Multimodal Assessment of Recurrent mTBI across the Lifespan
Abstract
:1. Introduction
2. Experimental Section
2.1. Participants
2.2. Study Outline
2.3. Cognitive Testing
Verbal Episodic Memory
Processing Speed
Verbal Fluency
Visuospatial Skills
Working Memory
Statistical Analysis of Cognitive Data
2.4. Magnetic Resonance Imaging (MRI)
2.4.1. Data Acquisition
2.4.2. Voxel-Based Morphometry
2.4.3. Tract-Based Spatial Statistics and Probabilistic Tractography
2.4.4. Analysis of RSFC Data
3. Results
3.1. Group Differences in Cognitive Data
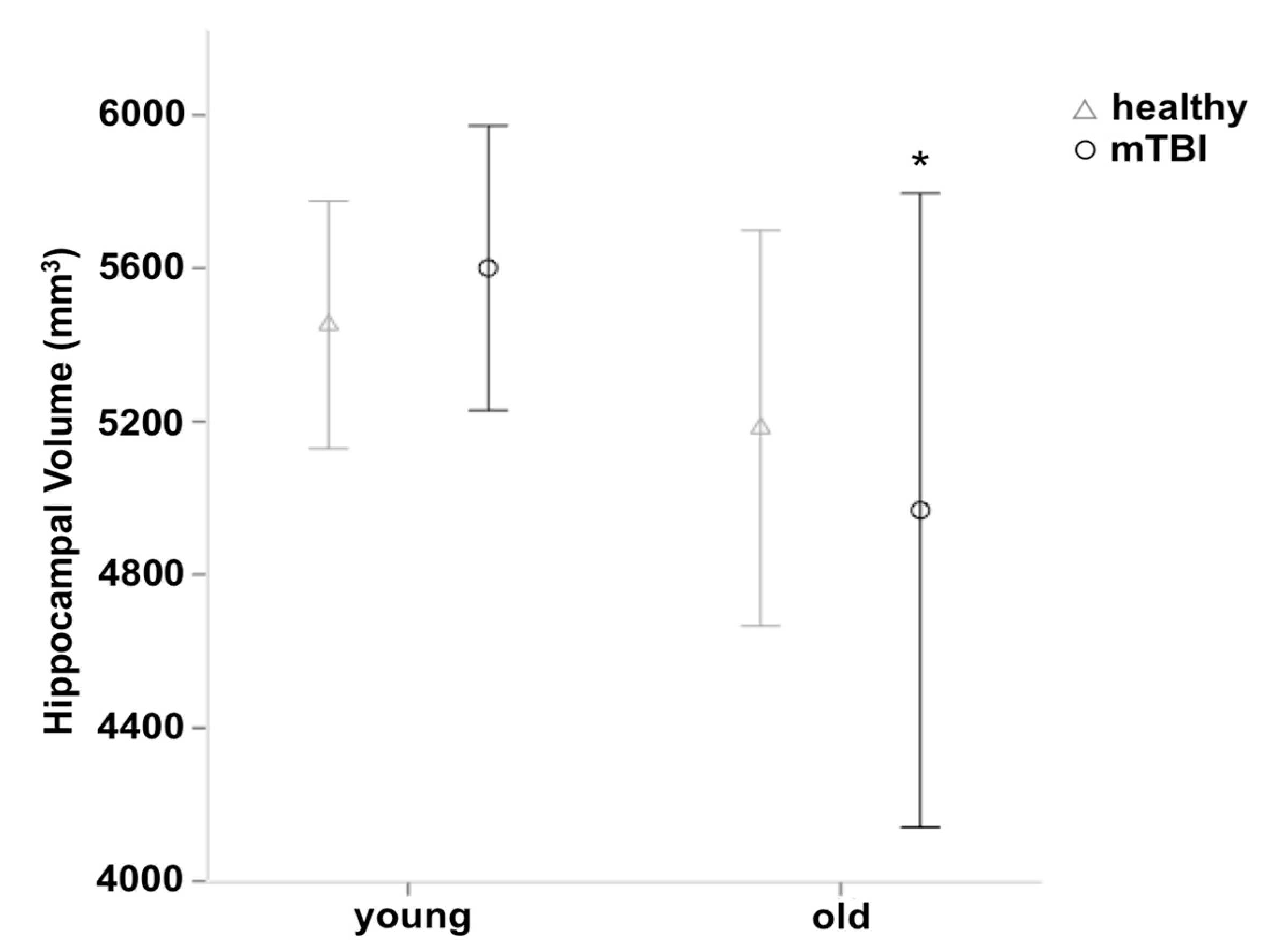
3.2. Group Differences in Gray Matter Volume
3.3. Group differences in DTI Measures
3.4. Group Differences in RSFC
4. Discussion
4.1. Cognitive Differences between Participants with mTBI and Healthy Controls
4.2. Regional Volume Changes in Participants with mTBI
4.3. Alterations in FA and MD Values in Participants with mTBI
4.4. Functional Connectivity in Participants with mTBI
4.5. Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Smith, S. Did Concussions Play Role in Lou Gehrig’s Disease? CNN 2010. Available online: http://edition.cnn.com/2010/HEALTH/08/17/als.lou.gehrigs.concussions/ (accessed on 30 January 2017).
- Smith, S. Ex-NFL Stars after Concussion: Lives Unraveled. CNN 2010. Available online: http://edition.cnn.com/2010/HEALTH/11/24/fred.mcneill.concussions/ (accessed on 30 January 2017).
- McCrea, M.; Guskiewicz, K.; Doncevic, S.; Helmick, K.; Kennedy, J.; Boyd, C.; Asmussen, S.; Ahn, K.W.; Wang, Y.; Hoelzle, J.; et al. Day of injury cognitive performance on the Military Acute Concussion Evaluation (MACE) by U.S. military service members in OEF/OIF. Mil. Med. 2014, 179, 990–997. [Google Scholar] [CrossRef] [PubMed]
- King, N.S.; Crawford, S.; Wenden, F.J.; Wade, D.T. The Rivermead Post Concussion Symptoms Questionnaire: A measure of symptoms commonly experienced after head injury and its reliability. J. Neurol. 1995, 242, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Kinnunen, K.M.; Greenwood, R.; Powell, J.H.; Leech, R.; Hawkins, P.C.; Bonnelle, V.; Patel, M.C.; Counsell, S.J.; Sharp, D.J. White matter damage and cognitive impairment after traumatic brain injury. Brain 2010, 134, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Konrad, C.; Geburek, A.J.; Rist, F.; Blumenroth, H.; Fischer, B.; Husstedt, I.; Arolt, V.; Schiffbauer, H.; Lohmann, H. Long-term cognitive and emotional consequences of mild traumatic brain injury. Psychol. Med. 2011, 41, 1197–1211. [Google Scholar] [CrossRef] [PubMed]
- Vanderploeg, R.D.; Curtis, G.; Belanger, H.G. Long-term neuropsychological outcomes following mild traumatic brain injury. J. Int. Neuropsychol. Soc. 2005, 11, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Wilke, S.; List, J.; Mekle, R.; Lindenberg, R.; Bukowski, M.; Ott, S.; Schubert, F.; Ittermann, B.; Flöel, A. No effect of anodal transcranial direct current stimulation on gamma-aminobutyric acid levels in patients with recurrent mild traumatic brain injury. J. Neurotrauma 2016, 33, 281–290. [Google Scholar] [CrossRef] [PubMed]
- List, J.; Ott, S.; Bukowski, M.; Lindenberg, R.; Flöel, A. Cognitive function and brain structure after recurrent mild traumatic brain injuries in young-to-middle-aged adults. Front. Hum. Neurosci. 2015, 9, 228. [Google Scholar] [CrossRef] [PubMed]
- Keightley, M.L.; Saluja, R.S.; Chen, J.K.; Gagnon, I.; Leonard, G.; Petrides, M.; Ptito, A. A functional magnetic resonance imaging study of working memory in youth after sports-related concussion: Is it still working? J. Neurotrauma 2014, 31, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, S.; De Beaumont, L.; Henry, L.C.; Boulanger, Y.; Evans, A.C.; Bourgouin, P.; Poirier, J. Théoret, H.; Lassonde, M.; Sports concussions and aging: A neuroimaging investigation. Cereb. Cortex 2013, 23, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Gomez, R.G.; White, D.A. Using verbal fluency to detect very mild dementia of the Alzheimer type. Arch. Clin. Neuropsychol. 2006, 21, 771–775. [Google Scholar] [CrossRef] [PubMed]
- De Beaumont, L.; Théoret, H.; Mongeon, D.; Messier, J.; Leclerc, S.; Tremblay, S.; Ellemberg, D.; Lassonde, M. Brain function decline in healthy retired athletes who sustained their last sports concussion in early adulthood. Brain 2009, 132, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Moretti, L.; Cristofori, I.; Weaver, S.M.; Chau, A.; Portelli, J.N.; Grafman, J. Cognitive decline in older adults with a history of traumatic brain injury. Lancet Neurol. 2012, 11, 1103–1112. [Google Scholar] [CrossRef]
- Plassman, B.L.; Havlik, R.J.; Steffens, D.C.; Helms, M.J.; Newman, T.N.; Drosdick, D.; Phillips, C.; Gau, B.A.; Welsh-Bohmer, K.A.; Burke, J.R. Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology 2000, 55, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Shively, S.; Scher, A.I.; Perl, D.P.; Diaz-Arrastia, R. Dementia resulting from traumatic brain injury. Arch. Neurol. 2012, 69, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Carroll, L.J.; Cassidy, J.D.; Holm, L.; Kraus, J.; Coronado, V.G. Methodological issues and research recommendations for mild traumatic brain injury: The WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J. Rehabil. Med. Med. 2004, 43, 113–125. [Google Scholar] [CrossRef]
- Keightley, M.L.; Sinopoli, K.J.; Davis, K.D.; Mikulis, D.J.; Wennberg, R.; Tartaglia, M.C.; Chen, J.K.; Tator, C.H. Is there evidence for neurodegenerative change following traumatic brain injury in children and youth? A scoping review. Front. Hum. Neurosci. 2014, 8, 139. [Google Scholar] [CrossRef] [PubMed]
- Wilde, E.A.; Merkley, T.L.; Bigler, E.D.; Max, J.E.; Schmidt, A.T.; Ayoub, K.W.; McCauley, S.R.; Hunter, J.V.; Hanten, G.; Li, X.; et al. Longitudinal changes in cortical thickness in children after traumatic brain injury and their relation to behavioral regulation and emotional control. Int. J. Dev. Neurosci. 2012, 30, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Monti, J.M.; Voss, M.W.; Pence, A.; McAuley, E.; Kramer, A.F.; Cohen, N.J. History of mild traumatic brain injury is associated with deficits in relational memory, reduced hippocampal volume, and less neural activity later in life. Front. Aging Neurosci. 2013, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Brezova, V.; Moen, K.G.; Skandsen, T.; Vik, A.; Brewer, J.B.; Salvesen, Ø.; Håberg, A.K. Prospective longitudinal MRI study of brain volumes and diffusion changes during the first year after moderate to severe traumatic brain injury. NeuroImage Clin. 2014, 5, 128–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lannsjö, M.; Raininko, R.; Bustamante, M.; von Seth, C.; Borg, J. Brain pathology after mild traumatic brain injury: An exploratory study by repeated magnetic resonance examination. J. Rehabil. Med. 2013, 45, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Slemmer, J.E. Repeated mild injury causes cumulative damage to hippocampal cells. Brain 2002, 125, 2699–2709. [Google Scholar] [CrossRef] [PubMed]
- King, J.B.; Lopez-Larson, M.P.; Yurgelun-Todd, D.A. Mean cortical curvature reflects cytoarchitecture restructuring in mild traumatic brain injury. NeuroImage Clin. 2016, 11, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.M.; Klimaj, S.; Toulouse, T.; Mayer, A.R. A prospective study of gray matter abnormalities in mild traumatic brain injury. Neurology 2013, 81, 2121–2127. [Google Scholar] [CrossRef] [PubMed]
- Rutgers, D.R.; Toulgoat, F.; Cazejust, J.; Fillard, P.; Lasjaunias, P.; Ducreux, D. White matter abnormalities in mild traumatic brain injury: A diffusion tensor imaging study. Am. J. Neuroradiol. 2008, 29, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Gale, S.D.; Johnson, S.C.; Bigler, E.D.; Blatter, D.D. Trauma-induced degenerative changes in brain injury: A morphometric analysis of three patients with preinjury and postinjury MR scans. J. Neurotrauma 1995, 12, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Henry, L.C.; Tremblay, J.; Tremblay, S.; Lee, A.; Brun, C.; Lepore, N.; Theoret, H.; Ellemberg, D.; Lassonde, M. Acute and chronic changes in diffusivity measures after sports concussion. J. Neurotrauma 2011, 28, 2049–2059. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.P.; Jang, S.H. Traumatic axonal injury of the corticospinal tract in the subcortical white matter in patients with mild traumatic brain injury. Brain Inj. 2015, 29, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Kraus, M.F.; Susmaras, T.; Caughlin, B.P.; Walker, C.J.; Sweeney, J.A.; Little, D.M. White matter integrity and cognition in chronic traumatic brain injury: A diffusion tensor imaging study. Brain 2007, 130, 2508–2519. [Google Scholar] [CrossRef] [PubMed]
- Inglese, M.; Makani, S.; Johnson, G.; Cohen, B.A.; Silver, J.A.; Gonen, O.; Grossman, R.I. Diffuse axonal injury in mild traumatic brain injury: A diffusion tensor imaging study. J. Neurosurg. 2005, 103, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Lipton, M.L.; Gellella, E.; Lo, C.; Gold, T.; Ardekani, B.A.; Shifteh, K.; Bello, J.A.; Branch, C.A. Multifocal white matter ultrastructural abnormalities in mild traumatic brain injury with cognitive disability: A voxel-wise analysis of diffusion tensor imaging. J. Neurotrauma 2008, 25, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.; Shifteh, K.; Gold, T.; Bello, J.A.; Lipton, M.L. Diffusion tensor imaging abnormalities in patients with mild traumatic brain injury and neurocognitive impairment. J. Comput. Assist. Tomogr. 2009, 33, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Geary, E.K.; Kraus, M.F.; Pliskin, N.H.; Little, D.M. Verbal learning differences in chronic mild traumatic brain injury. J. Int. Neuropsychol. Soc. 2010, 16, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Niogi, S.N.; Mukherjee, P.; Ghajar, J.; Johnson, C.; Kolster, R.A.; Sarkar, R.; Lee, H.; Meeker, M.; Zimmerman, R.D.; Manley, G.T.; et al. Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: A 3T diffusion tensor imaging study of mild traumatic brain injury. Am. J. Neuroradiol. 2008, 29, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Johnson, B.; Pennell, D.; Ray, W.; Sebastianelli, W.; Slobounov, S. Are functional deficits in concussed individuals consistent with white matter structural alterations: Combined FMRI & DTI study. Exp. Brain Res. 2010, 204, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Palacios, E.M.; Sala-Llonch, R.; Junque, C.; Roig, T.; Tormos, J.M.; Bargallo, N.; Vendrell, P. Resting-state functional magnetic resonance imaging activity and connectivity and cognitive outcome in traumatic brain injury. JAMA Neurol. 2013, 70, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Shumskaya, E.; Teuntje, M.J.C.; Norris, D.G.; Vos, P.E. Abnormal whole-brain functional networks in homogeneous acute mild traumatic brain injury. Neurology 2012, 79, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Milham, M.P.; Lui, Y.W.; Miles, L.; Reaume, J.; Sodickson, D.K.; Grossman, R.I.; Ge, Y. Default-mode network disruption in mild traumatic brain injury. Radiology 2012, 265, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Bharath, R.D.; Munivenkatappa, A.; Gohel, S.; Panda, R.; Saini, J.; Rajeswaran, J.; Shukla, D.; Bhagavatula, I.D.; Biswal, B.B. Recovery of resting brain connectivity ensuing mild traumatic brain injury. Front. Hum. Neurosci. 2015, 9, 513. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.C.; Covassin, T.; Nogle, S.; Doyle, S.; Russell, D.; Pearson, R.L.; Monroe, J.; Liszewski, C.M.; DeMarco, K.J.; Kaufman, D.I. A potential biomarker in sports-related concussion: Brain functional connectivity alteration of the default-mode network measured with sequential resting-state fMRI. J. Neurotrauma 2015, 32, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, N.; Pettemeridou, E.; Seimenis, I.; Eracleous, E.; Papacostas, S.S.; Papanicolaou, A.C.; Constantinidou, F. Assessing the relationship between neurocognitive performance and brain volume in chronic moderate-severe traumatic brain injury. Front. Neurol. 2016, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.; Folstein, S.; McHugh, P. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Hautzinger, M.; Keller, F.; Kühner, C. Beck Depression Inventar II (BDI 2); Harcourt Test Services: Frankfurt, Germany, 2006. [Google Scholar]
- Vanderploeg, R.D.; Curtiss, G.; Luis, C.A.; Salazar, A.M. Long-term morbidities following self-reported mild traumatic brain injury. J. Clin. Exp. Neuropsychol. 2007, 29, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Matser, E.J.T.; Lezak, M.D.; Jordan, B.D.; Traumatic, H.; In, B. Neuropsychological impairment in amateur soccer players. JAMA 1999, 282, 971–973. [Google Scholar] [CrossRef] [PubMed]
- Belanger, H.G.; Vanderploeg, R.D. The neuropsychological impact of sports-related concussion: A meta-analysis. J. Int. Neuropsychol. Soc. 2005, 11, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Helmstaedter, C.; Lendt, M.; Lux, S. Verbaler Lern- und Merkfähigkeitstest (VLMT); Beltz: Göttingen, Germany, 2001. [Google Scholar]
- Reitan, R.M. Validity of the Trail Making Test as an indicator or organic brain damage. Percept. Mot. Skills 1958, 8, 271–276. [Google Scholar] [CrossRef]
- Aschenbrenner, S.; Tucha, O.; Lange, K. Regensburger Wortflüssigkeits-Test, Handanweisung [Manual for the Regensburger Word Fluency Test]; Hogrefe: Göttingen, Germany, 2000. [Google Scholar]
- Osterrieth, P.A. Le test de copie d’une figure complex: Contribution a l’etude de la perception et de la memoire. Arch. Psychol. 1944, 30, 286–356. [Google Scholar]
- Härting, C.; Markowitsch, H.; Neufeld, H.; Calabrese, P.; Deisinger, K.; Kessler, J. Wechsler Gedächtnistest—Revidierte Fassung WMS-R, Deutsche Adaptation (Manual for the Wechsler Memory Scale—Revised, German Adaptation); Verlag Hans Huber Bern: Bern, Switzerland, 2000. [Google Scholar]
- Tzourio-Mazoyer, N.; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard, O.; Delcroix, N.; Mazoyer, B.; Joliot, M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002, 15, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Behrens, T.E.J.; Woolrich, M.W.; Jenkinson, M.; Johansen-Berg, H.; Nunes, R.G.; Clare, S.; Matthews, P.M.; Brady, J.M.; Smith, S.M. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn. Reson. Med. 2003, 50, 1077–1088. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Jenkinson, M.; Johansen-Berg, H.; Rueckert, D.; Nichols, T.E.; Mackay, C.E.; Watkins, K.E.; Ciccarelli, O.; Cader, M.Z.; Matthews, P.M. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage 2006, 31, 1487–1505. [Google Scholar] [CrossRef] [PubMed]
- Antonenko, D.; Külzow, N.; Cesarz, M.E.; Schindler, K.; Grittner, U.; Flöel, A. Hippocampal pathway plasticity is associated with the ability to form novel memories in older adults. Front. Aging Neurosci. 2016, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Panenka, W.J.; Lange, R.T.; Bouix, S.; Shewchuk, J.R.; Manraj, K.; Heran, S.; Brubacher, J.R.; Eckbo, R.; Shenton, M.E.; Iverson, G.L. Neuropsychological outcome and diffusion tensor imaging in complicated versus uncomplicated mild traumatic brain injury. PLoS ONE 2015, 10, e0122746. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.-G.; Zhang, Y.-F. DPARSF: A MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front. Syst. Neurosci. 2010, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Guskiewicz, K.M.; Ross, S.E.; Marshall, S.W. Postural stability and neuropsychological deficits after concussion in collegiate athletes. J. Athl. Train. 2001, 36, 263–273. [Google Scholar] [PubMed]
- McCauley, S.R.; Wilde, E.A.; Barnes, A.; Hanten, G.; Hunter, J.V.; Levin, H.S.; Smith, D.H. Patterns of early emotional and neuropsychological sequelae after mild traumatic brain injury. J. Neurotrauma 2014, 31, 914–925. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Asano, Y.; Shinoda, J. Decreased fractional anisotropy evaluated using tract-based spatial statistics and correlated with cognitive dysfunction in patients with mild traumatic brain injury in the chronic stage. Am. J. Neuroradiol. 2012, 33, 2117–2122. [Google Scholar] [CrossRef] [PubMed]
- Mckee, A.C.; Daneshvar, D.H. The neuropathology of traumatic brain injury. Handb. Clin. Neurol. 2015, 127, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Toledo, E.; Lebel, A.; Becerra, L.; Minster, A.; Linnman, C.; Maleki, N.; Dodick, D.W.; Borsook, D. The young brain and concussion: Imaging as a biomarker for diagnosis and prognosis. Neurosci. Biobehav. Rev. 2012, 36, 1510–1531. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, J.D.; Siddiqi, F.; Babb, J.S.; Bagley, L.J.; Mannon, L.J.; Sinson, G.P.; Grossman, R.I. Brain atrophy in mild or moderate traumatic brain injury: A longitudinal quantitative analysis. AJNR Am. J. Neuroradiol. 2002, 23, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Palacios, E.M.; Sala-Llonch, R.; Junque, C.; Fernandez-Espejo, D.; Roig, T.; Tormos, J.M.; Bargallo, N.; Vendrell, P. Long-term declarative memory deficits in diffuse TBI: Correlations with cortical thickness, white matter integrity and hippocampal volume. Cortex 2013, 49, 646–657. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xie, H.; Cotton, A.S.; Tamburrino, M.B.; Brickman, K.R.; Lewis, T.J.; Mclean, S.A.; Liberzon, I. Early cortical thickness change after mild traumatic brain injury following motor vehicle collision. J. Neurotrauma 2015, 32, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Wilde, E.A.; Newsome, M.R.; Bigler, E.D.; Pertab, J.; Merkley, T.L.; Hanten, G.; Scheibel, R.S.; Li, X.; Chu, Z.; Yallampalli, R.; et al. Brain imaging correlates of verbal working memory in children following traumatic brain injury. Int. J. Psychophysiol. 2011, 82, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Hudak, A.; Warner, M.; Marquez de la Plata, C.; Moore, C.; Harper, C.; Diaz-Arrastia, R. Brain morphometry changes and depressive symptoms after traumatic brain injury. Psychiatry Res. Neuroimaging 2011, 191, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Vogt, B.A.; Laureys, S. Posterior cingulate, precuneal & retrosplenial cortices: Cytology & components of the neural network correlates of consciousness. Prog. Brain Res. 2005, 150, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Hickie, I.; Naismith, S.; Ward, P.B.; Turner, K.; Scott, E.; Mitchell, P.; Wilhelm, K.; Parker, G. Reduced hippocampal volumes and memory loss in patients with early- and late-onset depression. Br. J. Psychiatry 2005, 186, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Schuff, N.; Woerner, N.; Boreta, L.; Kornfield, T.; Shaw, L.M.; Trojanowski, J.Q.; Thompson, P.M.; Jack, C.R.; Weiner, M.W. MRI of hippocampal volume loss in early Alzheimer’s disease in relation to ApoE genotype and biomarkers. Brain 2009, 132, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.C.; Mierzwa, A.J.; Marion, C.M.; Sullivan, G.M. White matter involvement after TBI: Clues to axon and myelin repair capacity. Exp. Neurol. 2016, 275, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Mierzwa, A.J.; Marion, C.M.; Sullivan, G.M.; McDaniel, D.P.; Armstrong, R.C. Components of myelin damage and repair in the progression of white matter pathology after mild traumatic brain injury. J. Neuropathol. Exp. Neurol. 2015, 74, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Green, R.E.A.; Colella, B.; Maller, J.J.; Bayley, M.; Glazer, J.; Mikulis, D.J. Scale and pattern of atrophy in the chronic stages of moderate-severe TBI. Front. Hum. Neurosci. 2014, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Bendlin, B.B.; Ries, M.L.; Lazar, M.; Alexander, A.L.; Dempsey, R.J.; Rowley, H.A.; Sherman, J.E.; Johnson, S.C. Longitudinal changes in patients with traumatic brain injury assessed with diffusion-tensor and volumetric imaging. Neuroimage 2008, 42, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, S.; Henry, L.C.; Bedetti, C.; Larson-Dupuis, C.; Gagnon, J.-F.; Evans, A.C.; Théoret, H.; Lassonde, M.; De Beaumont, L. Diffuse white matter tract abnormalities in history of sports-related concussions. Brain J. Neurol. 2014, 137, 2997–3011. [Google Scholar] [CrossRef] [PubMed]
- Maruta, J.; Palacios, E.M.; Zimmerman, R.D.; Ghajar, J.; Mukherjee, P. Chronic post-concussion neurocognitive deficits. I. Relationship with white matter integrity. Front. Hum. Neurosci. 2016, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Nudo, R.J. Recovery after brain injury: Mechanisms and principles. Front. Hum. Neurosci. 2013, 7, 887. [Google Scholar] [CrossRef] [PubMed]
- Nishibe, M.; Barbay, S.; Guggenmos, D.; Nudo, R.J. Reorganization of motor cortex after controlled cortical impact in rats and implications for functional recovery. J. Neurotrauma 2010, 27, 2221–2232. [Google Scholar] [CrossRef] [PubMed]
- Wilmoth, K.; LoBue, C.; Clem, M.; Didehbani, N.; Hart, J.; Womack, K.; Bell, K.; Batjer, H.; Cullum, C. Reliability of Self-Reported Concussion History in Older Adults with and Without Cognitive Impairment. Arch. Clin. Neuropsychol. 2016, 31, 573–583. [Google Scholar] [CrossRef]
- Didehbani, N.; Wilmoth, K.; Fields, L.; Lobue, C.; Strain, J.; Spence, J.; Dieppa, M.; Cullum, M.; Hart, J. Reliability of Self-Reported Concussion History in Retired NFL Players. Ann. Sport Med. Res. 2017, 4, 1115. [Google Scholar]
- Koerte, I.K.; Ertl-Wagner, B.; Reiser, M.; Zafonte, R.; Shenton, M.E. White matter integrity in the brains of professional soccer players without a symptomatic concussion. JAMA 2012, 308, 1859–1861. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.; Neuberger, T.; Gay, M.; Hallett, M.; Slobounov, S. Effects of subconcussive head trauma on the default mode network of the brain. J. Neurotrauma 2014, 31, 1907–1913. [Google Scholar] [CrossRef] [PubMed]
- Witte, A.V.; Köbe, T.; Kerti, L.; Rujescu, D.; Flöel, A. Impact of KIBRA Polymorphism on Memory Function and the Hippocampus in Older Adults. Neuropsychopharmacology 2016, 41, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Köbe, T.; Witte, A.V.; Schnelle, A.; Tesky, V.A.; Pantel, J.; Schuchardt, J.P.; Hahn, A.; Bohlken, J.; Grittner, U.; Flöel, A. Impact of resveratrol on glucose control, hippocampal structure and connectivity, and memory performance in patients with mild cognitive impairment. Front. Neurosci. 2017, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Sundermann, E.E.; Maki, P.M.; Rubin, L.H.; Lipton, R.B.; Landau, S.; Biegon, A. Female advantage in verbal memory: Evidence of sex-specific cognitive reserve. Neurology 2016, 87, 1916–1924. [Google Scholar] [CrossRef] [PubMed]
- Broglio, S.P.; Eckner, J.T.; Paulson, H.L.; Kutcher, J.S. Cognitive decline and aging: The role of concussive and subconcussive impacts. Exerc. Sport Sci. Rev. 2012, 40, 138–144. [Google Scholar] [CrossRef] [PubMed]

| Young Patients’ Characteristics | Older Patients’ Characteristics | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Age | Years of Education | No. of mTBI | Time since Last mTBI (month) | Cause of Last mTBI | Sex | Age | Years of Education | No. of mTBI | Time since Last mTBI (month) | Cause of Last mTBI |
| m | 27 | 17 | 2 | N/A | Ice hockey | f | 58 | 13 | 2 | 440 | Bicycle accident |
| m | 29 | 18 | 2 | 13 | Ice hockey | m | 69 | 18 | 4 | 106 | Bicycle accident |
| f | 22 | 15.5 | 4 | 27 | Skating | f | 55 | 13 | 2 | 366 | Ski accident |
| m | 21 | 15.5 | 3 | 33 | Rugby | f | 67 | 15.5 | 2 | 646 | Accident |
| f | 26 | 17.5 | 5 | 35-36 | Fall | m | 54 | 16 | 2 | 410 | Accident |
| m | 26 | 15.5 | 3 | 54 | Football | m | 80 | 13 | 3 | 580 | Vehicle accident |
| m | 24 | 17.5 | 2 | 27–30 | Football | f | 57 | 13 | 3 | 433 | Accident |
| m | 20 | 15.5 | 2 | 14 | Fall | m | 63 | 20 | 4 | 436 | Boxing |
| m | 21 | 15 | 2 | 9 | Soccer | f | 70 | 14 | 2 | 531 | Bicycle accident |
| m | 23 | 15 | 3 | 10 | Football | m | 57 | 18 | 3 | 62 | Accident |
| m | 21 | 15.5 | 2 | 22 | Football | f | 55 | 16 | 2 | 244 | Accident |
| m | 25 | 13 | 2 | 35–36 | Football | m | 55 | 18 | 2 | 204 | Handball |
| m | 23 | 10 | 2 | 12 | Football | f | 62 | 13 | 2 | 462 | Accident |
| m | 24 | 15.5 | 3 | 27–30 | Football | f | 59 | 17.5 | 2 | 489 | Assault |
| m | 25 | 13 | 6 | 7 | Football | m | 76 | 16 | 3 | 668 | Boxing |
| m | 30 | 18 | 7 | 11 | Mountainbike | m | 65 | 17.5 | 2 | 484 | Soccer |
| m | 24 | 15.5 | 2 | 32–44 | Kickboxing | m | 63 | 14.5 | 2 | 712 | Accident |
| Young (N = 38) | Old (N = 33) | All (N = 71) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mTBI | Healthy | T | p | mTBI | Healthy | T | p | mTBI | Healthy | T | p | |
| Demographic data | ||||||||||||
| Sex | 2 female, 15 male | 2 female, 19 male | - | - | 8 female, 9 male | 8 female, 8 male | - | - | 10 female, 24 male | 10 female, 27 male | - | - |
| Age | 24.2 ± 2.8 | 25.8 ± 5.4 | −1.9 | 0.3 | 62.7 ± 7.7 | 61.7 ± 5.9 | 0.4 | 0.7 | 43.4 ± 3.5 | 41.3 ± 3.1 | 0.5 | 0.7 |
| Education | 15.4 ± 2.0 | 15.1 ± 1.7 | 0.5 | 0.6 | 15.7 ± 2.3 | 15.5 ± 1.9 | 0.2 | 0.8 | 15.4 ± 2.1 | 15.3 ± 1.8 | 0.5 | 0.6 |
| MMSE | 29.8 ± 0.4 | 30.0 ± 0.0 | −1.9 | 0.1 | 29.5 ± 1.0 | 29.2 ± 1.4 | 0.8 | 0.4 | 29.7 ± 0.8 | 29.6 ± 1.0 | 0.1 | 0.7 |
| BDI | 2.5 ± 3.2 | 2.2 ± 2.4 | 0.3 | 0.8 | 2.7 ± 2.5 | 2.6 ± 2.3 | 0.2 | 0.9 | 2.6 ± 2.9 | 2.4 ± 2.3 | 0.4 | 0.7 |
| Verbal fluency | ||||||||||||
| RWT, Verbal fluency (s; food) | −0.95 ± 1.09 | 0 ± 1 | −2.68 | 0.01 | −0.12 ± 0.84 | 0 ± 1 | −0.37 | 0.72 | −0.42 ± 0.81 | 0 ± 1 | −1.88 | 0.06 |
| RWT, Verbal flexibility (gr; clothes/flowers) | −0.57 ± 0.87 | 0 ± 1 | −1.77 | 0.08 | −0.05 ± 1.31 | 0 ± 1 | −0.13 | 0.90 | −0.34 ± 0.94 | 0 ± 1 | −1.43 | 0.16 |
| RWT, overall Verbal fluency score | −0.99 ± 1.08 | 0 ± 1 | −2.79 | 0.01 | −0.08 ± 0.93 | 0 ± 1 | −0.23 | 0.82 | −0.43 ± 0.83 | 0 ± 1 | −1.92 | 0.06 |
| Independent Variables | Dependent Variables | df | F | p | η2 |
|---|---|---|---|---|---|
| Group | FA fornix | 1 | 1.12 | 0.29 | 0.02 |
| FA lUF | 1 | 0.05 | 0.82 | 0.00 | |
| FA rUF | 1 | 0.04 | 0.85 | 0.00 | |
| FA CC | 1 | 0.18 | 0.68 | 0.00 | |
| Age | FA fornix | 1 | 36.67 | 0.00 | 0.37 |
| FA lUF | 1 | 4.25 | 0.04 | 0.06 | |
| FA rUF | 1 | 2.09 | 0.15 | 0.03 | |
| FA CC | 1 | 30.64 | 0.00 | 0.33 | |
| Age*Group | FA fornix | 1 | 0.32 | 0.58 | 0.01 |
| FA lUF | 1 | 0.98 | 0.33 | 0.02 | |
| FA rUF | 1 | 0.18 | 0.67 | 0.00 | |
| FA CC | 1 | 0.01 | 0.91 | 0.00 |
| Independent Variables | Dependent Variables | df | F | p | η2 |
|---|---|---|---|---|---|
| Group | MD fornix | 1 | 0.17 | 0.68 | 0.00 |
| MD lUF | 1 | 0.00 | 1.00 | 0.00 | |
| MD rUF | 1 | 1.10 | 0.30 | 0.02 | |
| MD CC | 1 | 0.19 | 0.67 | 0.00 | |
| Age | MD fornix | 1 | 25.34 | 0.00 | 0.29 |
| MD lUF | 1 | 21.98 | 0.00 | 0.26 | |
| MD rUF | 1 | 12.79 | 0.00 | 0.17 | |
| MD CC | 1 | 9.14 | 0.00 | 0.13 | |
| Age*Group | MD fornix | 1 | 1.10 | 0.30 | 0.02 |
| MD lUF | 1 | 0.00 | 0.97 | 0.00 | |
| MD rUF | 1 | 0.00 | 0.98 | 0.00 | |
| MD CC | 1 | 0.68 | 0.41 | 0.01 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilke, S.; Prehn, K.; Taud, B.; List, J.; Flöel, A. Multimodal Assessment of Recurrent mTBI across the Lifespan. J. Clin. Med. 2018, 7, 95. https://doi.org/10.3390/jcm7050095
Wilke S, Prehn K, Taud B, List J, Flöel A. Multimodal Assessment of Recurrent mTBI across the Lifespan. Journal of Clinical Medicine. 2018; 7(5):95. https://doi.org/10.3390/jcm7050095
Chicago/Turabian StyleWilke, Skadi, Kristin Prehn, Benedikt Taud, Jonathan List, and Agnes Flöel. 2018. "Multimodal Assessment of Recurrent mTBI across the Lifespan" Journal of Clinical Medicine 7, no. 5: 95. https://doi.org/10.3390/jcm7050095





