1. Introduction
Salmonellosis is still an important cause of food-borne illness in humans. In 2006, a total of 165,023 confirmed cases of human salmonellosis were reported by the European Surveillance System [
1]. In Germany, 52,575 cases were notified in 2006.
Salmonella (
S.) Enteritidis and
S. Typhimurium were the serovars most frequently isolated from human cases. In 2006, 70% of all reported cases were associated with
S. Enteritidis and 24% with
S. Typhimurium. Beside these two major serovars,
S. Infantis and
S. Hadar (0.7% each),
S. Virchow,
S. Newport and
S. Derby (0.3 to 0.5% each) were the serovars reported next by frequency, reflecting altogether 1151 cases in 2006 [
2]. In 2011, the number of salmonellosis cases reported in the European Union (EU) had decreased to 95,548 cases [
3]. In Germany, a reduction by more than 50% to 24.512 cases was observed in 2011 compared to 2006 [
4]. Most probably, this reflects the efforts of the
Salmonella control program in laying hens, where
S. Enteritidis was the dominating serovar in Germany. The reduction in human cases was mainly due to the drop of cases caused by
S. Enteritidis to 45% (8764) of all cases. The proportion of cases caused by
S. Typhimurium increased in this period to 43%, whereas the absolute number decreased to 8346 cases. Farm animals and food of animal origin are an important source of human
Salmonella infection. Turkey and meat from turkeys are considered a relevant source of human infection [
5].
Before the baseline survey, data on the prevalence of
Salmonella along the turkey production chain were only available from the routine reporting systems underestimating most probably the true dimension of the problem. Among the Member States with
Salmonella positive production flocks, the observed occurrence of
Salmonella in turkey flocks ranged between 3.4% and 14.7% in 2006 [
1]. In contrast, in the baseline survey, on average 30.7% of the flocks tested positive [
6]. In Germany, in about 3.4% of the turkey flocks tested due to diagnostic investigations
Salmonella were detected [
7]. In 2006, the percentage of positive fresh turkey meat samples in the different countries varied from none to 14.3% [
1]. Of the turkey meat samples tested within official food control, 10.5% were contaminated with
Salmonella [
7].
Regulation (EC) No 2160/2003 and Directive 2003/99/EC foresee the collection of harmonized data in the EU and the implementation of an EU wide control strategy [
8,
9]. In this process, a baseline survey was carried out to determine the prevalence of
Salmonella in turkey flocks in the EU as foreseen in Decision 2006/662/EC and the technical specifications [
10,
11]. Based on the results of the baseline survey a preliminary target was set for the prevalence of
S. Enteritidis and
S. Typhimurium in turkey flocks and a sampling scheme was prescribed in Commission Regulation (EC) No 584/2008 [
12]. By the beginning of 2013, a final target together with a revised sampling scheme is set by Regulation (EU) No 1190/2012 [
13]. General elements of the National control programs are to be implemented in each Member State of the EU. These national control programs must cover the detection of
Salmonella following a minimum sampling and testing scheme recommended by international standardization bodies. In case
S. Enteritidis or
S. Typhimurium are detected in turkey breeding flocks, all birds, including day-old chicks in the flock and hatching eggs must be slaughtered or destroyed so as to reduce as much as possible the risk of spreading
Salmonella. Meat from fattening turkey flocks may not be placed on the market for human consumption unless it meets the criterion that
Salmonella spp. is absent in 25 grams (Commission Regulation (EC) No 2073/2005) [
14]. After the implementation of the control strategy in Germany, now the efficacy of the control program can be assessed against the target set.
The objectives of this study were (1) to assess the Salmonella prevalence in turkey flocks before and after the implementation of the control program in Germany and (2) to determine factors that are potentially associated with the presence of Salmonella in turkey flocks.
4. Discussion
One of the main findings of the baseline survey was that all turkey breeding flocks were negative in Germany. As shown by Mueller-Doblies
et al. [
20] a negative result in five pair boot swabs reflects that presence of
Salmonella in the flocks is very low. The favorable situation in breeding flocks may be related to the impact of
Salmonella vaccination which is regularly (82%) applied in German breeding flocks, but much less frequent in other countries (25% of the flocks in the EU). Results of the baseline survey on EU level showed that prevalence of
Salmonella spp. was higher in unvaccinated than in vaccinated breeding flocks [
6]. In Germany, more than 45 million turkey poults were hatched in 2007, around one third of them using imported hatching eggs. At the same time, less than 600.000 birds were imported but nearly 8 million birds exported [
22]. This reflects that around two third of the turkey poults fattened in Germany originated from German breeding flocks. In the baseline survey, on EU level, the observed prevalence of
Salmonella positive breeding flocks was 13.6%. Thus,
Salmonella may be introduced into fattening flocks by hatchings eggs from contaminated breeding flocks reflecting vertical transmission. In the EFSA analysis, a significant correlation between the prevalence estimates for breeding and fattening turkeys was observed [
23]. This comparison shows that German fattening flocks probably benefit from the favorable situation in German breeding flocks. However, as the origin of the individual chickens was not recorded during the baseline survey it can’t be proven that imported poults contributed to the
Salmonella findings in fattening flocks. In the United Kingdom, the risk of isolating
Salmonella spp. varied according to the company from which the poults were sourced [
24].
In the baseline survey, the observed prevalence rate for
Salmonella spp. was low with 10.3% in German fattening turkey flocks compared to the observed prevalence rate of
Salmonella positive flocks of 30.7% on EU level [
23]. In contrast, prevalence rates for
S. Enteritidis and
S. Typhimurium positive flocks were quite similar in Germany and on EU level with 3.7% and 3.8% [
23]. Only few flocks with the identical serovars and phage types were detected. This hints towards several independent sources from which
Salmonella were introduced into the fattening flocks. This may either be related to day old chicks of different origins or to horizontal introduction into the farms from non-turkey origins. A great diversity of
Salmonella sources for colonization of turkey flocks including infected chicks, feed and contaminated environment from previous flocks has already been described years ago [
25,
26]. Whereas in previous times frequently two or more different serovars were isolated in the same flock and also feed was frequently positive [
25], in our study the low infection rate and detection of a single serovar only even in all 15 flocks with five positive samples may hint towards an overall reduction of exposure and restriction to one major source for the individual flock. In a longitudinal study, carry-over of infection was observed in 44.8% of the positive houses, and introduction of new infection occurred in 8.4% of houses [
27].
S. Typhimurium,
S. Saintpaul and
S. Hadar were the most frequently observed serovars in the baseline survey in Germany. This is in line with global patterns from the EU study and US data [
23,
28] but differs also from some regional patterns, e.g. reported from UK or North Carolina [
29,
30]. The serovar pattern observed in Germany within the baseline survey was also in line with previous findings from diagnostic investigations in turkey flocks as well as in turkey meat [
31,
32]. All three serovars were among the most common serovars in turkey meat in Germany and in the EU in the same time period [
1,
32]. While
S. Typhimurium and
S. Hadar are also frequently isolated from other livestock species in Germany, such as pigs or broilers,
S. Saintpaul is predominantly found in turkeys. It has neither been isolated from broilers nor from laying hens or slaughtered pigs in the baseline surveys carried out [
33,
34]. Therefore, transmission of
S. Saintpaul from other species is unlikely even in regions with intensive livestock production such as the North–West of Germany and import of positive hatching eggs or day old chicks may be responsible for this pattern. On EU level, a tendency towards Member State specific clusters of
Salmonella spp. serovars was identified for flocks with fattening turkeys. This indicated on transmission of
Salmonella serovars mainly within the same Member State. For
S. Saintpaul a specific pathway from breeding flocks to fattening flocks in neighboring countries including Germany was identified in this study [
6].
Although there were substantial differences between the three regions of Germany with respect to the prevalence of
Salmonella, results of the logistic regression indicate that region could not be proved as a major factor contributing to the prevalence of
Salmonella in fattening turkeys. However, the sampling frame was not designed to detect regional differences within a Member State. In a study carried out in slaughter pigs, the pattern of regional distribution of prevalence rates was similar to that observed for turkeys [
33].
Whereas on EU level, the risk for
Salmonella infection increased with the size of the holding, this could not be confirmed in Germany. In other studies, several farm management related risk factors were identified [
24]. In France, the risk of
Salmonella contamination in fattening turkey flocks was decreased when floors were disinfected during decontamination procedures, when
Salmonella detection was carried out during rearing and when there was a metering pump in the house [
35].
In this study, risk for
Salmonella infection in free range flocks, which were mainly kept under organic conditions was significantly higher. This finding has to be considered with caution, as only 14 flocks had been included. In the EU study, the same finding was observed [
6]. In these flocks,
S. Kentucky or
S. Typhimurium was isolated. Both serotypes had also been detected in indoor flocks. A reason may be that animals housed with outdoor access are more prone to environmental contamination e.g., by feral birds or rodents. Good evidence for the importance of wildlife vectors, especially rodents and flies for the introduction of
Salmonella into flocks, its maintenance thereafter and the spread between flocks was described for laying hens but not for turkey flocks [
36,
37,
38,
39,
40].
In Germany, most positive flocks were detected in the first three month of the study (autumn). Likewise, on EU level, the risk of
Salmonella spp. in flocks with fattening turkeys was greater from October to December and January to March than from July to September [
6]. The reason for this observation remains unclear. In several studies dealing with
Salmonella contamination in turkey flocks, at slaughterhouses or retail meat, different seasonal patterns were observed. Some studies showed higher
Salmonella recovery rates in the spring/summer months than in the autumn/winter season [
41,
42] whereas others did not find such a pattern [
29,
43,
44]. In a longitudinal study covering the complete rearing period of turkeys,
Salmonella prevalence was increasing over time [
22]. Studies have demonstrated that increased pathogen loads in fecal shedding may occur during times of stress, such as high population housing [
38].
As the
Salmonella control program focuses on
S. Enteritidis and
S. Typhimurium, a prevalence of 3.7% of all fattening flocks positive for
S. Enteritidis or
S. Typhimurium was a further important finding of the baseline survey. Within a period of three years, starting in 2010, Member States of the EU have to meet a target for
S. Enteritidis and
S. Typhimurium of 1.0% [
12]. In the first two years of the new strategy, the very favorable situation in turkey breeding flocks was again confirmed in 2010 and 2011 in Germany. In the turkey fattening flocks, a substantially lower
Salmonella prevalence with a similar regional pattern was detected in the control program. A reduction of
Salmonella prevalence in turkey breeding flocks and fattening flocks was also recognized on EU level. There, in 2010 6.9% of the flocks tested positive for
Salmonella, 0.3% for
S. Enteritidis and
S. Typhimurium [
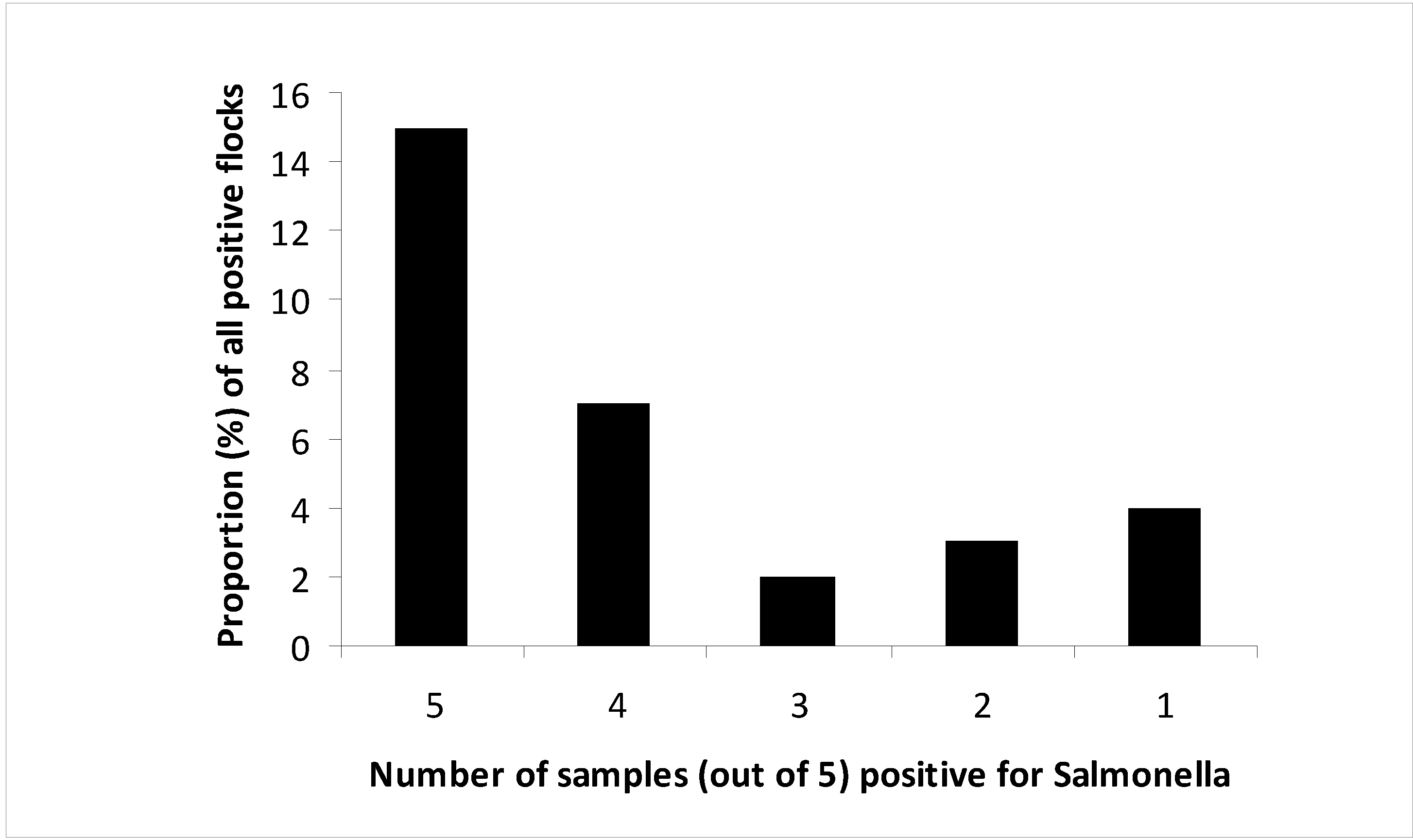
3]. As sample size was reduced from five pairs of boot swab samples in the baseline survey to two pairs of samples in the control program, reduced sensitivity of the sampling scheme may have contributed to the decrease in the observed prevalence rate. Analysis of the number of positive samples per flock in the baseline survey had shown that for more than half of the flocks not all five samples taken were
Salmonella positive. The European Food Safety Authority (EFSA) estimated on the basis of these results, that collecting only two instead of five samples would have led to a prevalence estimate on EU level for
S. Enteritidis and
S. Typhimurium of 2.8% instead of 3.8% [
23]. Mueller-Dobries
et al. [
20] showed in a comparative study on the sampling methods in turkey flocks that using only two pairs of boot swabs to sample all areas of the stable in comparison to splitting this to five compartments and five pairs of boot swabs had a sensitivity of around 80%. However, the reduction observed in Germany goes beyond the expected reduction by loss of sensitivity. In 2010, data in Germany and in the EU showed a significant reduction of
Salmonella prevalence in fattening flocks by around 60% compared to baseline survey results.
The reduced prevalence due to the control strategy in Germany is further confirmed by 2011 figures, where a further drop was observed for the overall Salmonella spp. prevalence and the prevalence of S. Enteritidis and S. Typhimurium using the same protocol as in 2010. The effect of risk based sampling, as required by legislation, may have led to a higher probability to find positive flocks. This may have lowered the difference in Salmonella prevalence from the first year of implementation to the second year of implementation, which is not significant. Several factors may have contributed to the decreasing tendency since the years 2006/2007. The baseline study and its outcome as well as the public discussions on the importance of Salmonella control in the laying hen and broiler sector made veterinarians and farmers aware of the Salmonella problem in the turkey sector. The regular testing of breeding flocks as well as fattening flocks combined with the pressure from the meat hygiene legislation and industry, marketing only Salmonella free poultry meat, were important drivers. Control measures taken by the individual farmers were not measured in Germany. But most probably, the requirement to restock birds only after cleaning and disinfection of the stable has been completed, to use feeding stuffs checked for Salmonella only, to apply strict hygiene regimes during the fattening period and to restock the farm with Salmonella free poults had been stepwise considered.
Major driver might have been the microbiological criterion and the pressure from the meat processing industry.
Salmonella carriage in turkey flocks is associated with the contamination level of turkey meat during slaughter and processing and thus subsequent introduction of
Salmonella into the food chain [
25,
45]. In Germany, the prevalence rate of
Salmonella positive turkey meat at retail dropped from 10.7% in 2006 and 6.0% in 2007 to 5.9% in 2010 and 3.4% in 2011 [
7,
32,
46,
47]. These data also point towards a benefit from the control strategy.
Among the serovars detected in turkey flocks in the baseline study in Germany,
S. Enteritidis and
S. Typhimurium were responsible for 94% of human salmonellosis cases in 2007 and 88% in 2011, comprising at least 17,120 cases in 2011. The other serovars identified in turkey flocks were identified in 1.1% (611) human cases in 2007 and 2.5% (623) human cases in 2011 reflecting a relative increase of relevance of these serovars for human infection. Due to the successful control of
S. Enteritidis in laying hens, the relative importance of
S. Typhimurium and other serovars was increasing considerably over the years. However,
S. Typhimurium is also frequently isolated from pigs and pork, indicating that turkey products may be not the only source of
S. Typhimurium in humans [
34]. Therefore, the impact of
Salmonella control in turkey flocks on the reduction of human
Salmonella infections needs to be assessed continuously.