1. Introduction
The complex physical and chemical phenomena associated with polyunsaturated oil or fatty acid (PUFA) oxidations remain very active research topics due to their importance in health maintenance. Indeed, due to their chemical structure, fat is very sensitive and frail. During the stages of production, storage or heating in food process, industrial oils and butters are susceptible to physicochemical and organoleptic deterioration, particularly because of oxidation and/or isomerization of unsaturated fatty acid chains leading to the formation of products harmful to health (peroxides, aldehydes, radicals, polymers, etc.) [
1,
2,
3].
Some synthetic antioxidants (butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), and tert-butylhydroquinone (TBHQ)) are currently used in food industry to slow down the activity of the oxidation catalysts or limit the spread of free radicals [
4,
5]. These artificial preservatives are nevertheless suspected of toxic, sensitizing, allergenic, and carcinogenic effects [
6].
Natural oil sources usually contain stabilizing compounds, which can be supplemented with various antioxidants to further extend storage or use lifetime. Mechanisms by which phenolics protect lipids from oxidation include reduction of reactive oxygen, nitrogen, or sulfur species, and by hydride donation to oxidized lipids to interrupt propagation events [
6]. The need to find new sources of oilseeds and natural preservatives of oils are the aims of much research into the native flora, especially the African one.
Previous ethnobotanical investigations on oilseeds from the South West of Burkina Faso had revealed that
Lannea microcarpa (African grape),
Sclerocarya birrea (A. Rich.) Hochst. (Marula), and
Pentadesma butyracea (butter tree) possess high potentials:
Lannea microcarpa plant organs (leaves, bark, fruits, mistletoe, and gum) are traditionally used against gastroenteritis, female infertility, high blood pressure, edema, coughing, poisoning, and burns;
Sclerocarya birrea (Marula) organs are used to treat diabetes, gastroenteritis, high blood pressure, and scurvy. The butter from
Pentadesma butyracea (butter tree) is a valuable alternative to Shea butter, with local people using it in food, in cosmetics, and for some therapeutic treatments [
7].
The oils from the oilseed of these three species are traditionally used for food, cosmetics, and phytotherapy by local people. This suggests that they could be valorized for fat stabilizing. Similarly, extraction residues rich with dietary components (lipids and proteins) have good potential for use as cattle feed.
The present study aimed to screen for agro-industrial potential (antioxidant, Shea butter stabilization, and feed capacity of the oilcake) of the oilseeds of Lannea microcarpa (African grape), Sclerocarya birrea (Marula), and Pentadesma butyracea (butter tree), and to correlate the activities with their phytochemicals.
2. Materials and Methods
2.1. Chemicals
To carry out our different activities (phytochemical screening and biological activities), we used solvents and various classic reagents. All reagents were of analytical grade. Folin-Ciocalteu reagent, sodium carbonate (Na2CO3), sodium hydroxide, sodium phosphate, gallic acid, quercetin, Aluminium trichloride (AlCl3), hydrochloric acid, magnesium turnings, corn starch and trichloro-acetic acid were purchased from Sigma Aldrich chemie (Steinheim, Germany); Ammonia, and potassium hexacyanoferrate were supplied by Fluka chemie (Buchs, Switzerland); sulfuric acid, acetic anhydride, ferric trichloride, chloroform, ethanol, methanol, petroleum ether and hexane were sourced from Prolabo (Paris, France); p-iodonitrotetrazolium chloride (INT) was sourced from Sigma-Aldrich (Hamburg, Germany); ascorbic acid, DNS (dinitrosalicylate), porcine pancreatic α-amylase, trypsin, and PABA (parabenzoic acid) were supplied by Labosi (Paris, France). Shea butter was purchased from a local company (Phycos industry, Ouagadougou, Burkina Faso).
2.2. Extraction
2.2.1. Extraction by Maceration
Flour (Ø 0.5 µm) obtained from grinding of the dried seeds (50 g) was macerated in 500 mL of 70% ethanol, for 48 h. Afterwards, the resulting extract (EE) was filtered throughout a Whatman paper n°1 and concentrated using rotary evaporator (Buchi Rotavapor R200, Buchi, Flawil, Switzerland) coupled with a vacuum pump at 60 °C.
2.2.2. Extraction by Percolation
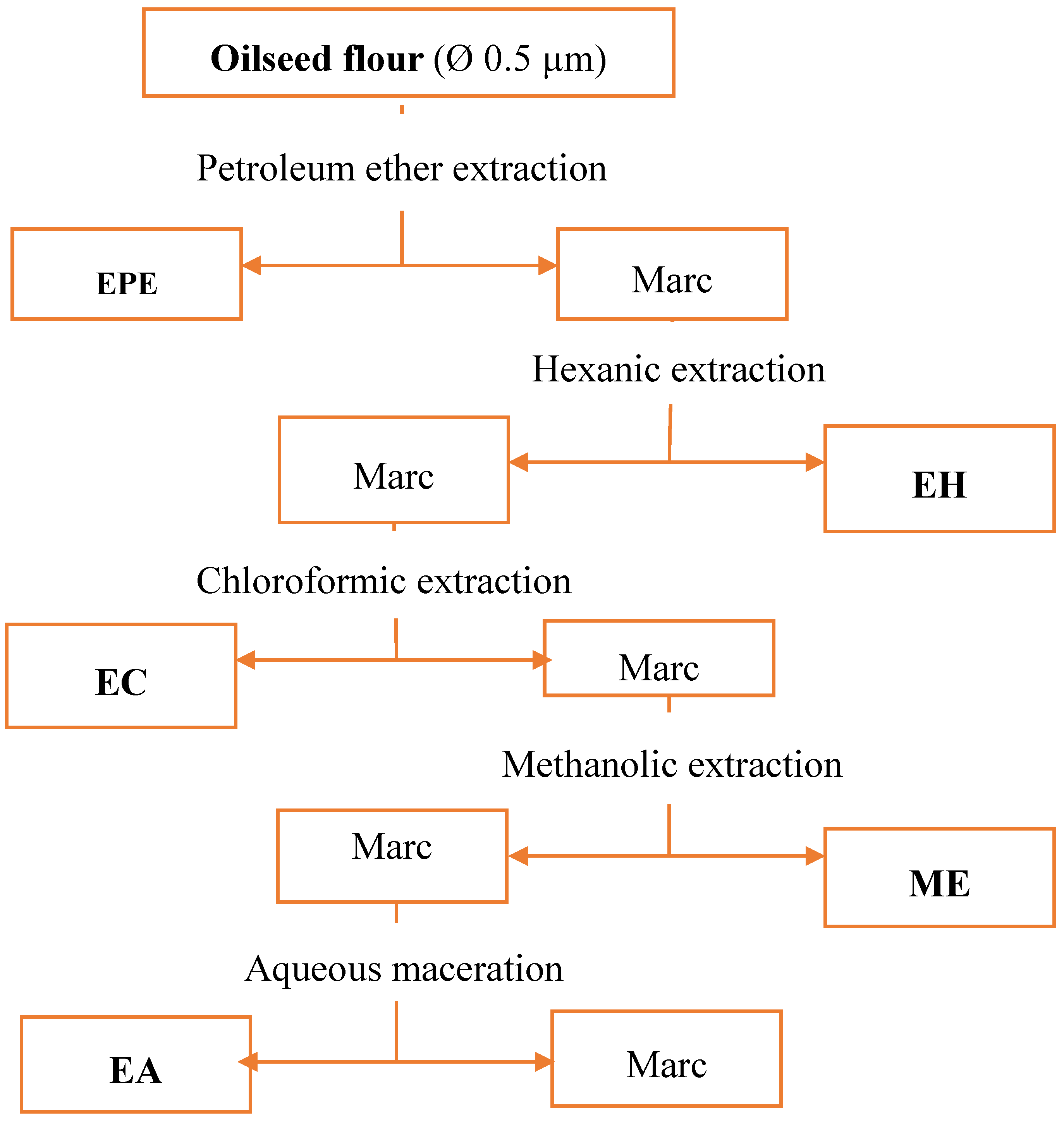
Flour (Ø 0.5 µm) obtained from grinding of the dried seeds (30 g) was mixed with 300 mL of solvent, and put in a Soxhlet apparatus (Fisherbrand NS29//NS45 32/40, Fischer, Strasburg, France) [
8]. Extractions were successively performed using the following order of organic solvents: petroleum ether, hexane, chloroform, and methanol. After a decoction for 2 h, the extracts were filtered using a Whatman paper. After pooling, the extracts were concentrated using rotary evaporator (Buchi Rotavapor R200), coupled with a vacuum pump at 60 °C. The diagram of extraction steps are summarized in
Figure 1.
2.3. Evaluation of Shea Butter Stabilizing Power: Rancimat Test
The Rancimat method is an accelerated shelf life test. Air is passed through the sample (solution of EPE, EH, EC extracts with or without Shea butter) in the reaction vessel at constant elevated temperature (120 °C). In this process fatty acids are oxidized. At the end of the test, volatile secondary reaction products are formed and transported into the measuring vessel by the air stream and absorbed in the measuring solution (deionized water). The continuously recorded electrical conductivity of the measuring solution is increasing due to the absorption of the reaction products. Thus, their appearance can be detected. The time until secondary reaction products are detected is called time of induction in Rancimat test, or TIR (in hour). It characterizes the stability to oxidation of oils and fats (ISO 6886, 2006). TIR is the time during which the fat resisted to oxidation. The percentage of resistance to Rancimat oxidation or the increase in the Rancimat induction time (ATIR %) of the sample is calculated using this formula:
where % ATIR = percentage of increase in Rancimat Induction Time for Shea butter; TIRsample = Rancimat induction time for the sample (butter + extract); TIRblk = Rancimat induction time for the pure butter sample.
For the routine assay, Shea butter (3 g) was mixed with the stabilizing solution to obtain 5% (w/w) of extract in Shea butter. The mixture was stirred and then used for the Rancimat test, which was conducted at 160 °C.
2.4. Antioxidant Activity Tests
2.4.1. DPPH Radical Scavenging Assay
Radical scavenging properties of the oilseed extracts against stable DPPH• (2,2′-diphenyl-1-picrylhydrazyl) radicals were determined spectrophotometrically at 517 nm using the method of Vélazquez [
9]. A series of 10 successive dilutions (by half) were done from a stock solution containing 10 mg/mL of extract in methanol (
w/
v). Then 100 μL of sample solution and 200 μL of DPPH solution (20 mg/L in methanol) were added in each tube. After 15 min in the dark at room temperature, the decrease in absorption was measured. The blank sample was composed of the same amount of methanol and DPPH• solution. All experiments were performed in triplicate. Radical scavenging capacities were calculated by the following formula:
where A
0 is the absorption of blank sample, and A is the absorption of tested extract solution. The extract concentration allowing a 50% scavenging activity (IC
50) was determined and expressed in μg/mL.
2.4.2. FRAP Assay
Ferric Reducing Antioxidant Power (FRAP) assay was carried out as described by Hinneburg [
10]. A volume of an extract solution (0.5 mL, 0.1 mg/mL) was added and mixed together with 1.25 mL, 0.2 M phosphate buffer pH 6.6 and 1.25 mL, 1% (
w/
v) aqueous potassium hexacyanoferrate (K
3Fe(CN)
6). After 30 min incubation at 50 °C, 1.25 mL, 10% trichloro-acetic acid was added. The mixture was centrifuged at 2000 rpm for 10 min. The supernatant (125 μL) was mixed with 125 μL 1% (
w/
v) aqueous FeCl
3 and 125 μL of distilled water. A control without extract was prepared under the same conditions. The absorbance was recorded at 700 nm. A calibration curve was carried out with ascorbic acid (0–200 mg/L). The Iron (III) reducing capacity was expressed as mg ascorbic acid equivalents (AAE)/g of extract (dry weight). The following formula was used:
where
C = concentration expressed as mg AAE/g of extract;
c = concentration read (mg AAE/L);
D = dilution factor;
CI = initial concentration of the extract.
2.5. Phytochemical Screening
The phytochemical screening consisted of a qualitative analysis of secondary metabolites present in the plant extracts samples using classical color or precipitation reactions.
2.5.1. Detection of the Main Phyto-Constituents by Tube Tests
a. Detection of tannins and phenolic compounds using the FeCl
3 test. A pinch of each hydroethanolic dried extract (EE) was dissolved with 2 mL of distilled water in a test tube, and then to 1 mL is added two to three drops of 1% solution of FeCl
3. The appearance of a dark blue color indicates the presence of gallic tannins, while catechin tannins give a blackish-green coloring [
11].
b. Detection of flavonoids by the Shibata test
. A pinch of each hydroethanolic dried extract (EE) was dissolved in 2 mL of 50% methanol. The solution was divided into two test tubes, one serving as a control, and the other for the test. In the test tube, some magnesium turnings were added with four drops of fuming HCl. A red color indicates the presence of flavonoid aglycone in the extract [
11].
c. Detection of triterpenes or sterols by the Libermann-Buchard test
. A pinch of each hydroethanolic dried extract (EE) was dissolved in a test tube containing a mixture of chloroform and acetic anhydride (1: 1
v/
v). H
2SO
4 (2 mL) was then gently poured in the bottom of the tube. If a red-brown or violet color appeared after 5 to 10 min (in the form of a ring, in the liquids separation zone), it indicates the presence of sterols and/or triterpene [
11].
d. Detection of saponines by the foam test. A pinch of each hydroethanolic dried extract (EE) was dissolved in a test tube containing 2 mL of distilled water. Then the solution obtained was stirred for 15 min. The appearance of a foam column, with at least 1 cm of height, persistent for 15 min, indicates the presence of saponines [
11].
f. Detection of coumarins
. A pinch of each hydroethanolic dried extract (EE) was dissolved in 2 mL of distilled water. The resulting solution was divided into two equal volumes in two test tubes; one serving as control, and the other as test tube in which was added 0.5 mL of 10% NH
4OH. The appearance of a blue or green fluorescence after excitation with a UV lamp at 65 nm indicated the presence of coumarins [
11].
g. Detection of anthraquinones by the Bornträger test. A pinch of each hydroethanolic dried extract (EE) was dissolved in a test tube with 1–2 mL of 25% NH
4OH. After stirring, the occurrence of a serum red color indicates the presence of emodol (revealing free or oxidized anthraquinones derivatives) [
11].
h. Detection of anthocyanins by the Piattelli Test
. A pinch of each hydroethanolic dried extract (EE) was dissolved in a test tube and divided into two parts. They were treated with either concentrated HCl or NaOH. Appearance of a red or blue color, according to whether acidic or alkaline medium was used, indicates the presence of anthocyanins [
11].
2.5.2. Total Phenolic Content Determination
Theses determinations were performed on methanol extracts (10 mg/mL). Total polyphenols were estimated with Folin-Ciocalteu reagent using gallic (GA) acid as standard [
12]. 25 μL of the test sample and 125 μL of Folin-Ciocalteu reagent (FCR) 0.2 N in distilled water were added to a test tube. After 5 min incubation, 100 μL of sodium carbonate (75 g/L) was added. After stirring and incubation in the dark for two hours, absorbance is read at 760 nm against control sample. The following formula was used to calculate the concentration expressed as gallic acid equivalent (GAE) in 100 mg of extract.
C = concentration expressed as mg GAE/g of extract;
c = concentration read (mg GAE/L);
D = Dilution factor;
Ci = initial concentration of the extract.
2.6. Evaluation of the Nutritional Quality of the Oilcake
2.6.1. Inhibition of α-Amylase
The α-amylase inhibitory activity was determined using the dinitrosalicylate assay (DNS) of Bernfield [
13,
14,
15]. Briefly, 200 µL of EE extract (10 mg/mL) and 500 µL, 20 mM sodium phosphate buffer pH 6.9 containing porcine pancreatic α-amylase (0.5 mg/mL) were incubated at 25 °C, for 10 min. Then, 500 µL of 1% corn starch solution in 0.02 mol/L sodium phosphate buffer (pH 6.9) was added to each tube. The reaction mixtures were incubated at 25 °C, for 10 min. The reaction was stopped with 1.0 mL of the basic DNS reagent. Then the mixture was incubated in a boiling water bath for 5 min, and cooled to room temperature. The reaction mixture was then diluted by adding 10 mL of distilled water and the absorbance measured at 540 nm. The inhibitory activity on α-amylase was expressed as a percent of inhibition, using the following equation:
where OD is the Optic density; OD
blank is the OD of the blank (starch + amylase); and OD
sample is the sample (starch + enzyme + extract).
2.6.2. Test of Trypsin Inhibition
This method is based on that described by Kakade [
16] and adapted by Malomo [
17]. Diluted sample suspension (1 mL) was pipetted in duplicate sets of test tubes and the volumes adjusted to 2 mL with distilled water. After adding 2 mL of trypsin solution (0.5 mg/mL) to each test tube, the tubes were placed in a water bath at 37 °C. Then 5 mL of
para-aminobenzoic acid (PABA) solution (previously heated to 37 °C) were added, and two minutes later, the volume was completed with 1 mL of 30% acetic acid. After mixing, the content is filtered (throughout a Whatman paper n°1) and the absorbance of the filtrate was measured at 410 nm against a reagent blank. The percentage of inhibition within each tube is calculated by the following equation:
where: OD is the Optic density; OD
blank is the OD of the blank (PABA+ trypsin); and OD
sample is the sample (starch + enzyme + extract).
2.7. Statistical Analysis
Data are expressed as mean ± SD from three separate observations. For in vitro antioxidant assays, a one way ANOVA test, followed by Tukey’s test (p < 0.05), was used to analyze the differences among EC50 of various fractions for different antioxidant assays.
4. Discussion
Analysis of radical scavenging activity (with DPPH radical) of extracts from African grape (
Lannea microcarpa), Marula (
Sclerocarya birrea), and Butter tree (
Pentadesma butyracea) has shown that this activity varies linearly with the extract concentration. These results corroborate those of Milianskas et al. [
20], who have shown that the total phenolics contribute over 90% in antioxidant activity of the extracts of various herbs, including
Salvia sp.,
Lavandula angustifolia,
Calendula officinalis,
Matricaria recutita,
Echinacea purpurea,
Rhaponticum carthamoides,
Juglans regia,
Melilotus officinalis,
Geranium macrorrhizum,
Potentilla fruticose, etc. [
4,
21].
The positive correlation between the reduction power on the ferric ions (FRAP) and antiradical activity of extracts (DPPH) shows that polar extracts (rich in polyphenols) have the best antioxidant activity [
4]. Indeed, Pearson linear correlation coefficients between the total polyphenol content and antioxidant capacity of the extracts are significantly positive for all three plant species (
R values are 0.984, 0.5867 and 0.7636 for
L. microcarpa,
S. birrea, and
P. butyracea, respectively). Thus, polyphenols are the dominant antioxidants in the extracts of these oilseeds.
Analysis of the results of the stabilization test of Shea butter by different extracts showed a gradual increase in preservation of Shea butter to oxidation (at 160 °C) depending on the antioxidant content of the extracts. Thus, comparing the three types of apolar extracts tested (petroleum ether, hexanic, and chloroformic), chloroformic extract is presented as the most optimal for the activities of radical scavenging (DPPH), the reduction of ferric ion (FRAP), and the stabilizing activity on Shea butter with Rancimat test. Natural oil sources usually contain preservative phenolics, which contribute in part to their preservation of oxidation [
2].
The study of linear correlations between the total polyphenol content of the different extracts examined and the stabilizing activity on Shea butter, using Pearson correlation test, gave interesting correlation coefficients of 0.724 and 0.984 for L. microcarpa and for P. butyracea, respectively.
From these results, it is obvious that polyphenols contribute massively to the protection against Rancimat oxidation of Shea butter. This explains that natural seed oils and weakly or not refined oils, such as olive oil rich in various phenolic compounds, are naturally protected by their endogenous phenolic antioxidants [
6,
22].
In the case of the extract of S. birrea, where the correlation between the total polyphenol content and the stabilizing activity on Shea butter has a middle value (R = 0.518), stabilization may be due to the contribution of other molecules, such as vitamins (tocopherols for example) and mineral elements, which have antioxidant activity.
Even if the polar extracts (methanol or hydro-ethanol) contain high rates of polyphenols, they have weak activity for Shea butter stabilization because of their weak miscibility with the butter. Perhaps these extracts contain high polar polyphenols or bound phenolics, as opposed to less polar organic solvents extracts (e.g., chloroform extracts with less polar polyphenols such as flavonols; also free phenolics or lipophilic vitamins such as tocopherols).
The aqueous ethanolic extract of an oilseed may contain all soluble eventual toxic substances of the oilcake. The extracts from all three oilseeds showed very low inhibition of α‑amylase and trypsin, which are key digestive enzymes of livestock. The results showed that 11.46 ≤ IC
50 α-amylase ≤ 34.17 mg/mL against 0.62 mg/mL for ascarbose (type 2 diabetes medicine), and 2.1 mg/mL for methanolic extract of leaves of
C. maxima [
12]. α-Amylase (α-1→4-
d-glucan 4-glucanohydrolase, EC 3.2.1.1) is an ubiquitous endo-enzyme that randomly splits α-(1→4)-linkages in polyglucans, such as starch and glycogen, yielding glucose, maltose, and maltodextrins [
2]. It is the major type of amylase found in mammals [
3]. Known α-amylase inhibitors are organic molecules, including proteins, polyphenols, and phytates, that impair the activity of the enzyme. The inhibition of this enzyme delays and reduces digestion of carbohydrates containing α-1,4-
d-glucose linkages, such as starch and other α‑glucans; thus it is an anti-nutritional factor for human and cattle.
For trypsin, the inhibition values are also weak (10.68 ≤ IC
50 ≤ 14.50 mg/mL). Trypsin (EC 3.4.21.4) is a serine protease found in the digestive system of many vertebrates. It is an endopeptidase involved in the breakdown of many different proteins, including as part of digestion in humans and other animals. It is used for numerous biotechnological processes. As a result, trypsin inhibitors that interfere with its activity can have an anti-nutritional effect [
12,
17]. The low anti-nutritional activity obtained with the oilseed extracts shows that the oilcakes of these three oilseed species may be easily digested by cattle. The oilcakes display high potential for livestock feed.
5. Conclusions
Results obtained show that the extracts of the three oilseeds possess interesting bio-preservative potential for stabilization of Shea butter. The chloroformic extracts contained fair polar (or middle polar) polyphenols, and gave the strongest antioxidant activities and higher stabilization of the shea butter. This shows that the polyphenols present in the extracts act to slow down the physico-chemical process of thermal degradation of oil during heating.
Among the three species used in this study, extracts of Lannea microcarpa revealed the greatest potential for bio-preservation of food and industrial oils and fats.
As oilcakes from industrial crop like soybean constitute the main source of protein for animal feed, in addition to being costly for the farmers of developing countries, the non-toxicity of the oilcake of the species studied here could allow them to become valued as feed.
Assessing resource availability and ensuring sustainable production of these native oilseeds in local communities may contribute to food security in West Africa.