4.1. Impact of Soil Heavy Metals on Performance and Mineral Flow of the Developing Wheat Crop
Non-permissible HM levels of soil A [
62,
63,
64] were accountable for phytotoxic concentrations (
Table 1; [
68,
69]) of Cd, Cu, and Zn in roots and of Zn in shoots of cv. JB Asano from the seedling state to maturity, as well as of Cd in the foliage of seedlings. Cadmium concentrations in the herbage violated the hygiene standards of forage (1 mg∙kg
−1; [
5]), whereas the high Zn load may be tolerable for monogastric livestock (500–1000 mg∙kg
−1) rather than for ruminants (300–500 mg∙kg
−1; [
71]). The strict indigenous HM control of seeds [
19,
20,
28,
72] at least held the grains obtained from a soil of 40 mg∙kg
−1 Cd to the permissible range of forage (
Table 1). The roots, herbage, and seeds grown on the non-contaminated soil C persisted in the range of ‘normal plant HM concentrations’ (
Table 1; [
62,
65]) and yielded food quality grains. In reference to these, phytotoxic effects of soil A reduced straw and grain production to 47.7% and 39.5%, respectively (
Table 2). Both the quantity of grains produced as well as their inherent attempts to gain the optimum but not maximum in (organics and) minerals essential for survival and germination [
27,
73] coined the mineral flow across the herbage.
In the sum of macronutrients and trace elements except Zn, soil-A grains reached around 94% and 90%, respectively, of the soil
C-grown seeds but were loaded up with Zn at the expense of Fe (derived from
Table 1). The soil A-grown straw reached 96.4% of the soil
C-grown biomass in the sum of trace elements except Zn. It dominated in the sum of macronutrients with 147% the soil-
C grown straw whose partial depletion resulted from the higher rates of mineral relocations from sink tissues to the 2.5-fold higher grain biomass.
The high demand of grains in soil C treatment depleted the minerals in root and shoot, whereas the few saturated soil A grains blocked mineral drain from the respective sink tissues (
Table 1 and
Table 2) and depreciated them to dead-end sinks of the less essential elements in the course of maturation. The gradual (seed fill-initiated) decline of the mineral load from root to seed is common to mature cereals obtained from non-contaminated soils [
74,
75,
76,
77]. However, a declining mineral load in growing plants may also be uptake-dependent. In rice, the acquisition of Ca, K, Mg, N, and P peaked at panicle initiation (i.e., at 56% of the plants’ development) to fall in a bell-shaped curve to zero at maturity [
78]. Similarly, the foliages of alfalfa and annual ryegrass showed diminishing mineral resources in later growth stages [
79,
80]. Maillard et al. [
81] recorded growth cessation in leaves of cereals, further crops, and trees at around 40% of their life span and initiation of senescence followed by a drain of nutrients at midlife. Ageing goldenrod (
Solidago gigantea Ait.) preferentially translocated KP to the perennial rhizomes [
82].
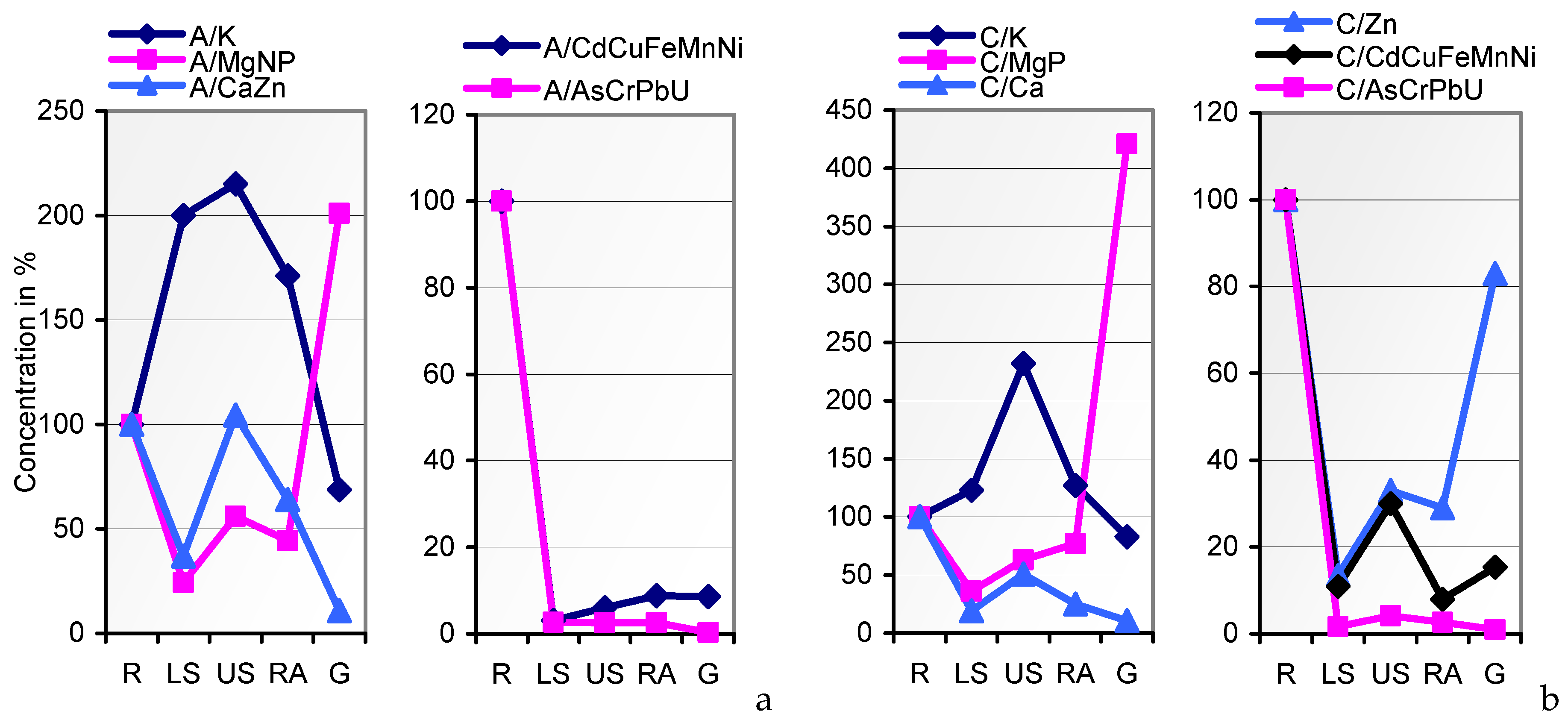
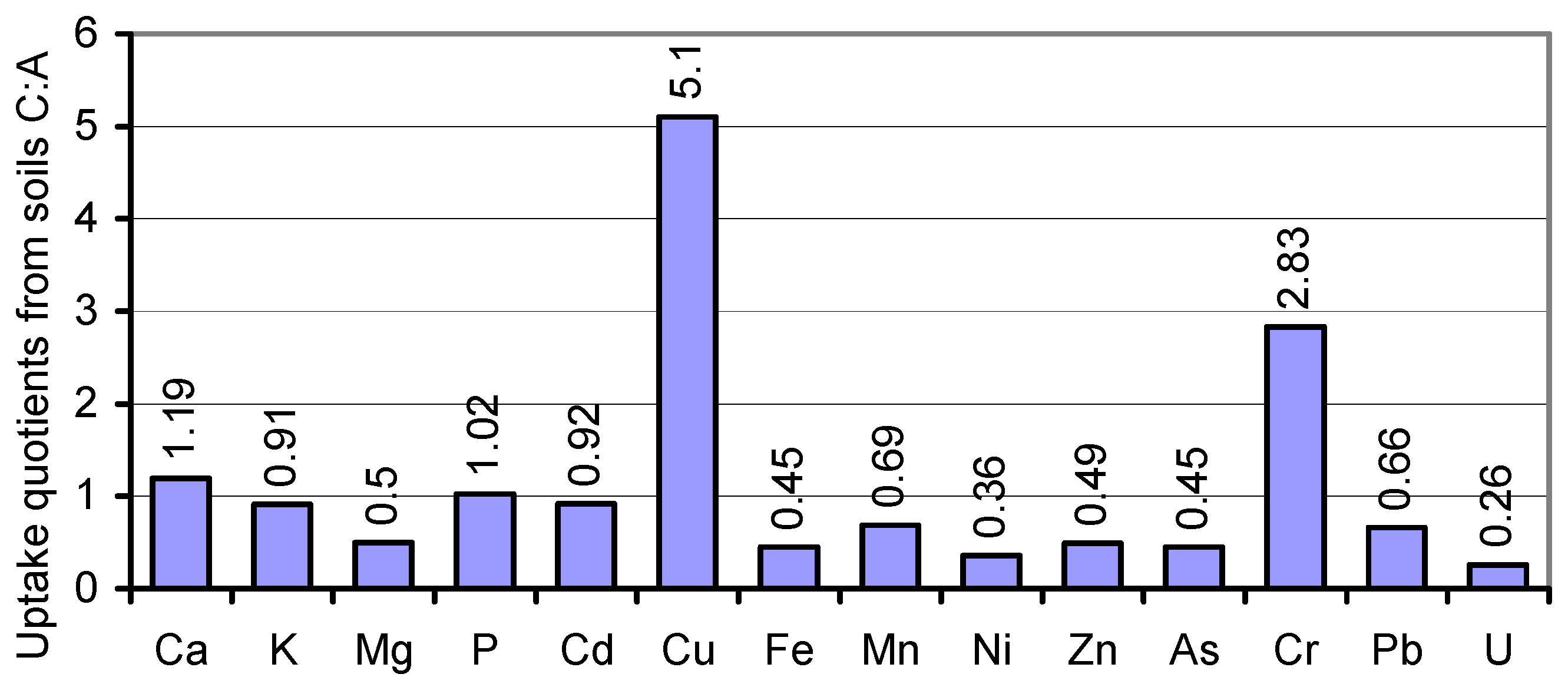
In the search for mineral uptake competitions in cv. JB Asano from the two variably composed soils A and C, we calculated the percentages of the elements per kg of the whole plant tissue (mg∙kg
−1 DW) to their total content in soil. Fixing soil A percentages at factor 1, the soil uptake rates of Ca, Cd, K, and P by soil
C-grown tissues coincided with those from soil A (
Figure 5). Unlike Cu and Cr, Mg and most of the traces were taken up at drastically lower rates from the resources in soil C. Attempts to level the Cu and Mg content of soil A- and soil
C-grown grains during seed fill in the inherently narrow span (
Table 1; [
27,
72]) may have caused the relatively elevated and reduced respective uptake of these elements, whereas variations in the uptake rates of other elements, for example, by mechanisms of uptake competition, shall not be derived from the data. The respective conclusions for a single organism should solely be drawn from lab-scale tests on defined media rather than on polymetallic soils. Thus, the impact of elevated HM concentrations in soil on the grain content of macronutrients cannot be postulated.
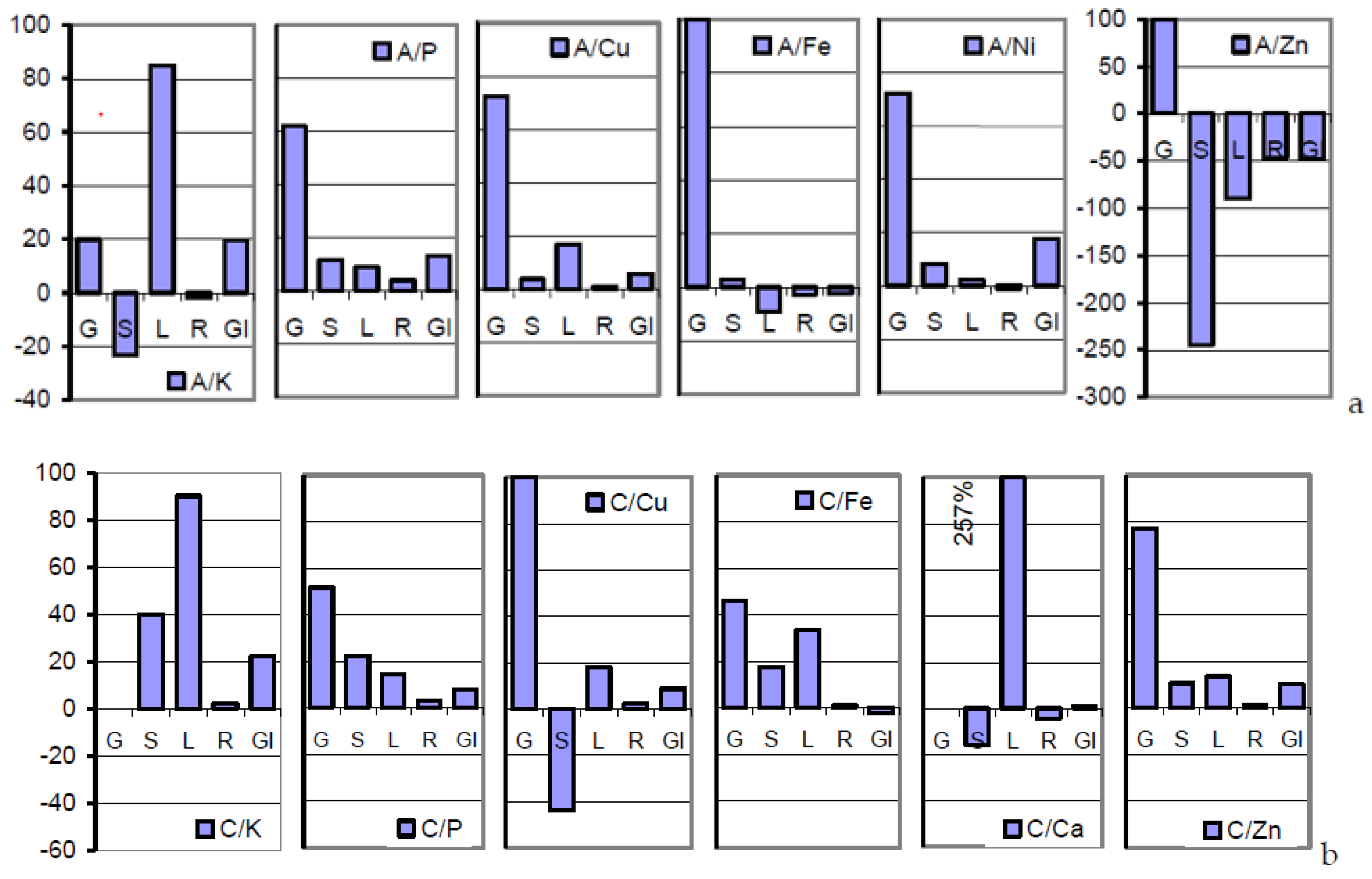
The mineral loads arrested in the sections of root, lower/upper stem, and rachis of the mature soil A-grown plant deviated drastically from those of the grain in a manner that is poorly accessible to interpretations. Severe incisions into the flow of all the minerals except K occurred at the root/lower stem interface (
Table 3;
Figure 1a). The concentrations predominantly rising in the upper shoot were once more dramatically down- or up-regulated at the extremely versatile rachis/grain interface in more or less successful attempts to realize the inherent seed target metallome of the cultivar [
19,
20,
21,
72]. Unlike the herbage, grains respond least to changes in agronomic conditions and the supply with soil minerals [
83,
84,
85] and nitrogen [
18,
23], and they stabilize their metallome inherently in a narrow range [
21,
23,
27]. This was also reported for the Fe and Zn content of soybean [
22], rice [
21], and pea plants [
20], although the respective concentrations in vegetative tissues rose drastically. Similarly, variable regimes of fertilization had little impact on the seed content of fatty acids and cellulose in peanut [
86], of amino acids, sugars, and phenolics in pea plants [
19,
87], and of starch and proteins in maize [
88]. Raising the N
org content in shoots of Chinese cabbage (
Brassica chinensis L.) to the 3.4- to 3.7-fold of water-treated plants by NH
4Cl application resulted in near-linear increases in the sum of the metalloprotein-associated transition metals, (Cd), Co, Cu, Mn, Ni, and Zn but not of Fe to 3.6- to 3.7-fold. Contributions of the individual elements ranged from 2.5× (Co, Cu, Ni) to >4× (Cd, Mn, Zn). Concentrations in Ca and Mg with their structural, metabolic, and enzyme regulatory functions [
89,
90] reached 2-fold. Concentrations in the sum of Fe, K, Na, V, and a group of non-essential metals did not exceed 1.1- to 1.2-fold as the individual contributions of Cr, Fe, and V diminished (to 0.45–0.75×) whereas those of Al, Ba, K, Li, Pb, Sr, Ti, and U slightly increased (to 1–1.8×) [
91]. The protein (N
org) content in grains from soil A ranged below that of the comparable reference grains (
Table 3) and could be partly responsible for their lower mineral load. Peterson et al. [
92] postulate the uptake promotion of Ca, Cu, Fe, Mg, Mn, P, S, and Zn but not of Cl and K by rising grain protein.
Table 4 and
Figure 1b document the congruity in the mode of mineral distribution and management in soil A- and soil C-grown plants. Therefore, the grains coincided with the widest in the content of macronutrients as well as in Cu and Fe (
Table 1) as shown by the narrow concentration spans around the inherent target level (values in parentheses). The corresponding concentration spans in soils and wheat grains amounted to, for example, 3.15 and 1.49 in Ca, respectively, 7.5 and 1.08 in Cu, respectively, 25 and 3.69 in Zn, respectively, but 32 and 24, respectively, in the less compensable Cd [
27].
In wheat grown on Tunisian mine tailings, soil, shoot, and grain concentrations varied by 27, 49, and 13× in Pb, respectively; 420, 43, and 30× in Cd, respectively; and 97, 35, and 2.4× in Zn, respectively [
83]. The field pea cv. Rocket responded to soil concentration spans of macro- and micronutrients in the range of 4.6 (Ca) to 109 (Zn) with variations of 1.31–2.40×, and to 475× in soil Cd with 15.9× in seeds [
19]. Hundreds of spelt wheat genotypes were grown at six locations in Turkey over three years. Notwithstanding large differences in their individual mineral uptakes, the median-value variations in grain protein and Ca, Cu, Fe, K, Mg, Mn, P, S, and Zn across all accessions ranged from as little as 1.16× (K) to 2.02× (Ca) [
85].
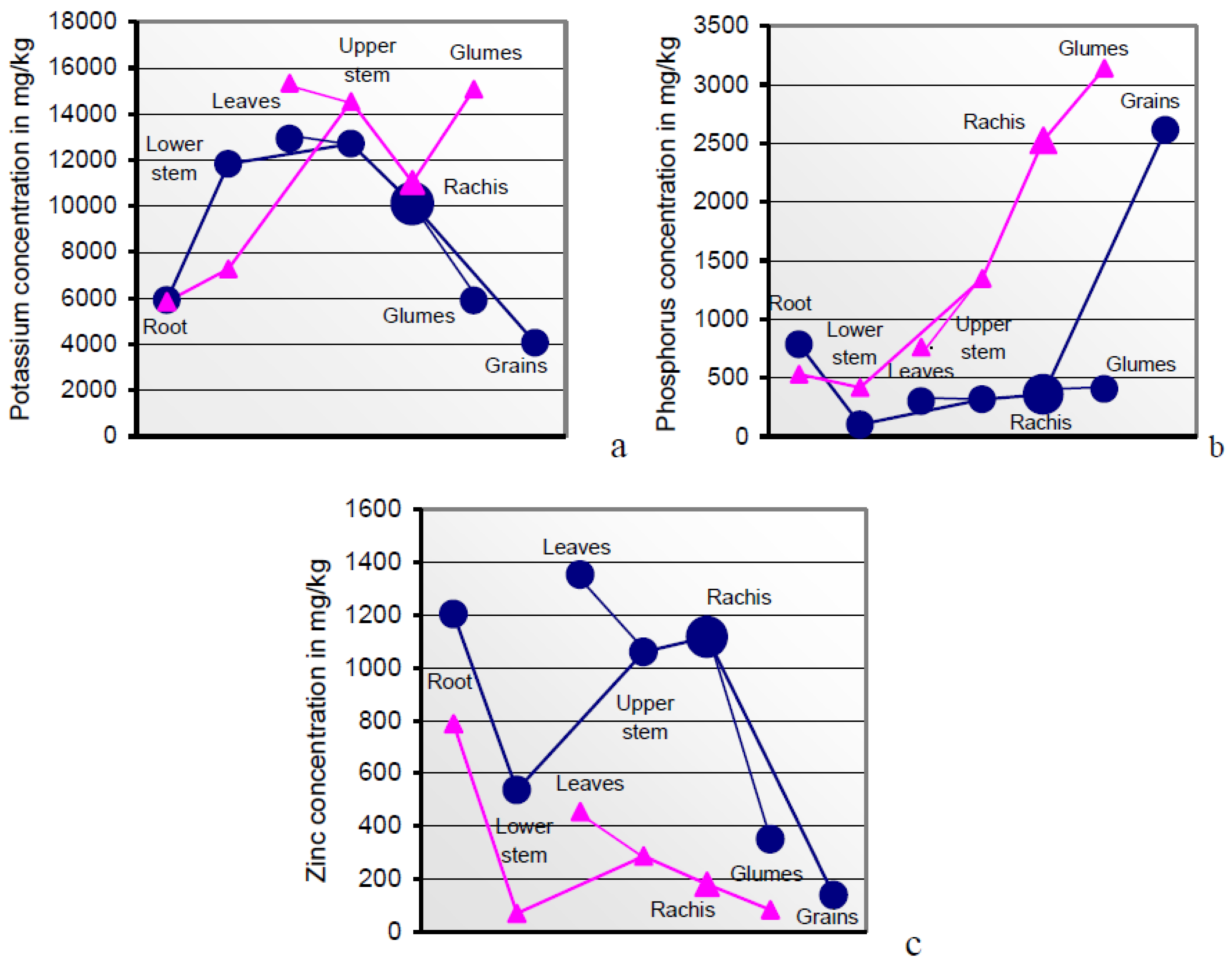
Higher resources in anthetic than in mature plant sections indicate mineral relocations from the gradually senescing sink tissues of the post-midlife herbage [
81] to the phloem-fed grains, superimposed by rapidly diminishing mineral uptake rates from the soil [
78]. Flag leaves with internodal sheaths and glumes were the sink tissues with the highest mineral concentrations (
Table 3 and
Table 4;
Figure 2 and
Figure 6). Sucrose and potassium are the major osmotic species [
93] to move the phloem sap via differences in the hydrostatic pressure from the donor tissues to the grains [
94].
It is part of the plant’s strategic and analytical top performance to identify the individual organic and mineral phloem constituents and to translocate them in delimited quantities to the grain in realizing its genetically pre-determined composition [
72]. The cytoplasm strands in sieve tubes actively capture the single phloem constituents in congruence with the current plant metabolic state [
90]. The
Figure 6a–c illustrate the order of surplus concentrations of K, P, but not Zn, across anthetic to mature sections of soil A-grown plants. Interestingly, the plant safely distinguishes between both the rachis-borne structures of grains and glumes in their roles as sink and source tissues.
4.2. Applicability of the Herbage from the Metalliferous Soil A
Compared to the herbage from soil C, the Cd, Cu, and Zn phytotoxicity of soil A reduced the harvestable biomass in cv. JB Asano to 40% and the total uptake of K and P from soil proportionally to 46% 33%, respectively (
Table 6). N, P, and K concentrations of the harvestable straw from both soils (
Table 6) corresponded the widest with the values reported for K (6.7–11.6 kg∙Mg
−1∙DW); P (0.38–1.3); and N (5) [
32,
95,
96,
97]. Contaminations of 1.69–4.24 mg∙kg
−1 DW in Cd and up to 1353 in Zn of the mature soil-A grown straw (
Table 1 and
Table 3) exclude its use as bedding material or roughage supplement to domestic animals [
5,
71]. This rule must not apply to the premature herbage. The Cd load of the (post-)winter herbage as well as of the soil itself is a serious hazard to winter grazing and thereby soil ingesting local herbivores (
Table 1; [
12,
98,
99]). Nevertheless, the rapidly growing green matter in the pre-tillering to the post-anthesis state shows a temporary and significant decline in the critical toxicants As, Cd, Cr, Pb, U, and Zn (
Table 1) and could potentially serve as a roughage supplement for the domestic livestock. This is supported by the facts that wheat herbage in the predominantly leafy and anthesis states varied in crude protein (24.4% and 11.8% of DW, respectively), hemicellulose (17.9 and 31.5, respectively), cellulose (18 and 27.3, respectively), lignin (1.9 and 4, respectively), and thus in digestibility (74.7 and 68.3, respectively) [
100]. Similarly, the metabolizable energy (ME) in the herbage cut at flowering, milk or dough stage, and maturity was 9, 8, and 7–8 MJ∙kg
−1∙DW in oats and 8, 8, and 6–7 MJ∙kg
−1∙DW in wheat, respectively [
101].
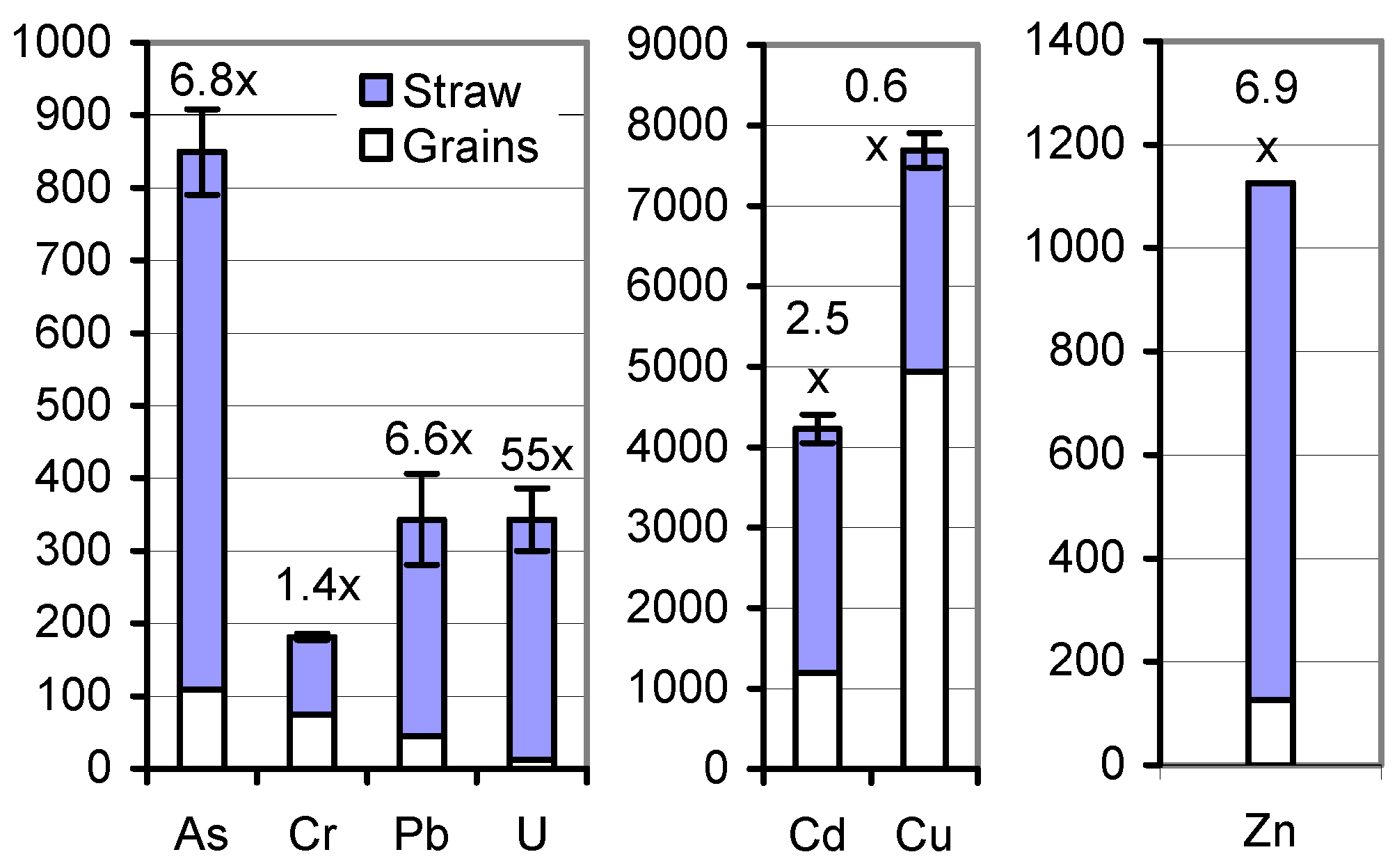
Returning the mature soil A-grown herbage to the cropland may be less problematic. In the sum of As, Cd, Cr, Cu, Pb, and U, and Zn (individually), 33.8 and 461 g ha
−1, respectively, came to roots and stubbles, 9.85 and 1927, respectively, to the entire harvestable rest straw, and, for comparison, 15.6 and 308, respectively, to the grains (
Table 5;
Figure 3). Processing the straw to biochar [
102,
103] may prevent the excessive propagation of mineral-immobilizing and nitrogen-binding microorganisms in alkaline soils while its positive effects on crop production of temperate zone soils are contested [
104]. Apart from mineral losses by volatilization during straw combustion at 400–800 °C [
96], 3.08 mg∙kg
−1 DW of Cd in the mature soil-A grown straw (
Table 1) would account for only 0.03‰–0.06‰ of the ash content reportedly ranging 4.65%–9.9% per∙DW [
32,
35,
96,
105]. In proportion to the macronutrients, Cd came to 11,280 mg∙per∙kg∙P, to 282 per∙kg∙K, and to 786 per∙kg∙N (refer to
Table 1). Comparable values for the root biomass amounted to 37,500 in P, 4975 in K, and 2673 in N. Ashed soil-A grown herbage is therefore no NPK fertilizer. Commercial high-purity mineral P fertilizers of 7–33 mg∙Cd per∙kg∙P
2O
5 constitute 16–75 mg∙Cd∙per∙kg∙P and are surpassed by values of 220–570 mg∙Cd∙per∙kg∙P in other products [
8,
106].
One can accept the use of the Cd-contaminated wheat straw in rates of 15%–45% as bulking material in the vermicomposting of sewage sludge (e.g., [
107]). Renouncing its use as a soil structure-improving material, however, does not really contribute to soil remediation. Consecutive wheat crops of 1070 years (Zn), 2400 (Cd), and 28,400 years (Pb) were necessary to capture 50% of the HM load of soil A by the whole plant (including roots) under the conditions of stable uptake rates (
Table 5). This corresponds with similar calculations [
10,
11,
12]. True soil remediation effects may come from the gradual downwash of mineral-clay complexes from the plough layer to subsoil strata [
8,
9]. Uranium waste rock deposits restored and turfed 30–35 years ago were initially treated with liquid manure and husbandry composts which released metal cation ligands and motile organic colloids. Across 34 test plots, the re-distribution of As, Cd, Co, Cr, Cu, Ni, Pb, U, and Zn resulted in mean concentrations of 108, 158, and 187 mg∙kg
−1 DW in the 0–3-cm sward layer, the 4–20-cm (plough) layer, and the 21–50-cm subsoil, respectively. In test plots not impaired by soil compaction and pseudogley formation, the ratings from the 0–3 cm sward layer, the 4–20 cm (plough) layer, and the 50–80 cm subsoil came to, for example, 42, 66, and 190 mg∙kg
−1∙DW, respectively, in As; 3.6, 4.8, and 23.5, respectively, in Cd; 31, 41, and 111, respectively, in Pb; and 459, 725, and 4104, respectively, in Zn with means of 76, 116, and 577, respectively, across all the elements [
9,
108].
The HM load of the soil A-grown herbage does not interfere with the extraction of green chemicals [
36,
109]. However, the fungi used in the preparatory delignification of the biomass feedstock for bioethanol production face the full toxicity of internal HM. The resulting oxidative stress leads to the damage of cellular biomolecules such as DNA, lipids, and proteins by reactive oxygen species and may result in cell death [
110,
111,
112] and restrictions in mycelial growth, enzymatic activity, and the rate of substrate conversion [
113,
114,
115]. Toxicity effects of Cd as pronounced in liquid media drastically diminish in the presence of soils, organic matter, and high concentrations of dissolved salts [
116]. In the solid-state fermentation of rice straw with the basidiomycete
Phanerochaete chrysosporium, Cd halved the activities of lignin peroxidase at 4 mg∙kg
−1 DW and of manganese peroxidase at 6 mg∙kg
−1; it also halved the degradation of lignin at 16 mg∙kg
−1, and of cellulose and hemicellulose at about 32 mg∙kg
−1 [
115]. The weight loss of straw incubated with
P. chrysosporium was optimum at Pb concentrations of 30 mg∙kg
−1, but proceeded, too, at 400 mg∙kg
−1 [
117]. The colonization of non-sterile soil by the basidiomycete
Pleurotus ostreatus reached limits at concentrations of 50–100 mg∙kg
−1 DW in Hg and 100–500 mg∙kg
−1 in Cd [
113]. An A-type soil lot of 41 mg∙kg
−1∙DW in Cd, 156 mg∙kg
−1 in Pb, and 3320 mg∙kg
−1 in Zn enabled normal mycelial growth and fructification by the lignicolous basidiomycetes
Hypholoma fasciculare, Kuehneromyces mutabilis, and
P. ostreatus [
118]. Lignocellulose fermenting fungi should therefore tolerate the full but moderate HM load of the soil-A grown wheat straw, whereas drastic HM dilution steps precede its use as feedstock in current bioethanol fermentation experiments at lab scale.
The preferred Simultaneous Saccharification and Fermentation (SSF) technology includes the hydrothermal pre-treatment at elevated temperature and atmospheric pressure predominantly in solid-liquid proportions of 1:10 (
w/
w) followed by the washing and drying of the solids. In the subsequent breakdown of cellulose and hemicellulose by commercial hydrolase enzymes and the concomitant microbial transformation of the liberated fermentable hexose sugars to ethanol, solid-liquid proportions of 1:10 to 1:20 are reported in the incubation media (e.g., [
35,
37,
48,
52]). According to Le et al. [
119], the retention of minerals in the hydrothermally pre-treated lignocellulose is dependent on pH, temperature, and treatment time. Initial pH values of 2–4 made Ca, K, Mg, Mn, P, and Zn levels fall to less than 20% by DW whereas Al, Cu, Fe, and Si concentrations did not change. These data match the widest with those obtained by experimental autoclaving of soil A-grown straw at 121 °C which was combined with a 1:15 dilution step at pH 3.9 (
Section 3.6). The treatment rendered the concentrations of the phytotoxicants Cd, Cu, and Zn non-critical.
The enzymatic hydrolysis of the pre-treated lignocellulose structures could promote release of the residual HM resources into the liquid incubation medium. Baker’s yeast (
Saccharomyces cerevisiae) as the preferred ethanologenic organism experienced 5% and EC50 growth inhibition in solution at 0.11 and 1.12 mg∙L
−1, respectively, in Cd; 55 and 96 mg∙L
−1, respectively, in Mn; 59 and 117 mg∙L
−1, respectively, in Ni; and 65 and 163 mg∙L
−1, respectively, in Zn during 12 h of incubation [
57]. The yeast exposed to 10 mg∙L
−1 Cd was temporarily inhibited to regain an almost normal growth rate [
120] apparently owing to its biosorption capacity in the order of 45 mg Cd
2+ per gram of biomass within 2 h [
121]. Exposure to Cu reduced the viability of the yeast cells to 88% at 0.32 mg∙L
−1 within 1 h and to 11% at 2.54 mg∙L
−1 within 5 min [
60]. Incubation at 30 °C for 24 h reduced the number of viable yeast cells from 18 × 10
9 per gram to 5–12 × 10
9 in the presence of 25 mg∙L
−1 Pb, and to 4–8 × 10
9 in the presence of 10 mg∙L
−1 Cd [
58]. Adding 50 µM Cd (5.6 mg∙L
−1) to a glucose/yeast extract medium reduced ethanol production by
S. cerevisiae from 25 to 2 g∙L
−1 within 10 h. Ethanol formation was not impaired even at 1 mM∙Cd in the medium if its uptake was competitively repressed by Ca
2+ supplements at the molar ratio of 50–100:1 [
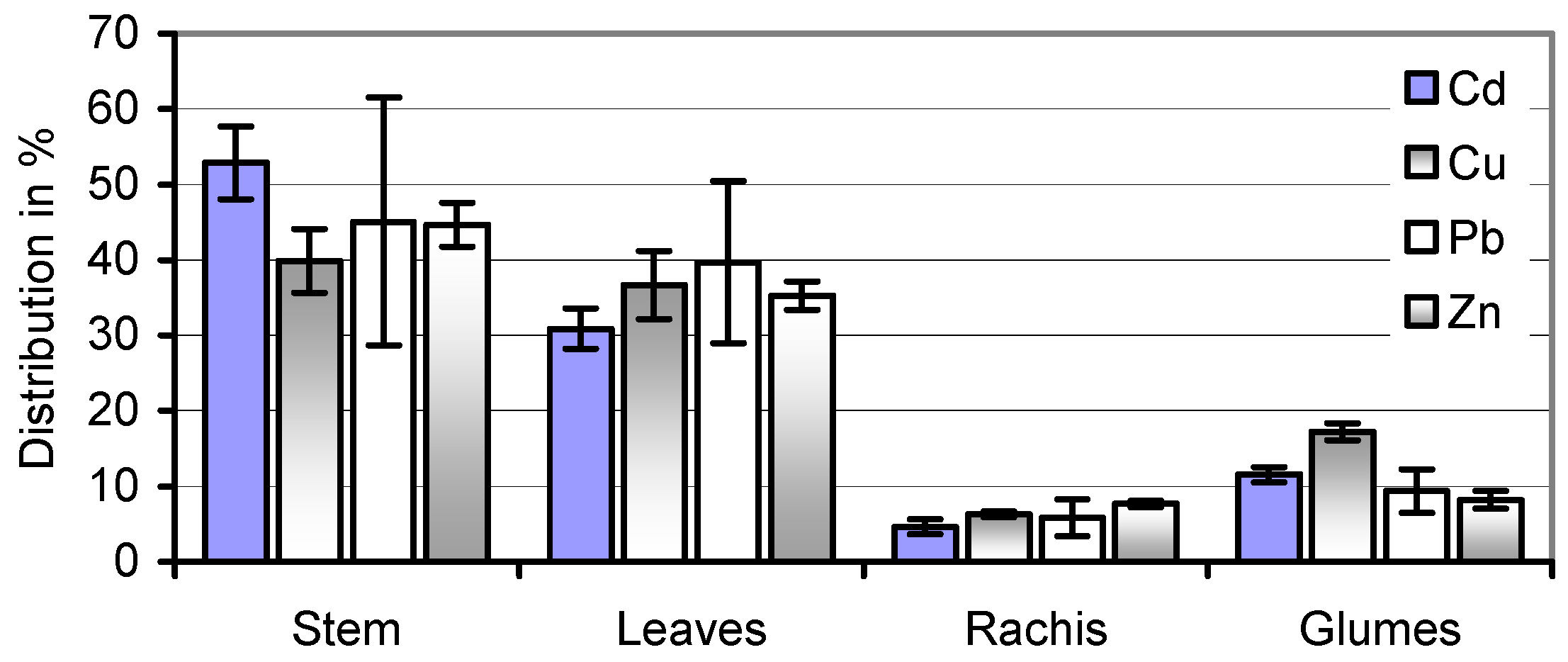
59]. The equal distribution of Cd, Cu, Pb, and Zn in stems and leaves of the soil A-grown straw (
Figure 4) makes speculations about a selective harvest of less-contaminated and therefore more valuable portions of the herbage illusory. At the same time, wet pre-treatment removes the majority of its potential HM toxicants.
Critical Cd and Cu concentrations may be obtained during the fermentation of ground whole-wheat grains in aqueous slurries. The suspensions may contain 10%–17% dry matter (
w/
w) at lab scale [
122,
123] and around 25% in practice [
124]. The combined effects of Cd and Cu concentrations reaching 3.13 and 12.3 mg∙kg
−1∙DW, respectively, in several soil A-grown wheat cvs. (
Table 3) could strain HM tolerance and vitality of yeast cells to the utmost. Their productivity could be impaired by the progressive acquisition of HM even from low concentrations in the incubation media during the 48–72-h batch fermentation process [
125].