Pinto Beans (Phaseolus vulgaris L.) as a Functional Food: Implications on Human Health
Abstract
:1. Introduction
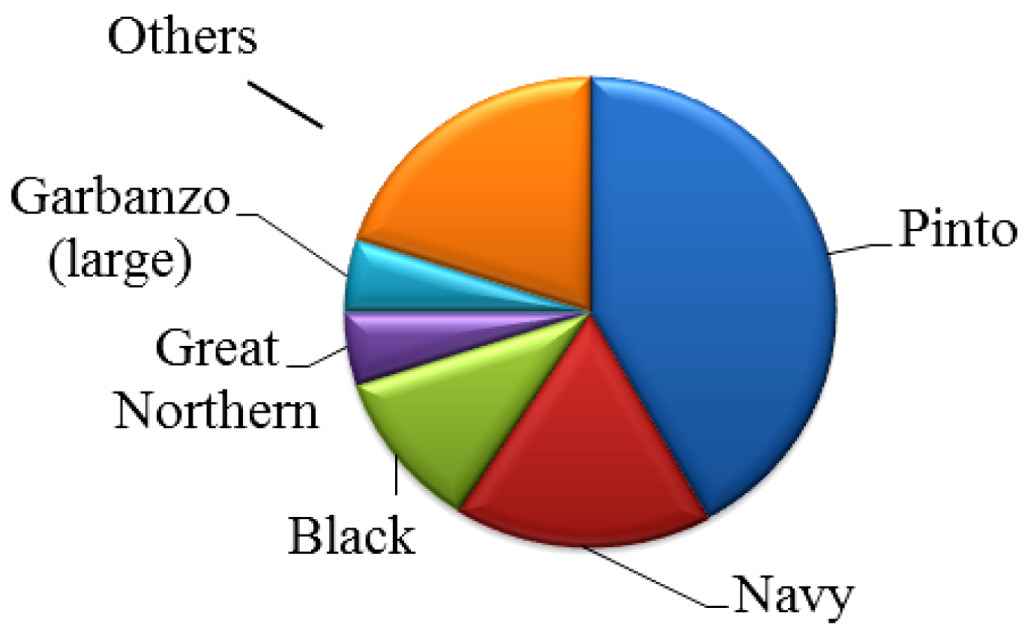
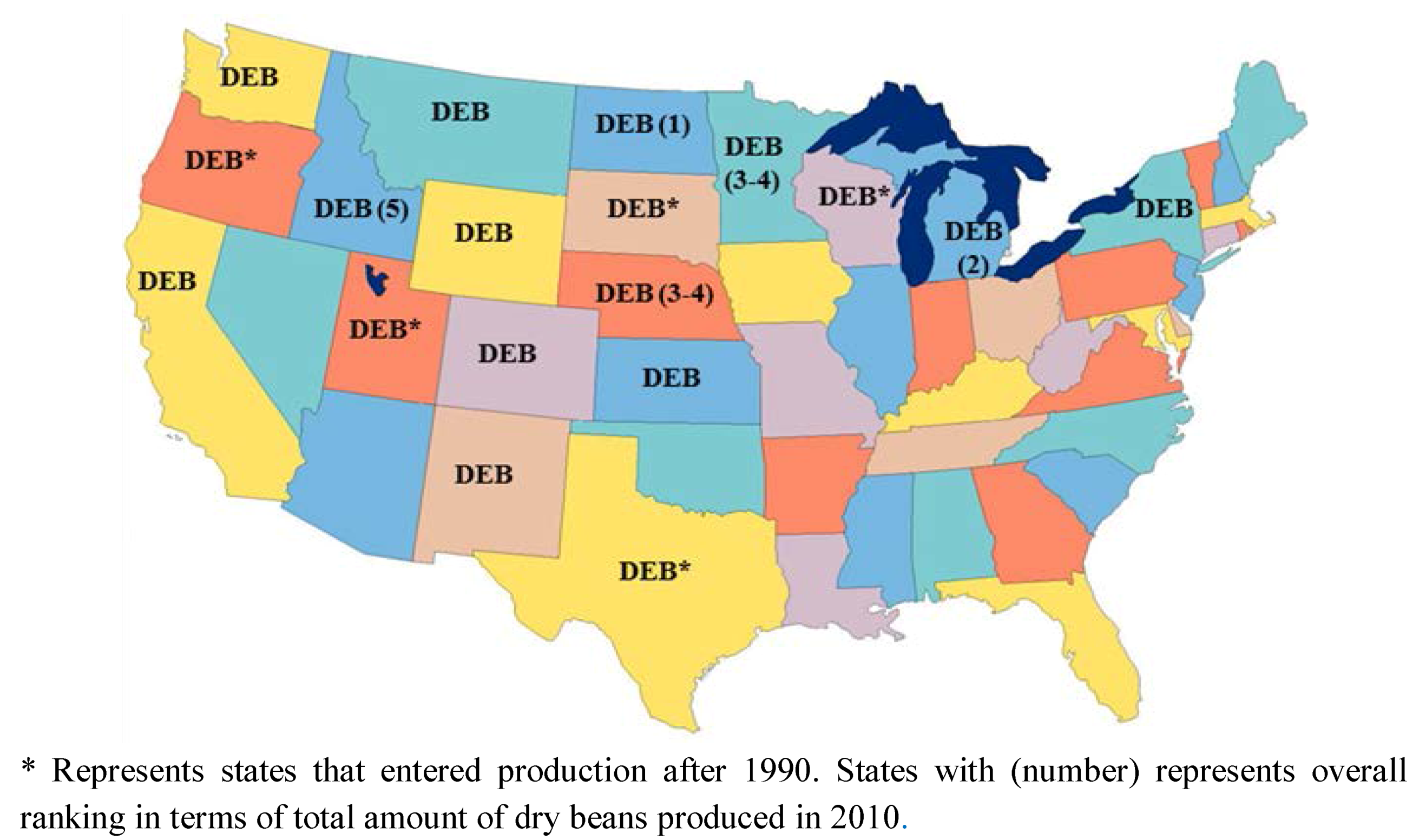
2. Agriculture Development of Dry Beans
3. Composition of Dry Beans
| Nutrient | Value per 100 g | |
|---|---|---|
| Raw | Cooked * | |
| Proximates | ||
| Energy | 347.00 kcal | 143.00 kcal |
| Protein | 21.42 g | 9.01 g |
| Total fat | 1.23 g | 0.65 g |
| Carbohydrate | 62.55 g | 26.22 g |
| Fiber, total dietary | 15.50 g | 9.00 g |
| Sugars, total | 2.11 g | 0.34 g |
| Minerals | ||
| Calcium (Ca) | 113 mg | 46 mg |
| Iron (Fe) | 5 mg | 2 mg |
| Magnesium (Mg) | 176 mg | 50 mg |
| Phosphorus (P) | 411 mg | 147 mg |
| Potassium (K) | 1393 mg | 436 mg |
| Sodium (Na) | 12 mg | 1 mg |
| Zinc (Zn) | 2 mg | 1 mg |
| Vitamins | ||
| Vitamin C (total ascorbic acid) | 6.30 mg | 0.80 mg |
| Thiamin | 0.71 mg | 0.19 mg |
| Riboflavin | 0.21 mg | 0.06 mg |
| Niacin | 1.17 mg | 0.32 mg |
| Vitamin B6 | 0.47 mg | 0.23 mg |
| Folate, DFE | 0.53 mg | 0.17 mg |
| Vitamin E (alpha-tocopherol) | 0.21 mg | 0.94 mg |
| Vitamin K (phylloquinone) | 5.6 μg | 3.5 μg |
| Lipids | ||
| Fatty acids (total saturated) | 0.24 g | 0.12 g |
| Fatty acids (total monounsaturated) | 0.23 g | 0.13 g |
| Fatty acids (total polyunsaturated) | 0.41 g | 0.24 g |
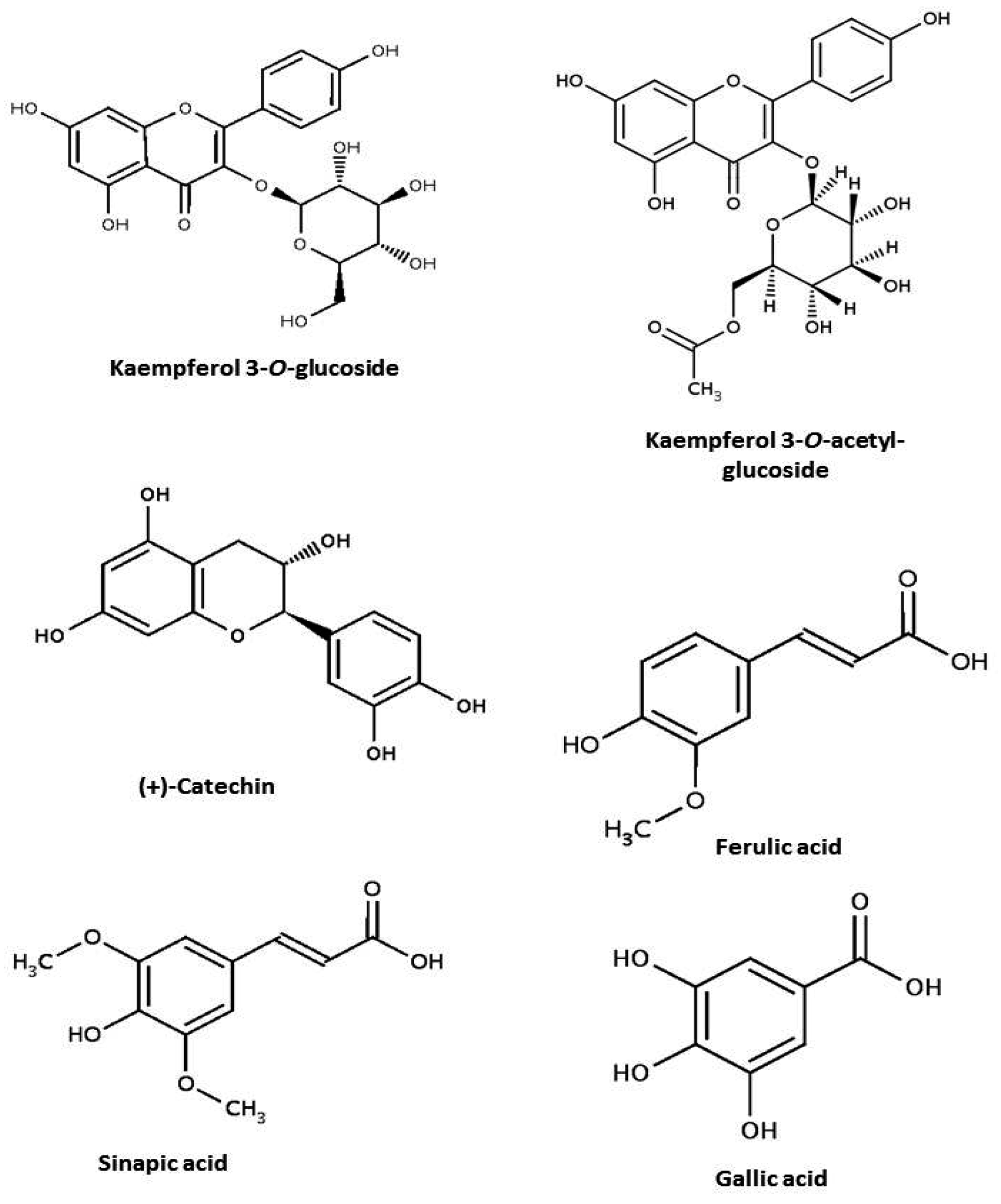
| Class | Subclass | Content (mg/100g) | Reference |
|---|---|---|---|
| Phenolic acids | p-coumaric acid | 4.9 a | Luthria & Pastor-Corrales [63] |
| Ferulic acid | 18.0 a | ||
| Sinapic acid | 7.8 a | ||
| Gallic acid | 8.7 | ||
| Flavonols | Kaempferol 3-O-glucoside | 14.8 | Xu & Chang [11] |
| Kaempferol 3-O-acetylglucoside | 3.0 |
4. Functional Food Properties of Pinto Beans: Protection against Chronic Cellular Stresses/Diseases
4.1. Oxidative Stress
| Stress-disease | Model | Treatment | Dosage | Main Outcomes | References |
|---|---|---|---|---|---|
| Oxidative stress and inflammation | In vitro (ORAC, COX and 150 LOX kits) | Hulls extracts from pinto and three types of beans | Pinto beans-158.20 mg/g a of total phenols and 3.04 mg/g of flavonols b | Total phenolic content and antioxidant activity of bean hulls were 6–8-fold higher than in whole beans. Extracts from pinto beans exhibited highest antioxidant capacity. Black and pinto beans exhibited the strongest COX-1 and COX-2 inhibitory effects. Pinto showed the strongest LOX inhibitory effect. | Oomah et al. [56] |
| Cancer and CVD | 40 men and 40 women aged 18–55 y with or without pre MetS | cooked pinto beans | 1/2 cup (130 g) of beans per day for 12 weeks | Propionate production was higher in treated groups than in control group. Eubacterium limosum was 50% lower in response bean consumption. Beans intake associated with lower blood total cholesterol in the controls (8%) and the pre-MetS group (4%). Bean consumption also resulted in lowered serum HDL-C and LDL-C in both groups. | Finley et al. [16] |
| CVD and diabetes mellitus | mildly insulin resistant adults (7 men, 9 women) | cooked pinto beans | 1/2 cup of beans daily for 8 weeks | Reduction of serum TC and LDL-C concentrations by over 8%. | Winham et al. [78] |
| Oxidative stress and bone resorption | 12-month-old male C57BL/6 mice | bean hull extract | 400 or 800 mg/kg for 3 months | BHE showed high antioxidant activity and its supplementation for 3 months decreased serum concentration of a bone resorption marker, and increased bone mineral density and trabecular thickness in the L3 vertebra in mice. | Cao et al. [77] |
| Colon cancer | 5 week old male F344 rats | Cooked pinto beans | Adjusted with beans to 18 g of protein/100 g of diet | Carcinogen azoxymethane induced rats fed a pinto bean rich diet had lower colon adenocarcinoma and tumor multiplicity. | Hughes et al. [69] |
| Oxidative stress and cancer cell proliferation | Human gastric adenocarcinoma AGS cells (CAA assay) and 9 human cancer cell lines (anti-proliferation assays) | Phenolic extracts from dry matured seeds of 13 food legumes, including pinto beans | 0.125, 0.25, 0.5, 1, 2, and 5 mg/mL of phenolic extract | Pinto beans, lentil and other beans exhibited dose-dependent inhibitory effects on cell proliferation of all tested cancer cell lines. Pinto beans showed the second strongest anti-proliferative activity of all analyzed legumes. Black soybean exhibited the greatest CAA with the lowest IC50 value followed by black bean and pinto bean. | Xu & Chang [79] |
| Oxidative stress and inflammation | macrophage cell line, RAW 264.7 | Protein hydrolysates Negro 8025 and Pinto Durango beans | 0.5–200 μM (based on soluble protein) | Hydrolysates of both varieties inhibited inflammation by modulation of NF-κB pathways (reducing its transactivation and the nuclear translocation of p65 subunit) | Oseguera-Toledo et al. [80] |
| Cardiovascular health | Normal young men | Canned pinto beans | 450 g/day | Average reduction in cholesterol levels (10%). | Shutler et al. [81] |
| Cardiovascular health | Hyperlipidemic men | Whole pinto beans | 120–162 g/day | Average reduction in serum cholesterol levels (10.4%). | Anderson et al. [82] |
| Blood glucose levels | In vitro digestibility | Pinto Bean Starches | Not applicable | Pinto bean digested at a slower rate compared to faba beans, which may be more effective in controlling glucose levels. | Ambigaipalan et al. [83] |
| Gastrointestinal health | In vitro fermentation | Polysaccharides from cooked pinto beans | Not applicable | Higher production of SCFA and altered pH. | Campos-Vega et al. [84] |
4.2. Inflammation
| Activity | Mechanism | Consequence |
|---|---|---|
| Antioxidant activity | - Radical scavenging | Reduction of free radicals and lipid peroxidation |
| - ROS generation inhibition- Pro | ||
| -oxidant enzyme inhibition | ||
| Modulation of inflammatory cells | - Modulation of enzymatic activitys | Reduction of inflammatory cells activation |
| - Modulation of secretory processe | ||
| Modulation of proinflammatory enzymes | - Inhibition of arachidonic acid enzymes | Reduction of inflammatory mediators (NO, leukotrienes, prostaglandins) |
| - Inhibition of NO synthase | ||
| Modulation of proinflammatory mediators | Modulation of cytokine production | Reduction of inflammatory cytokines (TNF-α, interleukins) |
| Modulation of proinflammatory gene expression | Modulation of signal transduction | Reduction of proinflammatory gene transcription |
4.3. Cardiovascular Health
4.4. Metabolic Syndrome
4.5. Cancer
4.6. Gastrointestinal Health
5. Conclusions
References
- Lyimo, M.; Mugula, J.; Elias, T. Nutritive composition of broth from selected bean varieties cooked for various periods. J. Sci. Food Agric. 1992, 58, 535–539. [Google Scholar] [CrossRef]
- Geil, P.B.; Anderson, J.W. Nutrition and health implications of dry beans: A review. J. Am. Coll. Nutr. 1994, 13, 549–558. [Google Scholar]
- Mitchell, D.C.; Lawrence, F.R.; Hartman, T.J.; Curran, J.M. Consumption of dry beans, peas, and lentils could improve diet quality in the US population. J. Am. Diet Assoc. 2009, 109, 909–913. [Google Scholar] [CrossRef]
- USDA Web site. Available online: http://www.ams.usda.gov/mnreports/lsaba.pdf (accessed on 29 November 2012).
- The Forum on Public Policy Web site. Available online: http://forumonpublicpolicy.com/archive06/uebersax.pdf (accessed on 29 November 2012).
- Landon, A. The “how” of the three sisters: The origins of agriculture in Mesoamerica and the human niche. NE Anthropol. 2008, 40, 110–124. [Google Scholar]
- FAO Web site. Available online: http://faostat.fao.org/site/339/default.aspx (accessed on 6 December 2012).
- U.S. Dry Bean Yields per Acre by State, 1950–2010 (Table 050). USDA Web site, 2011. Available online: http://usda.mannlib.cornell.edu/MannUsda/viewDocumentInfo.do?documentID=1394 (accessed on 25 November 2012).
- Nebraska Dry Bean Production by Class, 1919–2010 (Table 030). USDA Web site. 2011. Available online: http://usda.mannlib.cornell.edu/MannUsda/viewDocumentInfo.do?documentID=1394 (accessed on 25 November 2012).
- USDA Economic Research Service Web site. Available online: http://www.ers.usda.gov/ Briefing/ DryBeans/PDFs/DBnOutlook.pdf (accessed on 27 October 2012).
- Xu, B.; Chang, S.K.C. Total phenolic, phenolic acid, anthocyanin, flavan-3-ol, and flavonol profiles and antioxidant properties of pinto and black beans (Phaseolus vulgaris L.) as affected by thermal processing. J. Agric. Food Chem. 2009, 57, 4754–4764. [Google Scholar] [CrossRef]
- Lucier, G.; Lin, B.H.; Allshouse, J.; Kantor, L.S. Factor affecting dry bean consumption in the United States. Econ. Res. Serv. 2000, VGS-280, 26–34. [Google Scholar]
- USDA Web site. Available online: http://www.cnpp.usda.gov/Publications/USDAFoodPatterns/USDAFoodPatternsSummaryTable.pdf (accessed on 6 December 2012).
- USDA Web site. Available online: http://www.ams.usda.gov/AMSv1.0/getfile?dDocName=STELPRDC5098765 (accessed on 9 December 2012).
- Blaut, M. Relationship of prebiotics and food to intestinal microflora. Eur. J. Nutr. 2002, 41, I11–I16. [Google Scholar] [CrossRef]
- Finley, J.W.; Burrell, J.B.; Reeves, P.G. Pinto bean consumption changes SCFA profiles in fecal fermentations, bacterial populations of the lower bowel, and lipid profiles in blood of humans. J. Nutr. 2007, 137, 2391–2398. [Google Scholar]
- Lisa, T. Nutritional Information about Pinto Beans. Available online: http://www.livestrong.com/article/74379-nutritional-information-pinto-beans/ (accessed on 13 April 2012).
- Weinstein, S.J.; Hartman, T.J.; Stolzenberg-solomon, R.; Pietinen, P.; Barrett, M.J.; Taylor, P. R.; Virtamo, J.; Albanes, D. Null association between prostate cancer and serum folate, vitamin B6, vitamin B12, and homocysteine. Cancer Epidemiol. Biomarkers Prev. 2003, 12, 1271–1272. [Google Scholar]
- Zittoun, J. Anemias due to disorder of folate, vitamin B12 and transcobalamin metabolism. Rev. Prat. 1993, 43, 1358–1363. [Google Scholar]
- Engin, K.N. α-tocopherol: Looking beyond an antioxidant. Mol. Vis. 2009, 15, 855–860. [Google Scholar]
- Jiang, Q.; Christen, S.; Shigenaga, M.K.; Ames, B.N. γ-Tocopherol, the major form of vitamin E in the US diet deserves more attention. Am. Soc. Clin. Nutr. 2001, 74, 714–722. [Google Scholar]
- Singh, U.; Devaraj, S.; Jialal, I. Vitamin E, oxidative stress, and inflammation. Annu. Rev. Nutr. 2005, 25, 151–174. [Google Scholar] [CrossRef]
- Cockayne, S.; Adamson, J.; Lanham-New, S.; Shearer, M.J.; Gilbody, S.; Torgerson, D.J. Vitamin K and the prevention of fractures. Arch. Intern. Med. 2006, 166, 1256–1261. [Google Scholar] [CrossRef]
- Cheung, A.M.; Tile, L.; Lee, Y.; Tomlinson, G.; Hawker, G.; Scher, J.; Hu, H.; Veith, R.; Thompson, L.; Jamal, S.; et al. Vitamin K supplementation in postmenopausal women with osteopenia (ECKO Trial): A randomized controlled trial. PLoS Med. 2008, 5, 1–12. [Google Scholar] [CrossRef]
- Suehiro, T.; Sugimachi, K.; Matsumata, T.; Itasaka, H.; Taketomi, A.; Maeda, T. Protein induced by Vitamin K absence or antagonist II as a prognostic marker in hepatocellular carcinoma. Cancer 1994, 73, 2464–2471. [Google Scholar] [CrossRef]
- Nimptsch, K.; Rohrmann, S.; Nieters, A.; Linseisen, J. Serum undercarboxylated osteocalcin as biomarker of Vitamin K intake and risk of prostate cancer: A nested case-control study in the Heidelberg cohort of the European perspective investigation in cancer and nutrition. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 49–56. [Google Scholar] [CrossRef]
- Das, U.N. Essential fatty acids: Biochemistry, physiology and pathology. Biotechnol. J. 2006, 1, 420–439. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M. Omega-3 fatty acids and cardiovascular disease: New recommendations from the American heart association. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 151–152. [Google Scholar] [CrossRef]
- Leaf, A.; Kang, J.X.; Xiao, Y.-F. Fish oil fatty acids as cardiovascular drugs. Curr. Vasc. Pharmacol. 2008, 6, 1–12. [Google Scholar] [CrossRef]
- Harris, W.S.; Miller, M.; Tighe, A.P.; Davidson, M.H.; Schaefer, E.J. Omega-3 fatty acids and coronary heart disease risk: Clinical and mechanistic perspectives. Atherosclerosis 2008, 197, 12–24. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Essential fatty acids in health and chronic disease. Am. J. Clin. Nutr. 1999, 70, 560S–569S. [Google Scholar]
- Harper, C.R.; Jacobson, T.A. Beyond the Mediterranean diet: The role of omega-3 fatty acids in the prevention of coronary heart disease. Prev. Cardiol. 2003, 6, 136–146. [Google Scholar] [CrossRef]
- Winham, D.; Webb, D.; Barr, A. Beans and good health. Nutr. Today 2008, 5, 201–208. [Google Scholar] [CrossRef]
- USDA Web site. Available online: http://ndb.nal.usda.gov/ndb/foods/list?format=&count=&max=25&sort=&fg=Legumes+and+Legume+Products&man=&lfacet=&qlookup=&offset=25 (accessed on 28 November 2012).
- Reynoso-Camacho, R.; Ramos-Gomez, M.; Loarca-Pina, G. Bioactive Components in Common Beans (Phaseolus vulgaris L.). In Advances in Agricultural and Food Biotechnology; Guevara-González, R., Torres-pacheco, I., Eds.; Research Signpost: Trivandrum, India, 2006; pp. 217–236. [Google Scholar]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary polyphenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2012. [Google Scholar] [CrossRef]
- Clifford, M.N. Diet-derived phenols in plasma and tissues and their implications for health. Planta Med. 2004, 70, 1103–1114. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Current understanding of dietary polyphenols and their role in health and disease. Curr. Nutr. Food Sci. 2009, 5, 249–263. [Google Scholar] [CrossRef]
- Spencer, J.P.; Abd El Mohsen, M.M.; Minihane, A.M.; Mathers, J.C. Biomarkers of the intake of dietary polyphenols: Strengths, limitations and application in nutrition research. Br. J. Nutr. 2008, 99, 12–22. [Google Scholar]
- Scalbert, A.; Manach, C.; Morand, C.; Remesy, C. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Han, X.; Shen, T.; Lou, H. Dietary polyphenols and their biological significance. Int. J. Mol. Sci. 2007, 8, 950–988. [Google Scholar] [CrossRef]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significan. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef]
- Hollman, P.C.H. Absorption, bioavailability and metabolism of flavonoids. Pharm. Biol. 2004, 42, 74–83. [Google Scholar] [CrossRef]
- Beecher, G.R. Overview of dietary flavonoids: Nomenclature, occurrence and intake. J. Nutr. 2003, 133, 3248S–3254S. [Google Scholar]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar]
- Beninger, C.W.; Hosfield, G.L. Antioxidant activity of extracts, condensed tannin fractions, and pure flavonoids from Phaseolus vulgaris L. seed coat color genotypes. J. Agric. Food Chem. 2003, 51, 7879–7883. [Google Scholar] [CrossRef]
- Macz-Pop, G.A.; González-Paramás, A.M.; Pérez-Alonso, J.J.; Rivas-Gonzalo, J.C. New flavanol-anthocyanin condensed pigments and anthocyanin composition in guatemalan beans (Phaseolus spp.). J. Agric. Food Chem. 2006, 54, 536–542. [Google Scholar] [CrossRef]
- Amarowicz, R.; Pegg, R.B. Legumes as a source of natural antioxidants. Eur. J. Lipid Sci. Technol. 2008, 110, 865–878. [Google Scholar] [CrossRef]
- Thompson, M.; Brick, M.A.; McGinley, J.N.; Thompson, H.J. Chemical composition and mammary cancer inhibitory activity of dry bean. Crop Sci. 2009, 49, 179–176. [Google Scholar] [CrossRef]
- Lin, L.-Z.; Harnly, J.M.; Pastor-Corrales, M.S.; Luthria, D.L. The polyphenolic profiles of common bean (Phaseolus vulgaris L.). Food Chem. 2008, 107, 399–410. [Google Scholar] [CrossRef]
- Treutter, D. Managing phenol contents in crop plants by phytochemical farming and breeding—Visions and constraints. Int. J. Mol. Sci. 2010, 11, 807–857. [Google Scholar] [CrossRef]
- Madhujith, T.; Shahidi, F. Antioxidant potential of pea beans (Phaseolus vulgaris L.). J. Food Sci. 2005, 70, S85–S89. [Google Scholar] [CrossRef]
- Oomah, B.D.; Corbé, A.; Balasubramanian, P. Antioxidant and anti-inflammatory activities of bean (Phaseolus vulgaris L.) hulls. J. Agric. Food Chem. 2010, 58, 8225–8230. [Google Scholar] [CrossRef]
- Susan Marles, M.A.; Coulman, B.E.; Bett, E.K. Interference of condensed tannin in lignin analyses of dry Bean and forage crops. J. Agric. Food Chem. 2008, 56, 9797–9802. [Google Scholar] [CrossRef]
- Ferguson, L.R.; Chavan, R.R.; Harris, P.J. Changing concepts of dietary fiber: Implications for carcinogenesis. Nutr. Cancer 2001, 39, 155–169. [Google Scholar] [CrossRef]
- Doria, E.; Campion, B.; Sparvoli, F.; Tava, A.; Nielsen, E. Anti-nutrient components and metabolites with health implications in seeds of 10 common bean (Phaseolus vulgaris L. and Phaseolus lunatus L.) landraces cultivated in southern Italy. J. Food Compos. Anal. 2012, 26, 72–80. [Google Scholar] [CrossRef]
- Vucenik, I.; Shamsuddin, A.M. Protection against cancer by dietary IP6 and inositol. Nutr. Cancer 2006, 55, 109–125. [Google Scholar] [CrossRef]
- Bohn, L.; Meyer, A.S.; Rasmussen, S.K. Phytate: Impact on environment and human nutrition. A challenge for molecular breeding. J. Zhejiang Univ. Sci. B 2008, 9, 165–191. [Google Scholar]
- EMBL-EBI Web site. Available online: http://www.ebi.ac.uk/chebi/ (accessed on 10 December 2012).
- Luthria, D.L.; Pastor-Corrales, M.A. Phenolic acids content of fifteen dry edible bean (Phaseolus vulgaris L.) varieties. J. Food Compos. Anal. 2006, 19, 205–211. [Google Scholar] [CrossRef]
- WHO Web site. Available online: http://www.who.int/whr/2007/en/index.html (accessed on 28 November 2012).
- Darmadi-Blackberyy, I.; Wahiqvist, M.L.; Kouris-Blazos, B.; Steen, W.; Lukiot, W.; Horie, Y.; Hoire, K. Legumes: The most important dietary predictor of survival in older people of different ethnicities. Asia Pac. J. Clin. Nutr. 2004, 13, 217–220. [Google Scholar]
- Bazzano, L.A.; Jiang, H.; Ogden, L.G.; Loria, C.; Vupputuri, S.; Myers, L.; Whelton, P.K. Legume consumption and risk of coronary heart disease in US mean and women. Arch. Intern. Med. 2001, 161, 2573–2578. [Google Scholar] [CrossRef]
- Hertog, M.G.; Feskens, E.J.; Hollman, P.C.; Katan, M.B.; Kromhout, D. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen elderly study. Lancet 1993, 342, 1007–1011. [Google Scholar]
- Hangen, L.; Bennink, M.R. Consumption of black beans and navy beans (Phaseolus vulgaris) reduced azoxymethane-induced colon cancer in rats. Nutr. Cancer 2002, 44, 37–41. [Google Scholar]
- Hughes, J.S.; Ganthavorn, C.; Wilson-Sanders, S. Dry beans inhibit azoxymethane-induced colon carcinogenesis in F344 rats. J. Nutr. 1997, 127, 2328–2333. [Google Scholar]
- Correa, P. Epidemiological correlations between diet and cancer frequency. Cancer Res. 1981, 41, 3685–3690. [Google Scholar]
- Fang, Y.-Z.; Yang, S.; Wu, G. Free radicals, antioxidants, and nutrition. Nutrition 2002, 18, 872–879. [Google Scholar] [CrossRef]
- Ellis, E.M. Reactive carbonyls and oxidative stress: Potential for therapeutic intervention. Pharmacol. Ther. 2007, 115, 13–24. [Google Scholar] [CrossRef]
- Manach, C.; Mazur, A.; Scalbert, A. Polyphenols and prevention of cardiovascular diseases. Curr. Opin. Lipidol. 2005, 16, 77–84. [Google Scholar]
- Middleton, E.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar]
- Espín, J.C.; García-Conesa, M.T.; Tomás-Barberán, F.A. Nutraceuticals: Facts and fiction. Phytochemistry 2007, 68, 2986–3008. [Google Scholar] [CrossRef]
- Yi, W.; Fischer, J.; Krewer, G.; Akoh, C.C. Phenolic compounds from blueberries can inhibit colon cancer cell proliferation and induce apoptosis. J. Agric. Food Chem. 2005, 53, 7320–7329. [Google Scholar] [CrossRef]
- Cao, J.J.; Gregoire, B.R.; Sheng, X.; Liuzzi, J.P. Pinto bean hull extract supplementation favorably affects markers of bone metabolism and bone structure in mice. Food Res. Int. 2010, 43, 560–566. [Google Scholar] [CrossRef]
- Winham, D.M.; Hutchins, A.M.; Johnston, C.S. Pinto bean consumption reduces biomarkers for heart disease risk. J. Am. Coll. Nutr. 2007, 26, 243–249. [Google Scholar]
- Xu, B.; Chang, S.K.C. Comparative study on antiproliferation properties and cellular antioxidant activities of commonly consumed food legumes against nine human cancer cell lines. Food Chem. 2012, 134, 1287–1296. [Google Scholar] [CrossRef]
- Oseguera-Toledo, M.E.; de Mejia, E.G.; Dia, V.P.; Amaya-Llano, S.L. Common bean (Phaseolus vulgaris L.) hydrolysates inhibit inflammation in LPS-induced macrophages through suppression of NF-κB pathways. Food Chem. 2011, 127, 1175–1185. [Google Scholar] [CrossRef]
- Shutler, S.M.; Bircher, G.M.; Tredger, J.A.; Morgan, L.M.; Walker, A.F.; Low, A.G. The effect of daily baked beans (Phaseolus vulgaris) consumption on the plasma lipid levels of young, normo-cholesterolaemicmen. Br. J. Nutr. 1989, 61, 257–265. [Google Scholar] [CrossRef]
- Anderson, J.W.; Gustafson, N.J.; Spencer, D.B.; Tietyen, J.; Bryant, C.A. Serum lipid response of hypercholesterolemic men to single and divided doses of canned beans. Am. J. Clin. Nutr. 1990, 51, 1013–1019. [Google Scholar]
- Ambigaipalan, P.; Hoover, R.; Donner, E.; Liu, Q.; Jaiswal, S.; Chibbar, R.; Nantanga, K.K.M.; Seetharaman, K. Structure of faba bean, black bean and pinto bean starches at different levels of granule organization and their physicochemical properties. Food Res. Int. 2011, 44, 2962–2974. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Reynoso-Camacho, R.; Pedraza-Aboytes, G.; Acosta-Gallegos, J.A.; Guzman-Maldonado, S.H.; Paredes-Lopez, O.; Oomah, B.D.; Loarca-Piña, G. Chemical composition and in vitro polysaccharide fermentation of different beans (Phaseolus vulgaris L.). J. Food Sci. 2009, 74, T59. [Google Scholar]
- Beninger, C.W.; Gu, L.; Prior, R.L.; Junk, D.C.; Vandenberg, A.; Bett, K.E. Changes in polyphenols of the seed coat during the after-darkening process in pinto beans (Phaseolus vulgaris L.). J. Agric. Food Chem. 2005, 53, 7777–7782. [Google Scholar]
- Nathan, C. Points of control in inflammation. Nature 2002, 420, 846–852. [Google Scholar] [CrossRef]
- Barton, G.M. A calculated response: Control of inflammation by the innate immune system. J. Clin. Investig. 2008, 118, 413–420. [Google Scholar] [CrossRef]
- Dinarello, C. Anti-inflammatory agents: Present and future. Cell 2010, 140, 935–950. [Google Scholar] [CrossRef]
- Kleinert, H.; Schwarz, P.M.; Forstermann, U. Regulation of the expression of inducible nitric oxide synthase. Biol. Chem. 2003, 500, 255–266. [Google Scholar]
- Alderton, W.K.; Cooper, C.E.; Knowles, R.G. Nitric oxide synthases: Structure, function and inhibition. Biochem. J. 2001, 357, 593–615. [Google Scholar] [CrossRef]
- García-Lafuente, A.; Guillamón, E.; Villares, A.; Rostagno, M.A.; Martínez, J.A. Flavonoids as anti-inflammatory agents: Implications in cancer and cardiovascular disease. Inflamm. Res. 2009, 58, 537–552. [Google Scholar] [CrossRef]
- Yoon, J.-H.; Baek, S.J. Molecular targets of dietary polyphenols with anti-inflammatory properties. Yonsei Med. J. 2005, 46, 585–596. [Google Scholar] [CrossRef]
- Havsteen, B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef]
- Middleton, E.; Kandaswami, C. Effects of flavonoids on immune and inflammatory cell functions. Biochem. Pharmacol. 1992, 43, 1167–1179. [Google Scholar] [CrossRef]
- Libby, P. Inflammation in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2045–2051. [Google Scholar] [CrossRef]
- Wang, S.; Wu, D.; Matthan, N.R.; Lamon-Fava, S.; Lecker, J.L.; Lichtenstein, A.H. Reduction in dietary omega-6 polyunsaturated fatty acids: Eicosapentaenoic acid plus docosahexaenoic acid ratio minimizes atherosclerotic lesion formation and inflammatory response in the LDL receptor null mouse. Atherosclerosis 2009, 204, 147–155. [Google Scholar] [CrossRef]
- Venter, C.S.; Vorster, H.H.; Cummins, J.H. Effects of dietary propionate on carbohydrate and lipid metabolism in healthy volunteers. Am. J. Gastroenterol. 1990, 85, 549–553. [Google Scholar]
- Hopps, E.; Noto, D.; Caimi, G.; Averna, M.R. A novel component of the metabolic syndrome: The oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 72–77. [Google Scholar] [CrossRef]
- Tapsell, L.C. Diet and metabolic syndrome: Where does resistant starch fit in? J. AOAC Int. 2004, 87, 756–760. [Google Scholar]
- Beyer-Sehlmeyer, G.; Glei, M.; Hartmann, E.; Hughes, R.; Persin, C.; Böhm, V.; Schubert, R.; Jahreis, G.; Pool-Zobel, B.L. Butyrate is only one of several growth inhibitors produced during gut flora-mediated fermentation of dietary fiber sources. Br. J. Nutr. 2007, 90, 1057–1070. [Google Scholar]
- St-Onge, M.P.; Farnworth, E.R.; Jones, P.J. Consumption of fermented and nonfermented dairy products: Effects on cholesterol concentrations and metabolism. Am. J. Clin. Nutr. 2000, 71, 674–681. [Google Scholar]
- The Bean Institute Web site. Available online: http://beaninstitute.com/dry-beans-in-the-diet-may-benefit-people-with-diabetes/ (accessed on 14 September 2012).
- Lajolo, F.M.; Finardi, F.; Menezes, E.W. Amylase Inhibitors in Phaseolus vulgaris Beans. Food Technol. 1991, 45, 119–121. [Google Scholar]
- Lajolo, F.M.; Genovese, M.I. Nutritional significance of lectins and enzyme inhibitors from legumes. J. Agric. Food Chem. 2002, 50, 6592–6598. [Google Scholar] [CrossRef]
- Livesey, G.; Taylor, R.; Hulshof, T.; Howlett, J. Glycemic response and health—A systematic review and meta-analysis: Relations between dietary glycemic properties and health outcomes. Am. J. Clin. Nutr. 2008, 87, 258S–268S. [Google Scholar]
- CDC Web site. Available online: http://www.cdc.gov/Features/Cancer Statistics/ (accessed on 16 February 2012).
- ACS Web site. Available online: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-031941.pdf (accessed on 30 October 2012).
- Bobe, G.; Barrett, K.G.; Mentor-Marcel, R.A.; Saffiotti, U.; Young, M.R.; Colburn, N.H.; Albert, P.S.; Bennink, M.R.; Lanza, E. Dietary cooked navy beans and their fractions attenuate colon carcinogenesis in azoxymethane-induced Ob/Ob mice. Nutr. Cancer 2008, 60, 373–381. [Google Scholar] [CrossRef]
- Bawadi, H.; Bansode, R.R.; Trappey, A.; Truax, R.E.; Losso, J.N. Inhibition of Caco-2 colon, MCF-7 and Hs578T breast, and DU 145 prostatic cancer cell proliferation by water-soluble black bean condensed tannins. Cancer Lett. 2005, 218, 153–162. [Google Scholar] [CrossRef]
- Nijveldt, R.J.; Nood, E.; Hoorn, D.E.C.; Boelens, P.G.; Norren, K.; Leeuwen, P.A.M. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar]
- Garbisa, S.; Sartor, L.; Biggin, S.; Salvato, B.; Benelli, R.; Albini, A. Tumor gelatinases and invasion inhibited by the green tea flavanol epigallocatechin-3-gallate. Cancer 2001, 91, 822–831. [Google Scholar] [CrossRef]
- Govers, M.J.; Gannon, N.J.; Dunshea, F.R.; Gibson, P.R.; Muir, J.G. Wheat bran affects the site of fermentation of resistant starch and luminal indexes related to colon cancer risk: A study in pigs. Gut 1999, 45, 840–847. [Google Scholar] [CrossRef]
- Feregrino-Pérez, A.; Berumen, L.C.; García-Alcocer, G.; Guevara-Gonzalez, R.G.; Ramos-Gomez, M.; Reynoso-Camacho, R.; Acosta-Gallegos, J.; Loarca-Piña, G. Composition and chemopreventive effect of polysaccharides from common beans (Phaseolus vulgaris L.) on azoxymethane-induced colon cancer. J. Agric. Food Chem. 2008, 56, 8737–8744. [Google Scholar]
- Le Leu, R.K.; Brown, I.L.; Hu, Y.; Morita, T.; Esterman, A.; Young, G.P. Effect of dietary resistant starch and protein on colonic fermentation and intestinal tumourigenesis in rats. Carcinogenesis 2007, 28, 1052S–1057S. [Google Scholar]
- Diamant, M.; Blaak, E.E.; de Vos, W.M. Do nutrient-gut-microbiota interactions play a role in human obesity, insulin resistance and type 2 diabetes? Obes. Rev. 2011, 12, 272–281. [Google Scholar] [CrossRef]
- Collins, M.D.; Gibson, G.R. Probiotics, prebiotics, and synbiotics: Approaches for modulating the microbial ecology of the gut. Am. J. Clin. Nutr. 1999, 69, 1052–1057. [Google Scholar]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar]
- Roberfroid, M.B. Prebiotics: Preferential substrates for specific germs? 2001 73, 406S–409S.
- Pereira, D.I.A.; Mccartney, A.L.; Gibson, G.R. An in vitro study of the probiotic potential of a bile-salt-hydrolyzing Lactobacillus fermentum strain, and determination of its cholesterol-lowering properties. Appl. Environ. Microbiol. 2003, 69, 4743–4752. [Google Scholar] [CrossRef]
- Delzenne, N.M.; Kok, N. Effects of fructans-type prebiotics on lipid metabolism. Am. J. Clin. Nutr. 2001, 73, 456S–458S. [Google Scholar]
- Henningsson, Å.M.; Margareta, E.; Nyman, G.L.; Björck, I.M.E. Content of short-chain fatty acids in the hindgut of rats fed processed bean (Phaseolus vulgaris) flours varying in distribution and content of indigestible carbohydrates. Br. J. Nutr. 2007, 86, 379–389. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Câmara, C.R.S.; Urrea, C.A.; Schlegel, V. Pinto Beans (Phaseolus vulgaris L.) as a Functional Food: Implications on Human Health. Agriculture 2013, 3, 90-111. https://doi.org/10.3390/agriculture3010090
Câmara CRS, Urrea CA, Schlegel V. Pinto Beans (Phaseolus vulgaris L.) as a Functional Food: Implications on Human Health. Agriculture. 2013; 3(1):90-111. https://doi.org/10.3390/agriculture3010090
Chicago/Turabian StyleCâmara, Cristiane R. S., Carlos A. Urrea, and Vicki Schlegel. 2013. "Pinto Beans (Phaseolus vulgaris L.) as a Functional Food: Implications on Human Health" Agriculture 3, no. 1: 90-111. https://doi.org/10.3390/agriculture3010090





