.001 |
| White blood cell (103 uL–1) | ||||
| - <4 vs. 4–10 | 2.27 (1.90–2.70) | <0.001 | 1.42 (1.18–1.71) | <0.001 |
| - >10 vs. 4–10 | 1.13 (1.04–1.23) | 0.006 | 1.07 (0.98–1.17) | 0.120 |
| Serum sodium (mmol L–1) | ||||
| - <135 vs. 135–145 | 2.43 (2.20–2.67) | <0.001 | 1.68 (1.52–1.86) | <0.001 |
| - >145 vs. 135–145 | 2.37 (2.03–2.77) | <0.001 | 1.90 (1.62–2.24) | <0.001 |
| Serum potassium (mEq L–1) | ||||
| - <3.5 vs. 3.5–5 | 1.52 (1.36–1.71) | <0.001 | 1.37 (1.21–1.54) | <0.001 |
| - >5 vs. 3.5–5 | 2.91 (2.47–3.41) | <0.001 | 1.63 (1.37–1.93) | <0.001 |
| History of hypertension | 1.39 (1.25–1.56) | <0.001 | 1.24 (1.10–1.40) | <0.001 |
| History of diabetes mellitus | 1.10 (0.95–1.26) | 0.194 | - | - |
| History of ischemic heart disease | 1.56 (1.37–1.78) | <0.001 | 1.38 (1.19–1.60) | <0.001 |
| Diagnosis of cancer | 1.43 (1.37–1.49) | <0.001 | 1.47 (1.41–1.54) | <0.001 |
| CRP/albumin (dichotomized) | ||||
| - ≤1.58 | 1 | - | 1 | - |
| - >1.58 | 2.06 (1.90–2.25) | <0.001 | 1.49 (1.36–1.62) | <0.001 |
| CRP/albumin (continuous) * | 1.08 (1.08–1.09) | <0.001 | 1.08 (1.07–1.09) | <0.001 |
| CRP/Albumin for 30-Day Mortality | CRP/Albumin for 1-Year Mortality | |||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-Value * | Hazard Ratio (95% CI) | p-Value * | |
| Cardiovascular or thoracic surgery (n = 4642) | 1.07 (1.03–1.12) | <0.001 | 1.11 (1.08–1.13) | <0.001 |
| General surgery (n = 2663) | 1.02 (0.98–1.08) | 0.336 | 1.07 (1.05–1.10) | <0.001 |
| Neuro or spine surgery (n = 2134) | 1.08 (0.99–1.18) | 0.082 | 1.09 (1.03–1.16) | 0.003 |
| Orthopedic, OBGY, urologic surgery (n = 1873) | 1.17 (1.10–1.24) | <0.001 | 1.10 (1.06–1.14) | <0.001 |
| Plastic, OPH, ENT, DT, procedures (n = 520) | 1.11 (1.04–1.18) | 0.001 | 1.05 (1.00–1.09) | 0.036 |
| Diagnosis of cancer (n = 3875) | 1.10 (1.06–1.14) | <0.001 | 1.08 (1.06–1.10) | <0.001 |
| ICU admission after emergency surgery (n = 1290) | 1.02 (0.96–1.09) | 0.474 | 1.05 (1.02–1.10) | 0.006 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, T.K.; Ji, E.; Na, H.-s.; Min, B.; Jeon, Y.-T.; Do, S.-H.; Song, I.-A.; Park, H.-P.; Hwang, J.-W. C-Reactive Protein to Albumin Ratio Predicts 30-Day and 1-Year Mortality in Postoperative Patients after Admission to the Intensive Care Unit. J. Clin. Med. 2018, 7, 39. https://doi.org/10.3390/jcm7030039
Oh TK, Ji E, Na H-s, Min B, Jeon Y-T, Do S-H, Song I-A, Park H-P, Hwang J-W. C-Reactive Protein to Albumin Ratio Predicts 30-Day and 1-Year Mortality in Postoperative Patients after Admission to the Intensive Care Unit. Journal of Clinical Medicine. 2018; 7(3):39. https://doi.org/10.3390/jcm7030039
Chicago/Turabian StyleOh, Tak Kyu, Eunjeong Ji, Hyo-seok Na, Byunghun Min, Young-Tae Jeon, Sang-Hwan Do, In-Ae Song, Hee-Pyoung Park, and Jung-Won Hwang. 2018. "C-Reactive Protein to Albumin Ratio Predicts 30-Day and 1-Year Mortality in Postoperative Patients after Admission to the Intensive Care Unit" Journal of Clinical Medicine 7, no. 3: 39. https://doi.org/10.3390/jcm7030039





