Long-Term Increase of Radiographic Damage and Disability in Patients with RA in Relation to Disease Duration in the Era of Biologics. Results from the SCQM Cohort
Abstract
:1. Introduction
2. Methods
2.1. Study Population and Design
2.2. Exposure of Interest
2.3. Inclusion/Exclusion Criteria
2.4. Outcome Parameters
2.5. Statistical Analysis
3. Results
3.1. Patients
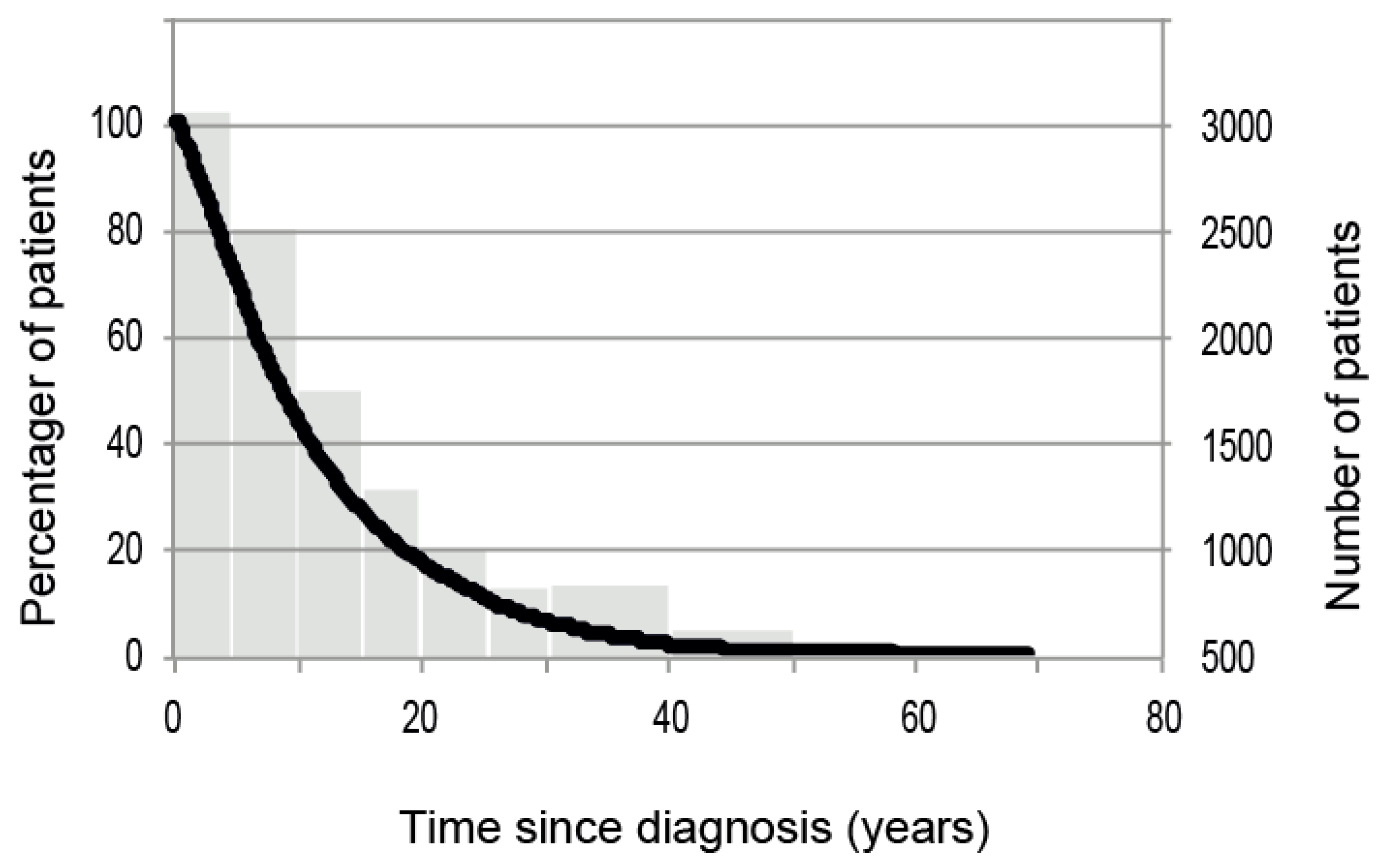
3.2. Distribution of Disease Duration
3.3. Demographical Data
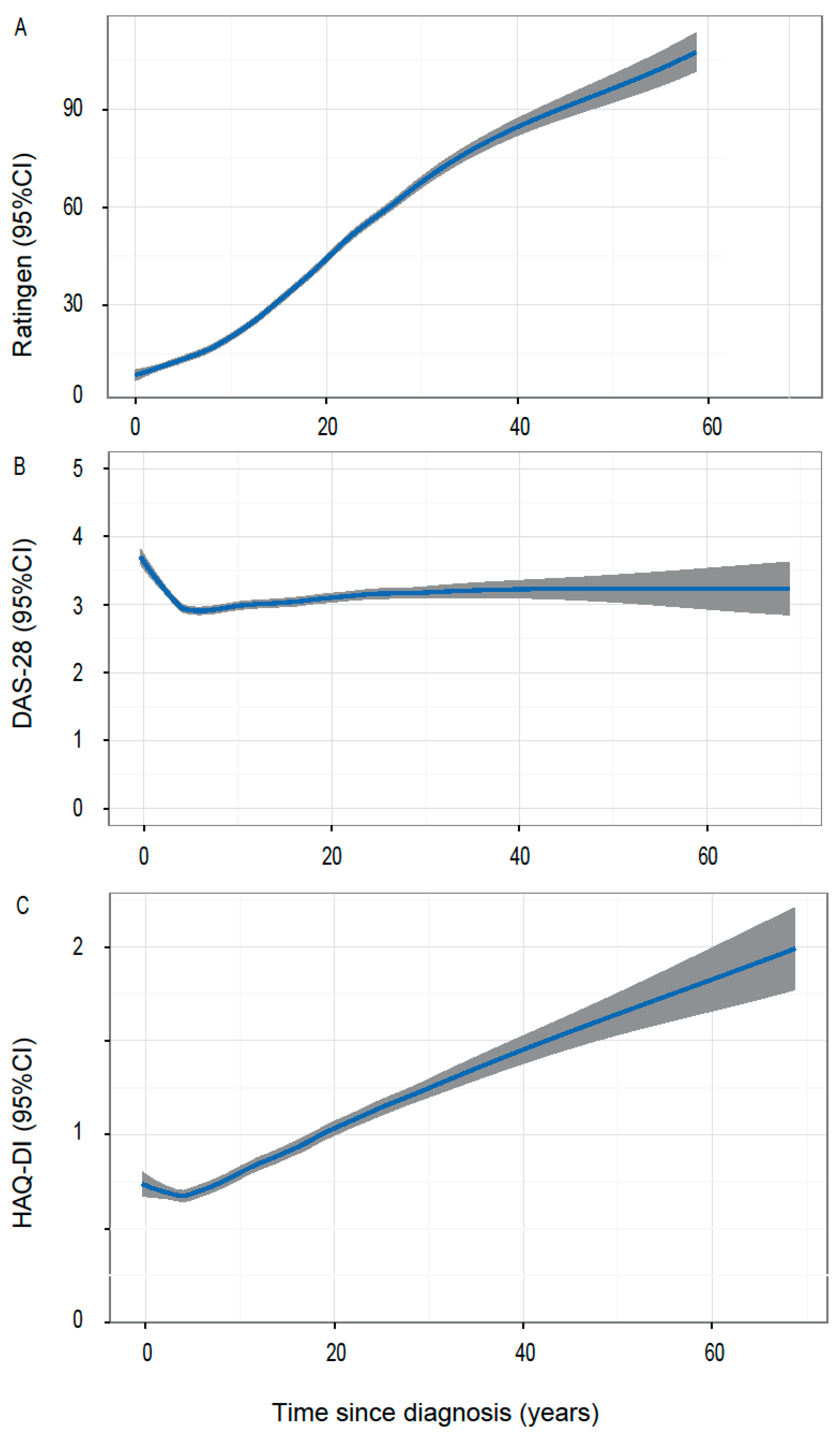
3.4. Primary Outcome
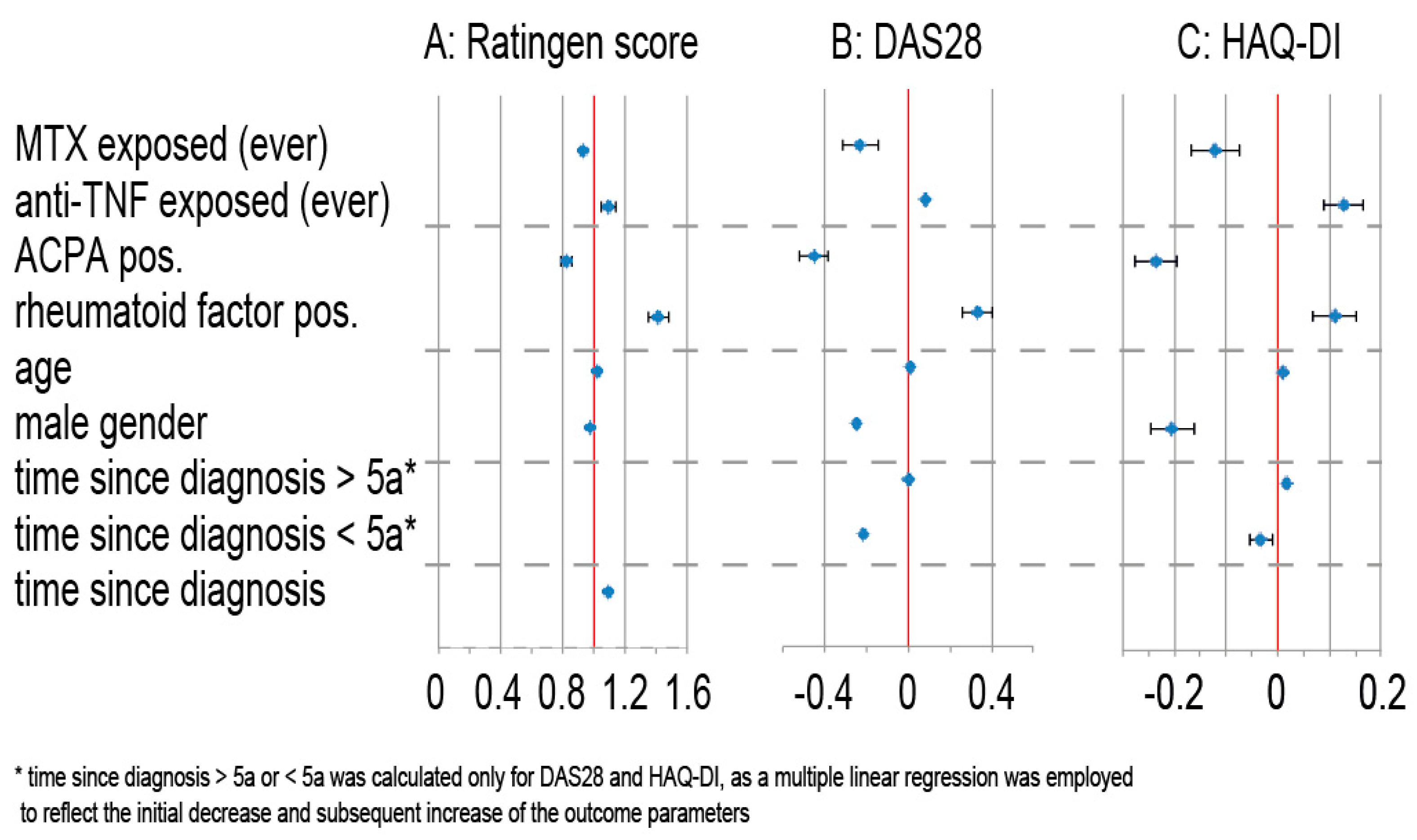
3.5. Secondary Outcomes
4. Discussion
4.1. The Extent of Radiographic Damage has Decreased in Comparison to Earlier Analyses
4.2. Radiographic Destruction and Use of TNF Antagonists
4.3. ACPA Positivity Associated with Milder Disease Course
4.4. Disconnect between Disability and Radiographic Destruction
4.5. Extra-Articular Manifestations Increase in Parallel to Disease Duration
4.6. Strengths and Limitations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wolfe, F.; Sharp, J.T. Radiographic outcome of recent-onset rheumatoid arthritis: A 19-year study of radiographic progression. Arthritis Rheumatol. 1998, 41, 1571–1582. [Google Scholar] [CrossRef]
- Mau, W.; Bornmann, M.; Weber, H.; Weidemann, H.F.; Hecker, H.; Raspe, H.H. Prediction of permanent work disability in a follow-up study of early rheumatoid arthritis: Results of a tree structured analysis using RECPAM. Br. J. Rheumatol. 1996, 35, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Hawley, D.J. The longterm outcomes of rheumatoid arthritis: Work disability: A prospective 18 year study of 823 patients. J. Rheumatol. 1998, 25, 2108–2117. [Google Scholar] [PubMed]
- Gordon, D.A.; Stein, J.L.; Broder, I. The extra-articular features of rheumatoid arthritis. A systematic analysis of 127 cases. Am. J. Med. 1973, 54, 445–452. [Google Scholar] [CrossRef]
- Jäntti, J.; Aho, K.; Kaarela, K.; Kautiainen, H. Work disability in an inception cohort of patients with seropositive rheumatoid arthritis: A 20 year study. Rheumatol. Oxf. Engl. 1999, 38, 1138–1141. [Google Scholar] [CrossRef]
- Sokka, T.; Kautiainen, H.; Pincus, T.; Verstappen, S.M.; Aggarwal, A.; Alten, R.; Andersone, D.; Badsha, H.; Baecklund, E.; Belmonte, M.; et al. Work disability remains a major problem in rheumatoid arthritis in the 2000s: Data from 32 countries in the QUEST-RA study. Arthritis Res. Ther. 2010, 12, R42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verstappen, S.M.M.; Bijlsma, J.W.J.; Verkleij, H.; Buskens, E.; Blaauw, A.A.M.; Ter Borg, E.J.; Jacobs, J.W.G. Overview of work disability in rheumatoid arthritis patients as observed in cross-sectional and longitudinal surveys. Arthritis Rheumatol. 2004, 51, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.L. Radiological progression in established rheumatoid arthritis. J. Rheumatol. Suppl. 2004, 69, 55–65. [Google Scholar] [PubMed]
- Siegmeth, W.; Eberl, R.; Tausch, G.; Raith, W. Comparative study of the effect of the antimetabolic agent methotrexate in psoriasis, psoriatic arthropathy and rheumatoid arthritis. Verh. Dtsch. Ges. Rheumatol. 1969, 1, 383–387. [Google Scholar] [PubMed]
- Leirisalo-Repo, M.; Kautiainen, H.; Laasonen, L.; Korpela, M.; Kauppi, M.J.; Kaipiainen-Seppanen, O.; Luosujarvi, R.; Luukkainen, R.; Karjalainen, A.; Blafield, H.; et al. Infliximab for 6 months added on combination therapy in early rheumatoid arthritis: 2-year results from an investigator-initiated, randomised, double-blind, placebo-controlled study (the NEO-RACo Study). Ann. Rheum. Dis. 2013, 72, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Grigor, C.; Capell, H.; Stirling, A.; McMahon, A.D.; Lock, P.; Vallance, R.; Porter, D.; Kincaid, W. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): A single-blind randomised controlled trial. Lancet Lond. Engl. 2004, 364, 263–269. [Google Scholar] [CrossRef]
- Maini, R.; St Clair, E.W.; Breedveld, F.; Furst, D.; Kalden, J.; Weisman, M.; Smolen, J.; Emery, P.; Harriman, G.; Feldmann, M.; et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: A randomised phase III trial. ATTRACT Study Group. Lancet Lond. Engl. 1999, 354, 1932–1939. [Google Scholar] [CrossRef]
- Smolen, J.S.; Breedveld, F.C.; Burmester, G.R.; Bykerk, V.; Dougados, M.; Emery, P.; Kvien, T.K.; Navarro-Compan, M.V.; Oliver, S.; Schoels, M.; et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann. Rheum. Dis. 2016, 75, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.B.; Emery, P.; Greenwald, M.W.; Dougados, M.; Furie, R.A.; Genovese, M.C.; Keystone, E.C.; Loveless, J.E.; Burmester, G.-R.; Cravets, M.W.; et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheumatol. 2006, 54, 2793–2806. [Google Scholar] [CrossRef] [PubMed]
- Kremer, J.M.; Genant, H.K.; Moreland, L.W.; Russell, A.S.; Emery, P.; Abud-Mendoza, C.; Szechinski, J.; Li, T.; Ge, Z.; Becker, J.-C.; et al. Effects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis: A randomized trial. Ann. Intern. Med. 2006, 144, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, N.; Hashimoto, J.; Miyasaka, N.; Yamamoto, K.; Kawai, S.; Takeuchi, T.; Murata, N.; van der Heijde, D.; Kishimoto, T. Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): Evidence of clinical and radiographic benefit from an X ray reader-blinded randomised controlled trial of tocilizumab. Ann. Rheum. Dis. 2007, 66, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Kievit, W.; Fransen, J.; Malefijt, M.C.D.; den Broeder, A.A.; van Riel, P.L.C.M. Treatment changes and improved outcomes in RA: An overview of a large inception cohort from 1989 to 2009. Rheumatol. Oxf. Engl. 2013, 52, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Filippini, M.; Bazzani, C.; Atzeni, F.; Puttini, P.S.; Marchesoni, A.; Favalli, E.G.; Caporali, R.; Cavagna, L.; Gorla, R. Effects of anti-TNF alpha drugs on disability in patients with rheumatoid arthritis: Long-term real-life data from the Lorhen Registry. BioMed Res. Int. 2014, 2014, 416892. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Allaire, S.; Michaud, K. The prevalence and incidence of work disability in rheumatoid arthritis, and the effect of anti-tumor necrosis factor on work disability. J. Rheumatol. 2007, 34, 2211–2217. [Google Scholar] [PubMed]
- Sokka, T.; Kautiainen, H.; Häkkinen, A.; Hannonen, P. Radiographic progression is getting milder in patients with early rheumatoid arthritis. Results of 3 cohorts over 5 years. J. Rheumatol. 2004, 31, 1073–1082. [Google Scholar] [PubMed]
- Heikkilä, S.; Isomäki, H. Long-term outcome of rheumatoid arthritis has improved. Scand. J. Rheumatol. 1994, 23, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Finckh, A.; Choi, H.K.; Wolfe, F. Progression of radiographic joint damage in different eras: Trends towards milder disease in rheumatoid arthritis are attributable to improved treatment. Ann. Rheum. Dis. 2006, 65, 1192–1197. [Google Scholar] [CrossRef] [PubMed]
- Rau, R.; Wassenberg, S.; Herborn, G.; Stucki, G.; Gebler, A. A new method of scoring radiographic change in rheumatoid arthritis. J. Rheumatol. 1998, 25, 2094–2107. [Google Scholar] [PubMed]
- Uitz, E.; Fransen, J.; Langenegger, T.; Stucki, G. Clinical quality management in rheumatoid arthritis: Putting theory into practice. Swiss Clinical Quality Management in Rheumatoid Arthritis. Rheumatol. Oxf. Engl. 2000, 39, 542–549. [Google Scholar] [CrossRef]
- Van Gestel, A.M.; Haagsma, C.J.; van Riel, P.L. Validation of rheumatoid arthritis improvement criteria that include simplified joint counts. Arthritis Rheumatol. 1998, 41, 1845–1850. [Google Scholar] [CrossRef]
- Fries, J.F.; Spitz, P.; Kraines, R.G.; Holman, H.R. Measurement of patient outcome in arthritis. Arthritis Rheumatol. 1980, 23, 137–145. [Google Scholar] [CrossRef]
- Abasolo, L.; Ivorra-Cortes, J.; Leon, L.; Jover, J.A.; Fernandez-Gutierrez, B.; Rodriguez-Rodriguez, L. Influence of demographic and clinical factors on the mortality rate of a rheumatoid arthritis cohort: A 20-year survival study. Semin. Arthritis Rheumatol. 2016, 45, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Landewé, R.; van der Heijde, D.; Klareskog, L.; van Vollenhoven, R.; Fatenejad, S. Disconnect between inflammation and joint destruction after treatment with etanercept plus methotrexate: Results from the trial of etanercept and methotrexate with radiographic and patient outcomes. Arthritis Rheumatol. 2006, 54, 3119–3125. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Avila, J.C.M.; Aletaha, D. Tocilizumab inhibits progression of joint damage in rheumatoid arthritis irrespective of its anti-inflammatory effects: Disassociation of the link between inflammation and destruction. Ann. Rheum. Dis. 2012, 71, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Van der Helm-van Mil, A.H.; Verpoort, K.N.; Breedveld, F.C.; Toes, R.E.; Huizinga, T.W. Antibodies to citrullinated proteins and differences in clinical progression of rheumatoid arthritis. Arthritis Res. Ther. 2005, 7, R949–R958. [Google Scholar] [CrossRef] [PubMed]
- Forslind, K.; Ahlmen, M.; Eberhardt, K.; Hafstrom, I.; Svensson, B. Prediction of radiological outcome in early rheumatoid arthritis in clinical practice: Role of antibodies to citrullinated peptides (anti-CCP). Ann. Rheum. Dis. 2004, 63, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Alasti, F.; Smolen, J.S. Rheumatoid factor, not antibodies against citrullinated proteins, is associated with baseline disease activity in rheumatoid arthritis clinical trials. Arthritis Res. Ther. 2015, 17, 229. [Google Scholar] [CrossRef] [PubMed]
- Sokka, T.; Kankainen, A.; Hannonen, P. Scores for functional disability in patients with rheumatoid arthritis are correlated at higher levels with pain scores than with radiographic scores. Arthritis Rheumatol. 2000, 43, 386–389. [Google Scholar] [CrossRef]
- Molenaar, E.T.H.; Voskuyl, A.E.; Dijkmans, B.A.C. Functional disability in relation to radiological damage and disease activity in patients with rheumatoid arthritis in remission. J. Rheumatol. 2002, 29, 267–270. [Google Scholar] [PubMed]
- Bombardier, C.; Barbieri, M.; Parthan, A.; Zack, D.J.; Walker, V.; Macarios, D.; Smolen, J.S. The relationship between joint damage and functional disability in rheumatoid arthritis: A systematic review. Ann. Rheum. Dis. 2012, 71, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Aoki, Y.; Terayama, K.; Sonobe, M.; Takahashi, H.; Saito, M.; Nakagawa, K. Health assessment questionnaire-disability index (HAQ-DI) score at the start of biological disease-modifying antirheumatic drug (bDMARD) therapy is associated with radiographic progression of large joint damage in patients with rheumatoid arthritis. Mod. Rheumatol. 2017, 27, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekara, S.; Shobha, V.; Dharmanand, B.G.; Jois, R.; Kumar, S.; Mahendranath, K.M.; Haridas, V.; Prasad, S.; Singh, Y.; Daware, M.A.; et al. Reduced incidence of extra-articular manifestations of RA through effective disease control: Karnataka Rheumatoid Arthritis Comorbidity (KRAC) study. Int. J. Rheum. Dis. 2017, 20, 1694–1703. [Google Scholar] [CrossRef] [PubMed]
- Nikiphorou, E.; Norton, S.; Carpenter, L.; Dixey, J.; Walsh, D.A.; Kiely, P.; Young, A. Secular Changes in Clinical Features at Presentation of Rheumatoid Arthritis: Increase in Comorbidity but Improved Inflammatory States. Arthritis Care Res. 2017, 69, 21–27. [Google Scholar] [CrossRef] [PubMed]

| Disease Duration (Years) | Number (n) | Age (Years, Mean) | Female * (%) | BMI * (kg/m2, Mean) | Pos. Fam. History * (%) | RF Pos. * (%) | ACPA Pos. * (%) | Rheumatoid Nodules * (%) | Extra-art. Manifest * (%) | MTX Pre-exposed * (%) | TNF Pre-exposed * (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| all | 7850 | 59.9 | 74.4 | 26.0 | 20.6 | 70.8 | 62.2 | 22.7 | 72.9 | 81.9 | 57.7 |
| <5 | 2535 | 57.0 | 69.2 | 26.2 | 15.0 | 60.8 | 54.4 | 7.1 | 52.3 | 83.1 | 42.4 |
| ≥5–10 | 1990 | 58.8 | 74.2 | 26.4 | 21.5 | 69.6 | 63.9 | 15.6 | 61.8 | 84.1 | 61.8 |
| ≥10–15 | 1250 | 60.8 | 76.7 | 26.2 | 22.1 | 75.6 | 66.5 | 26.3 | 81.1 | 83.5 | 65.2 |
| ≥15–20 | 788 | 62.2 | 77.0 | 25.7 | 25.4 | 79.3 | 64.0 | 36.3 | 84.5 | 81.2 | 69.3 |
| ≥20–25 | 500 | 63.3 | 79.2 | 25.5 | 25.6 | 81.1 | 73.2 | 41.1 | 85.7 | 75.1 | 67.2 |
| ≥25–30 | 325 | 65.3 | 81.8 | 24.8 | 24.3 | 85.5 | 74.7 | 51.5 | 86.3 | 76.3 | 67.8 |
| ≥30–40 | 333 | 66.5 | 82.9 | 25.2 | 26.7 | 79.6 | 70.2 | 50.8 | 91.0 | 77.0 | 64.4 |
| ≥40 | 129 | 71.5 | 86.0 | 24.9 | 31.0 | 84.3 | 69.4 | 56.7 | 66.7 | 66.7 | 62.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heinimann, K.; Von Kempis, J.; Sauter, R.; Schiff, M.; Sokka-Isler, T.; Schulze-Koops, H.; Müller, R. Long-Term Increase of Radiographic Damage and Disability in Patients with RA in Relation to Disease Duration in the Era of Biologics. Results from the SCQM Cohort. J. Clin. Med. 2018, 7, 57. https://doi.org/10.3390/jcm7030057
Heinimann K, Von Kempis J, Sauter R, Schiff M, Sokka-Isler T, Schulze-Koops H, Müller R. Long-Term Increase of Radiographic Damage and Disability in Patients with RA in Relation to Disease Duration in the Era of Biologics. Results from the SCQM Cohort. Journal of Clinical Medicine. 2018; 7(3):57. https://doi.org/10.3390/jcm7030057
Chicago/Turabian StyleHeinimann, Katja, Johannes Von Kempis, Rafael Sauter, Michael Schiff, Tuulikki Sokka-Isler, Hendrik Schulze-Koops, and Rüdiger Müller. 2018. "Long-Term Increase of Radiographic Damage and Disability in Patients with RA in Relation to Disease Duration in the Era of Biologics. Results from the SCQM Cohort" Journal of Clinical Medicine 7, no. 3: 57. https://doi.org/10.3390/jcm7030057




