Influence of Composted Dairy Manure and Perennial Forage on Soil Carbon and Nitrogen Fractions during Transition into Organic Management
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Soil Sampling
2.3. Soil Total Carbon and Nitrogen
2.4. Mineralizable (Active) Carbon and Nitrogen Fractions
2.5. Resistant Soil C and N Fraction
2.6. Intermediate (Slow) Soil C and N Fraction
2.7. Statistical Analysis
3. Results and Discussion
3.1. Soil Organic C (SOC) and Soil Total N (STN)
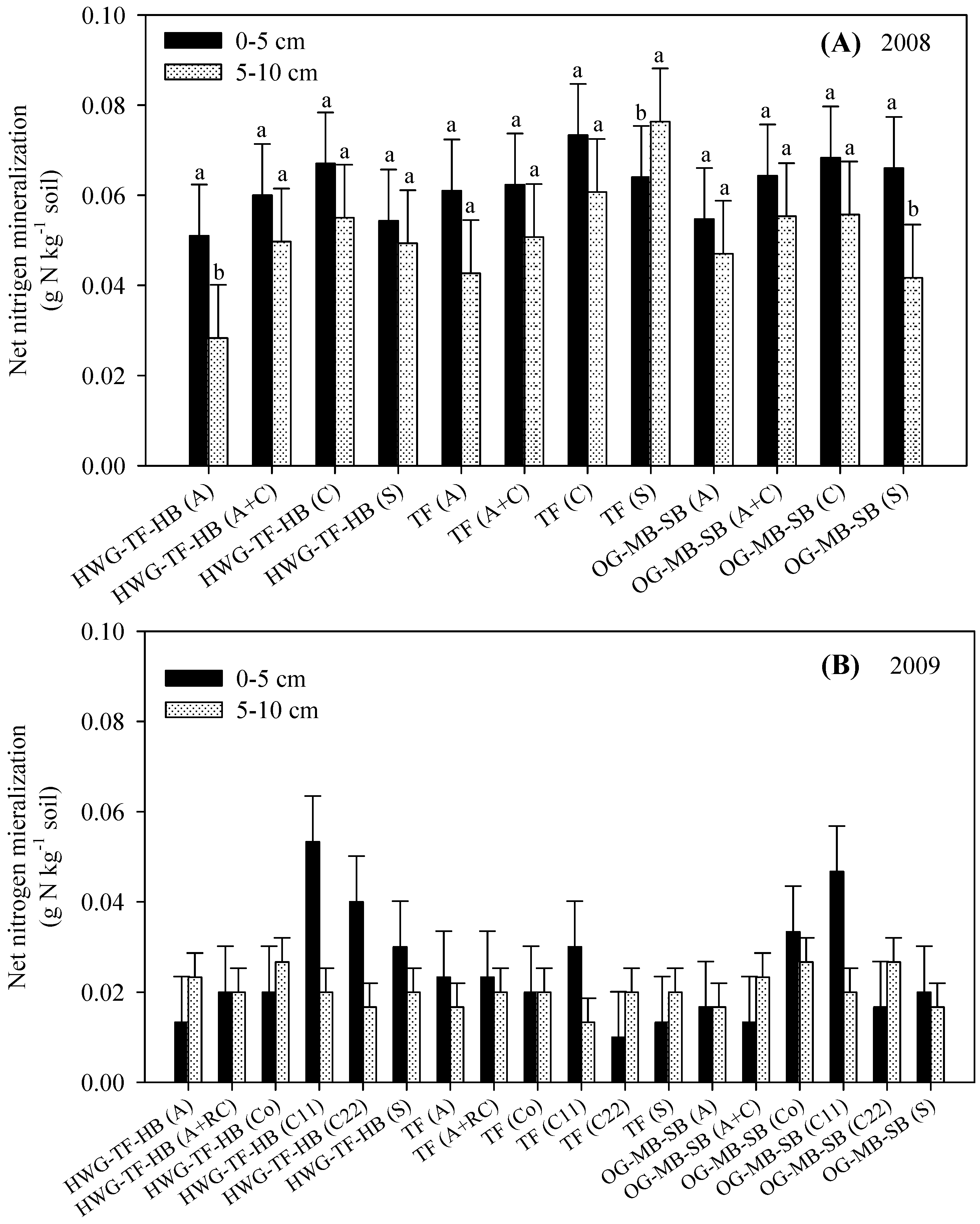
3.2. Net Nitrogen Mineralization (Nmin)
3.3. Potential Mineralizable C (Co) and Mineralization Rate Constant (kc)
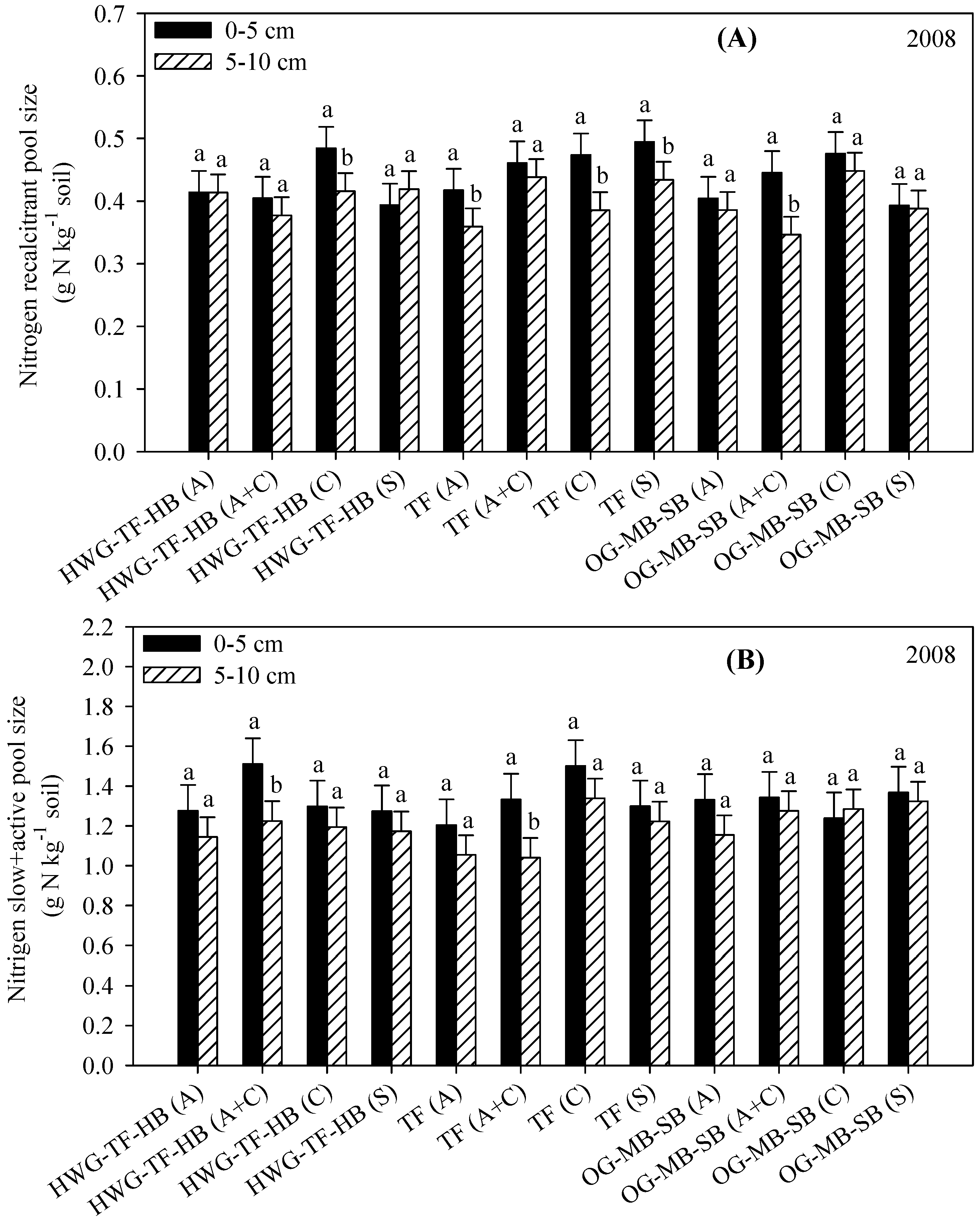
3.4. Resistant (Acid Hydrolysis) and Slow Soil C and N Fractions
3.5. Relationship between Soil C and N Fractions
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Economic Research Service. Table 2, Unites State Certified Organic Farmland Acreage, Livestock Numbers, and Farm Operations. Table 4, Certified Organic Producers, Pasture, and Cropland. Number of Certified Operations, by State, 2000–2011. Total Acreage of Certified Organic Pasture and Cropland by State, 1997 and 2000–2011; USDA, Economic Research Service: Washington, DC, USA, 2013. Available online: http://www.ers.usda.gov/data-products/organic-production.aspx (accessed on 24 October 2013).
- Sacco, D.; Moretti, B.; Monaco, S.; Grignani, C. Six-year trasition from conventional to organic farming: Effects on crop production and soil quality. Eur. J. Agron. 2015, 69, 10–20. [Google Scholar] [CrossRef]
- McBride, W.D.; Greene, C. Characteristics, Costs, and Issues for Organic Dairy Farming; Economic Res. Rep. 82; USDA-Economic Research Service: Washington, DC, USA, 2009. Available online: https://www.ers.usda.gov/webdocs/publications/err82/11004_err82_1_.pdf?v=41079 (accessed on 19 April 2017).
- Lui, K.; Hammermeiste, A.M.; Warman, P.R.; Drury, C.F.; Martin, R.C. Assessing soil nitrogen availability in contrasting cropping system at the end of transition to organic production. Can. J. Soil Sci. 2011, 91, 493–501. [Google Scholar]
- U.S. Department of Agriculture; National Organic Program. Title 7 Code of Federal Regulations, Part 205; USDA: Washington, DC, USA, 2016. Available online: http://www.ecfr.gov (accessed on 9 May 2016).
- Cusick, P.R.; kelling, K.A.; Powell, J.M.; Munoz, G.R. Estimates of residual dairy manure nitrogen availability using various techniques. J. Environ. Qual. 2006, 35, 2170–2177. [Google Scholar] [CrossRef] [PubMed]
- Endelman, J.B.; Reeve, J.R.; Drost, D.T. A new decay series for organic crop production. Agron. J. 2010, 102, 457–463. [Google Scholar] [CrossRef]
- Cook, J.L.; Brummer, J.E.; Meiman, P.L.; Gourd, T. Colorado Forage Guide Bulletin # 563A, Colorado State University, 2014. Available online: http://www.ext.colostate.edu/sam/forage-guide.pdf (accessed on 19 April 2017).
- Lynch, D.H.; Voroney, R.P.; Warman, P.R. Nitrogen availability from composts for humid region perennial grass and legume-grass forage production. J. Environ. Qual. 2004, 33, 1509–1520. [Google Scholar] [CrossRef]
- Antil, R.S.; Bar-Tal, A.; Fine, P.; Hadas, A. Predicting nitrogen and carbon mineralization of composted manure and sewage sludge in soil. Compost Sci. Util. 2011, 19, 33–43. [Google Scholar] [CrossRef]
- Ros, G.H.; Temminghoff, E.J.M.; Hoffland, E. Nitrogen mineralization: A review and meta-analysis of the predictive value of soil test. Eur. J. Soil Sci. 2011, 62, 162–173. [Google Scholar] [CrossRef]
- Wander, M. Soil organic matter fractions and their relevance to soil function. In Soil Organic Matter in Sustainable Agriculture; Magdoff, F., Weil, R.R., Eds.; CRC Press: Boca Raton, FL, USA, 2004; pp. 67–102. [Google Scholar]
- Davidson, E.A.; Janssens, J.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Ros, G.H.; Hanegraaf, M.C.; Hoffland, E.; van-Riemsdijk, W.H. Predicting soil N mineralization: Relavence of organic matter fractions and soil properties. Soil Biol. Biochem. 2011, 43, 1714–1722. [Google Scholar] [CrossRef]
- Olk, D.C.; Gregorich, E.G. Overview of the symposium proceedings, Meaningful pools in determining soil carbon and nitrogen dynamics. Soil Sci. Soc. Am. J. 2006, 70, 967–974. [Google Scholar] [CrossRef]
- Smith, P.; Smith, J.U.; Powlson, D.S.; McGill, W.B.; Arah, J.R.M.; Chertov, O.G.; Coleman, K.; Franko, U.; Frolking, S.; Jenkinson, D.S.; et al. A comparison of the performance of nine soil organic matter models using datasets from seven long-term experiments. Geoderma 1997, 81, 153–225. [Google Scholar] [CrossRef]
- Collins, H.P.; Elliott, E.T.; Paustian, K.; Bundy, L.G.; Dick, W.A.; Hunggins, D.R.; Smucker, A.J.M.; Paul, E.A. Soil carbon pools and fluxes in long-term corn belt agroecosystems. Soil Biol. Biochem. 2000, 32, 157–168. [Google Scholar] [CrossRef]
- Paul, E.A.; Morris, S.J.; Conant, R.T.; Plante, A.F. Does the acid hydrolysis-incubation method measure meaningful soil organic carbon pool? Soil Sci. Soc. Am. J. 2006, 70, 1023–1035. [Google Scholar] [CrossRef]
- Belay-Tedla, A.; Zhou, X.; Su, B.; Wan, S.; Luo, Y. Labile, recalcitrant, and microbial carbon and nitrogen pools of tallgrass prairie soil in the US Great Plains subjected to experimental warming and clipping. Soil Biol. Biochem. 2009, 41, 110–116. [Google Scholar] [CrossRef]
- Jha, P.; De, A.; Lakaria, B.J.; Biswas, A.K.; Singh, M.; Reddy, K.S.; Rao, A.S. Soil carbon pools, mineralization and flexes associated with land use changes in vertisols of the central India. Natl. Acad. Sci. Lett. 2012, 35, 475–483. [Google Scholar] [CrossRef]
- Dungait, J.A.J.; Hopkins, D.W.; Gregory, A.S.; Whitmore, A.P. Soil organic matter turnover is governed by accessibility not recalcitrance. Glob. Chang. Biol. 2012, 18, 1781–1796. [Google Scholar] [CrossRef]
- National Climatic Data Center. 2005. Available online: http://www.ncdc.noaa.gov/cdo-web/datasets/ANNUAL/stations/COOP:053005/detail (accessed on 19 April 2017).
- National Resources Conservation Service (NRCS). Fort Collins Series. Available online: https://soilseries.sc.egov.usda.gov/OSD_Docs/F/FORT_COLLINS.html (accessed on 19 April 2017).
- Hurisso, T.T.; Davis, J.G.; Brummer, J.E.; Stromberger, M.E.; Mikha, M.M.; Haddix, M.L.; Booher, M.R.; Paul, E.A. Rapid changes in microbial biomass and aggregate size distribution in response to changes in organic matter management in grass pasture. Geoderma 2013, 193, 68–75. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis; Part 3: Chemical Methods-SSSA Book Series No. 5; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; SSSA Inc.: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Franzluebbers, A.J.; Haney, R.L.; Honeycutt, C.W.; Schomber, H.H.; Hons, F.M. Flush of carbon dioxide following reweting of dried soil relates to active organic pools. Soil Sci. Soc. Am. J. 2000, 64, 613–623. [Google Scholar] [CrossRef]
- Mikha, M.M.; Rice, C.W. Tillage and manure effects on soil and aggregate-associated carbon and nitrogen. Soil Sci. Soc. Am. J. 2004, 68, 809–816. [Google Scholar] [CrossRef]
- Mikha, M.M.; Rice, C.W.; Milliken, G.A. Carbon and nitrorgen mineralization as affected by drying and wetting cycles. Soil Biol. Biochem. 2005, 37, 339–347. [Google Scholar] [CrossRef]
- Stanford, G.; Smith, S.J. Nitrogen mineralization potentials of soil. Soil Sci. Soc. Am. Proc. 1972, 36, 465–472. [Google Scholar] [CrossRef]
- Molina, J.A.E.; Clapp, E.C.; Larson, W.E. Potential mineralizable nitrogen in soil: The simple exponential model does not apply for the first 12 weeks of incubation. Soil Sci. Soc. Am. J. 1980, 44, 442–443. [Google Scholar] [CrossRef]
- Davidson, E.A.; Eckert, R.W.; Hart, S.C.; Firestone, M.K. Direct extraction of microbial biomass nitrogen from forest and grassland soils of California. Soil Biol. Biochem. 1989, 21, 773–778. [Google Scholar] [CrossRef]
- Bundy, L.G.; Meisinger, J.J. Nitrogen availability indices. In Methods of Soil Analysis; Part 2: Microbiological and Biochemical Properties-SSSA Book Series No. 5; Weaver, R.W., Angle, S., Bottomley, P., Bezdicek, D., Smith, S., Tabatabai, A., Wollum, A., Eds.; SSSA Inc.: Madison, WI, USA, 1994; pp. 951–984. [Google Scholar]
- Hart, S.C.; Stark, J.M.; Davidson, E.A.; Firestone, M.K. Nitrogen mineralization, immobilization, and nitrification. In Methods of Soil Analysis; Part 2: Microbiological and Biochemical Properties-SSSA Book Series No. 5; Weaver, R.W., Angle, S., Bottomley, P., Bezdicek, D., Smith, S., Tabatabai, A., Wollum, A., Eds.; SSSA Inc.: Madison, WI, USA, 1994; pp. 985–1018. [Google Scholar]
- Plante, A.F.; Conant, R.T.; Paul, E.A.; Paustian, K.; Six, J. Acid hydrolysis of easily dispersed and microaggregate-derived silt- and clay-sized fractions to isolate resistant soil organic matter. Eur. J. Soil Sci. 2006, 57, 456–467. [Google Scholar] [CrossRef]
- SAS Institute. SAS/STAT User’s Guide; Version 9.3; SAS Inc.: Cary, NC, USA, 2010. [Google Scholar]
- Fan, J.; Ding, W.; Xiang, J.; Qin, S.; Zhang, J.; Ziadi, N. Carbon sequestration in an intensively cultivated candy loam soil in the north China plain as affected by compost and inorganic fertilizer application. Geoderma 2014, 230, 22–28. [Google Scholar] [CrossRef]
- Kaur, T.; Brar, B.S.; Dhillo, N.S. Soil organic matter dynamics as affected by long-term use of organic and inorganic fertilizers under maize-wheat cropping system. Nutr. Cycl. Agroecosyst. 2008, 81, 59–69. [Google Scholar] [CrossRef]
- Mikha, M.M.; Hergert, G.W.; Benjamin, J.G.; Jabro, J.D.; Nielsen, R.A. Long-term manure impacts on soil aggregates and aggregate-associated carbon and nitrogen. Soil Sci. Soc. Am. 2015, 79, 626–636. [Google Scholar] [CrossRef]
- McDonald, N.T.; Watson, C.J.; Lalor, S.T.J.; Laughlin, R.J.; Wall, D.P. Evaluation of soil test for predicting nitrogen mineralization in temperate grassland soils. Soil Sci. Soc. Am. 2014, 78, 1051–1064. [Google Scholar] [CrossRef]
- Hurisso, T.T.; Davis, J.G.; Brummer, J.E.; Stromberger, M.E.; Mikha, M.M.; Booher, M.R. Short-term nitrogen mineralization during transition from conventional to organic management is unchanged by composted dairy manure addition in perennial forage. Org. Agric. 2012, 2, 219–232. [Google Scholar] [CrossRef]
- Saviozzi, A.; Vanni, G.; Cardelli, R. Carbon mineralization kinetics in soils under urban environment. Appl. Soil Ecol. 2014, 73, 64–69. [Google Scholar] [CrossRef]
- Dind, X.; Han, X.; Liang, Y.; Qiao, Y.; Li, L.; Li, N. Changes in soil organic carbon pools after 10 years of continue manuring combined with chemical fertilizer in a Mollisol in China. Soil Tillage Res. 2012, 122, 36–41. [Google Scholar]
- Cavalli, D.; Bechini, L.; Gallina, P.M. Measurinh and modeling soil carbon respiration following repeated dairy slurry application. Soil Sci. Soc. Am. 2014, 78, 1414–1425. [Google Scholar] [CrossRef]
- Mikha, M.M.; Rice, C.W.; Benjamin, J.G. Estimating soil mineralizable nitrogen under different management practices. Soil Sci. Soc. Am. 2006, 70, 1522–1531. [Google Scholar] [CrossRef]
- Rabbi, S.M.F.; Wilson, B.R.; Lockwood, P.V.; Daniel, H.; Young, I.M. Soil organic carbon mineralization rates in aggregates under contrasting land uses. Geoderma 2014, 216, 10–18. [Google Scholar] [CrossRef]
- En, C.; Al-Kaisi, M.M.; Liange, W.; Changhuuan, D.; Deti, X. Soil organic carbon mineralization as affected by cyclical temperature fluctuation in a Karst region of southwestern China. Pedosphere 2015, 25, 512–523. [Google Scholar]
- Paul, E.A.; Morris, S.J.; Bohm, S. The determination of soil C pools sizes and turnover rates: Biophysical fractionation and traces. In Assessment Methods Soil Carbon; Kimble, J.M., Follett, R.F., Stewart, B.A., Eds.; CRC Press: Boca Raton, FL, USA, 2001; pp. 193–205. [Google Scholar]
- Collins, H.P.; Smith, J.L.; Fransen, S.; Alva, A.K.; Kruger, C.E.; Granatstein, D.M. Carbon sequestration under irrigated switchgrass (Panicum virgatum L.) production. Soil Sci. Soc. Am. J. 2010, 74, 2049–2058. [Google Scholar] [CrossRef]
- Kendall, R.A.J.; Long, D.S.; Collins, H.P.; Pierce, F.J.; Chatterjee, A.; Smith, J.L.; Young, S.L. Soiln carbon dynamics of transition to pasific northwest cellulosic ethanol feedstock production. Soil Sci. Soc. Am. 2015, 79, 272–281. [Google Scholar] [CrossRef]
| Species | Scientific Name | Variety | Seeding Rate kg ha−1 |
|---|---|---|---|
| (WG-TF-B) | |||
| Hybrid wheatgrass | Elymus hoffmainni K.B. Jensen & K.H. Asay | ’Newhy’ | 9.0 |
| Tall fescue | Festuca arundinacea Schreb. | ’Fawn’ Endophyte-free | 7.3 |
| Hybrid brome | Bromus inermis Leyss. x beibersteinii Roem. & Schult. | ’Bigfoot’ | 10.1 |
| (OG-MB-SB) | |||
| Orchardgrass | Dactylis glomerata L. | ’Crown Royale’ | 3.3 |
| Meadow brome | Bromus biebersteinii Roem. & Schult. | 'Paddock' | 11.2 |
| Smooth brome | Bromus inermis Leyss. | ‘Lincoln’ | 5.6 |
| Legumes | |||
| Alfalfa | Medicago sativa L. | ‘Ranger’ | 9.0 |
| Sainfoin | Onobrychis viciaefolia Scop. | ‘Sandhills’ | 22.4 |
| Treatment | Nitrogen Source | SOC | STN | ||
|---|---|---|---|---|---|
| Soil Depth (cm) | |||||
| 0–5 | 5–10 | 0–5 | 5–10 | ||
| g kg−1 | |||||
| HWG-TF-HB † | Sainfoin | 13.38 a § | 13.96 a | 1.67 a | 1.59 a |
| TF †† | Sainfoin | 14.99 a | 13.33 a | 1.79 a | 1.66 a |
| OG-MB-SB ‡ | Sainfoin | 14.55 a | 13.83 a | 1.76 a | 1.71 a |
| HWG-TF-HB | Alfalfa | 14.15 a | 13.58 a | 1.69 a | 1.56 a |
| TF | Alfalfa | 13.38 a | 10.52 b | 1.62 a | 1.42 b |
| OG-MB-SB | Alfalfa | 14.00 a | 11.58 b | 1.74 a | 1.54 b |
| HWG-TF-HB | Compost ¶ | 14.67 a | 12.68 b | 1.78 a | 1.61 a |
| TF | Compost | 16.18 a | 14.03 b | 1.98 a | 1.72 b |
| OG-MB-SB | Compost | 14.68 a | 13.41 a | 1.72 a | 1.73 a |
| HWG-TF-HB | Alfalfa + Compost | 16.15 a | 13.06 b | 1.92 a | 1.60 b |
| TF | Alfalfa + Compost | 16.17 a | 11.33 b | 1.79 a | 1.48 b |
| OG-MB-SB | Alfalfa + Compost | 15.69 a | 12.30 b | 1.79 a | 1.62 a |
| PR > F | |||||
| Treatment | 0.3587 | 0.5570 | |||
| Depth (cm) | <0.0001 | <0.0001 | |||
| 0–5 | 14.83 a | 1.77 a | |||
| 5–10 | 12.80 b | 1.60 b | |||
| Treatment × Depth | 0.7857 | 0.3278 | |||
| Treatment | Nitrogen Source | SOC | STN | ||
|---|---|---|---|---|---|
| Soil Depth (cm) | |||||
| 0–5 | 5–10 | 0–5 | 5–10 | ||
| g kg−1 | |||||
| HWG-TF-HB † | Sainfoin | 13.30 a § | 10.61 b | 1.60 a | 1.26 b |
| TF †† | Sainfoin | 13.99 a | 10.84 b | 1.58 a | 1.35 b |
| OG-MB-SB ‡ | Sainfoin | 14.82 a | 10.86 b | 1.69 a | 1.38 b |
| HWG-TF-HB | Alfalfa | 14.45 a | 10.82 b | 1.56 a | 1.39 a |
| TF | Alfalfa | 13.24 a | 10.90 b | 1.53 a | 1.43 a |
| OG-MB-SB | Alfalfa | 12.94 a | 10.10 b | 1.59 a | 1.32 b |
| HWG-TF-HB | Compost (0 Mg ha−1) ¶ | 15.85 a | 11.63 b | 1.86 a | 1.45 b |
| TF | Compost (0 Mg ha−1) | 15.58 a | 12.36 b | 1.80 a | 1.49 b |
| OG-MB-SB | Compost (0 Mg ha−1) | 15.94 a | 11.23 b | 1.92 a | 1.42 b |
| HWG-TF-HB | Compost (11.2 Mg ha−1) | 15.11 a | 11.35 b | 1.75 a | 1.32 b |
| TF | Compost (11.2 Mg ha−1) | 15.55 a | 10.89 b | 1.88 a | 1.31 b |
| OG-MB-SB | Compost (11.2 Mg ha−1) | 15.10 a | 10.96 b | 1.65 a | 1.40 b |
| HWG-TF-HB | Compost (22.4 Mg ha−1) | 12.94 a | 11.43 a | 1.42 a | 1.35 a |
| TF | Compost (22.4 Mg ha−1) | 14.43 a | 11.67 b | 1.70 a | 1.45 b |
| OG-MB-SB | Compost (22.4 Mg ha−1) | 15.46 a | 11.35 b | 1.68 a | 1.40 b |
| HWG-TF-HB | Alfalfa + Compost (R) ‡‡ | 15.49 a | 11.72 b | 1.75 a | 1.40 b |
| TF | Alfalfa + Compost (R) | 15.96 a | 10.26 b | 1.74 a | 1.27 b |
| OG-MB-SB | Alfalfa + Compost (R) | 14.21 a | 11.20 b | 1.71 a | 1.40 b |
| PR > F | |||||
| Treatment | 0.2859 | 0.3442 | |||
| Depth (cm) | <0.0001 | <0.0001 | |||
| 0–5 | 14.67 a | 1.69 a | |||
| 5–10 | 11.12 b | 1.38 b | |||
| Treatment × Depth | 0.4656 | 0.2374 | |||
| Treatment | Nitrogen Source | Potential Mineralizable C (Co) | Mineralization Rate Constant (kc) | ||
|---|---|---|---|---|---|
| Soil Depth (cm) | |||||
| 0–5 | 5–10 | 0–5 | 5–10 | ||
| mg kg−1 | day−1 | ||||
| HWG-TF-HB † | Sainfoin | 1029.9 a § | 680.0 a | 0.0304 a | 0.0426 a |
| TF †† | Sainfoin | 1600.2 a | 943.3 b | 0.0188 a | 0.0321 a |
| OG-MB-SB ‡ | Sainfoin | 1399.4 a | 939.1 a | 0.0255 a | 0.0328 a |
| HWG-TF-HB | Alfalfa | 1408.1 a | 1055.6 a | 0.0273 a | 0.0266 a |
| TF | Alfalfa | 1255.1 a | 853.0 a | 0.0275 a | 0.0384 a |
| OG-MB-SB | Alfalfa | 1480.8 a | 1076.4 a | 0.1066 a | 0.0318 a |
| HWG-TF-HB | Compost ¶ | 1355.6 a | 784.1 b | 0.0852 a | 0.0370 a |
| TF | Compost | 1995.2 a | 983.3 b | 0.0196 a | 0.0297 a |
| OG-MB-SB | Compost | 1887.4 a | 1056.5 b | 0.0213 a | 0.0274 a |
| HWG-TF-HB | Alfalfa + Compost | 1456.6 a | 997.3 a | 0.0226 a | 0.0331 a |
| TF | Alfalfa + Compost | 1742.7 a | 967.3 b | 0.0223 a | 0.0317 a |
| OG-MB-SB | Alfalfa + Compost | 1710.4 a | 907.3 b | 0.0227 a | 0.3470 a |
| PR > F | |||||
| Treatment | 0.4664 | 0.5170 | |||
| Depth (cm) | <0.0001 | 0.7663 | |||
| 0–5 | 1526.8 a | 0.0365 a | |||
| 5–10 | 936.9 b | 0.0332 a | |||
| Treatment × Depth | 0.7857 | 0.5859 | |||
| Treatment | Nitrogen Source | Potential Mineralizable C (Co) | Mineralization Rate Constant (kc) | ||
|---|---|---|---|---|---|
| Soil Depth (cm) | |||||
| 0–5 | 5–10 | 0–5 | 5–10 | ||
| mg kg−1 | day−1 | ||||
| HWG-TF-HB † | Sainfoin | 788.3 a § | 378.6 b | 0.0445 a | 0.0593 a |
| TF †† | Sainfoin | 615.8 a | 321.0 a | 0.0670 a | 0.0668 a |
| OG-MB-SB ‡ | Sainfoin | 720.0 a | 383.8 b | 0.0554 a | 0.0533 a |
| HWG-TF-HB | Alfalfa | 725.3 a | 361.8 b | 0.0467 a | 0.0638 a |
| TF | Alfalfa | 924.3 a | 341.4 b | 0.0440 a | 0.0665 a |
| OG-MB-SB | Alfalfa | 852.9 a | 450.9 b | 0.0485 a | 0.0524 a |
| HWG-TF-HB | Compost (0 Mg ha−1) ¶ | 829.3 a | 344.1 b | 0.0538 a | 0.0630 a |
| TF | Compost (0 Mg ha−1) | 934.8 a | 360.6 b | 0.0411 b | 0.0679 a |
| OG-MB-SB | Compost (0 Mg ha−1) | 1196.3 a | 337.5 b | 0.0474 a | 0.0632 a |
| HWG-TF-HB | Compost (11.2 Mg ha−1) | 892.8 a | 403.4 b | 0.0464 a | 0.0568 a |
| TF | Compost (11.2 Mg ha−1) | 596.0 a | 379.0 a | 0.0595 a | 0.0594 a |
| OG-MB-SB | Compost (11.2 Mg ha−1) | 707.5 a | 344.0 b | 0.0790 a | 0.0547 a |
| HWG-TF-HB | Compost (22.4 Mg ha−1) | 729.9 a | 394.7 b | 0.0597 a | 0.0626 a |
| TF | Compost (22.4 Mg ha−1) | 842.3 a | 380.2 b | 0.0471 a | 0.0596 a |
| OG-MB-SB | Compost (22.4 Mg ha−1) | 728.5 a | 424.9 a | 0.0706 a | 0.0505 a |
| HWG-TF-HB | Alfalfa + Compost (R) ‡‡ | 911.9 a | 277.6 b | 0.0450 b | 0.0702 a |
| TF | Alfalfa + Compost (R) | 977.7 a | 372.2 b | 0.0450 a | 0.0631 a |
| OG-MB-SB | Alfalfa + Compost (R) | 1023.5 a | 343.3 b | 0.0317 b | 0.0579 a |
| PR > F | |||||
| Treatment | 0.7630 | 0.6113 | |||
| Depth (cm) | <0.0001 | 0.0043 | |||
| 0–5 | 833.2 a | 0.0518 b | |||
| 5–10 | 366.6 b | 0.0606 a | |||
| Treatment × Depth | 0.4656 | 0.2280 | |||
| Treatment | Nitrogen Source | Resistant C Pool (Cr) | Slow C Pool (Cs) | ||
|---|---|---|---|---|---|
| Soil Depth (cm) | |||||
| 0–5 | 5–10 | 0–5 | 5–10 | ||
| g kg−1 | g kg−1 | ||||
| HWG-TF-HB † | Sainfoin | 5.23 a§ | 5.14 a | 7.11 a | 8.14 a |
| TF †† | Sainfoin | 7.03 a | 5.20 b | 6.37 a | 7.19 a |
| OG-MB-SB ‡ | Sainfoin | 5.13 a | 6.00 a | 8.02 a | 6.90 a |
| HWG-TF-HB | Alfalfa | 6.68 a | 6.20 a | 6.07 a | 6.33 a |
| TF | Alfalfa | 5.28 a | 4.85 a | 6.84 a | 4.82 a |
| OG-MB-SB | Alfalfa | 5.17 a | 4.69 a | 7.35 a | 5.81 a |
| HWG-TF-HB | Compost ¶ | 6.25 a | 4.58 b | 7.07 a | 7.32 a |
| TF | Compost | 6.00 a | 4.79 b | 8.19 a | 8.25 a |
| OG-MB-SB | Compost | 6.72 a | 5.72 a | 6.07 a | 6.63 a |
| HWG-TF-HB | Alfalfa + Compost | 6.08 a | 6.17 a | 8.61 a | 5.89 b |
| TF | Alfalfa + Compost | 5.06 a | 4.75 a | 9.65 a | 5.61 b |
| OG-MB-SB | Alfalfa + Compost | 6.43 a | 5.17 b | 7.55 a | 6.21 a |
| PR > F | |||||
| Treatment | 0.4911 | 0.6140 | |||
| Depth (cm) | 0.0002 | 0.028 | |||
| 0–5 | 5.92 a | 7.382 a | |||
| 5–10 | 5.27 b | 6.592 b | |||
| Treatment × Depth | 0.0481 | 0.1352 | |||
| Treatment | Nitrogen Source | Resistant C Pool (Cr) | Slow C Pool (Cs) | ||
|---|---|---|---|---|---|
| Soil Depth (cm) | |||||
| 0–5 | 5–10 | 0–5 | 5–10 | ||
| g kg−1 | g kg−1 | ||||
| HWG-TF-HB † | Sainfoin | 4.68 a § | 4.85 a | 7.83 a | 5.39 b |
| TF †† | Sainfoin | 5.14 a | 6.07 a | 8.23 a | 5.45 b |
| OG-MB-SB ‡ | Sainfoin | 4.56 a | 4.20 a | 9.53 a | 6.28 b |
| HWG-TF-HB | Alfalfa | 4.95 a | 4.79 a | 8.48 a | 5.66 b |
| TF | Alfalfa | 5.01 a | 4.43 a | 7.30 a | 6.13 a |
| OG-MB-SB | Alfalfa | 5.35 a | 3.81 b | 6.74 a | 5.84 a |
| HWG-TF-HB | Compost (0 Mg ha−1) ¶ | 5.64 a | 3.95 b | 9.38 a | 7.33 b |
| TF | Compost (0 Mg ha−1) | 5.20 a | 4.05 a | 9.44 a | 7.93 a |
| OG-MB-SB | Compost (0 Mg ha−1) | 6.83 a | 4.89 b | 7.91 a | 6.00 a |
| HWG-TF-HB | Compost (11.2 Mg ha−1) | 8.01 a | 4.42 b | 6.20 a | 6.53 a |
| TF | Compost (11.2 Mg ha−1) | 6.24 a | 5.06 a | 8.72 a | 5.45 b |
| OG-MB-SB | Compost (11.2 Mg ha−1) | 5.36 a | 5.54 a | 9.03 a | 5.08 b |
| HWG-TF-HB | Compost (22.4 Mg ha−1) | 5.43 a | 3.86 b | 6.78 a | 7.17 a |
| TF | Compost (22.4 Mg ha−1) | 6.97 a | 5.73 a | 6.62 a | 5.56 a |
| OG-MB-SB | Compost (22.4 Mg ha−1) | 6.43 a | 5.64 a | 8.30 a | 5.29 b |
| HWG-TF-HB | Alfalfa + Compost (R) ‡‡ | 5.61 a | 4.62 a | 8.97 a | 6.82 b |
| TF | Alfalfa + Compost (R) | 5.78 a | 3.92 b | 9.21 a | 4.96 b |
| OG-MB-SB | Alfalfa + Compost (R) | 4.87 a | 4.29 a | 8.32 a | 6.57 a |
| PR > F | |||||
| Treatment | 0.004 | 0.3226 | |||
| Depth (cm) | <0.0001 | <0.0001 | |||
| 0–5 | 5.671 a | 8.166 a | |||
| 5–10 | 4.618 b | 6.081 b | |||
| Treatment × Depth | 0.1099 | 0.1085 | |||
| Measurement | SOC | Co | Cs |
|---|---|---|---|
| 2008 | |||
| 0–5 cm | |||
| Soil organic C (SOC) | |||
| Mineralizable C (Co) | 0.4428 ** | ||
| Slow C (Cs) | 0.6770 *** | −0.0797 | |
| Resistant C (Cr) | 0.2442 | 0.3239 * | −0.5022 ** |
| 5–10 cm | |||
| Soil organic C (SOC) | |||
| Mineralizable C (Co) | 0.1372 | ||
| Slow C (Cs) | 0.7517 *** | −0.0885 | |
| Resistant C (Cr) | 0.3066 | 0.1781 | −0.3900 * |
| 2009 | |||
| 0–5 cm | |||
| Soil organic C (SOC) | |||
| Mineralizable C (Co) | 0.3856 ** | ||
| Slow C (Cs) | 0.7588 *** | 0.1182 | |
| Resistant C (Cr) | 0.2857 * | 0.1645 | −0.3905 ** |
| 5–10 cm | |||
| Soil organic C (SOC) | |||
| Mineralizable C (Co) | −0.3843 ** | ||
| Slow C (Cs) | 0.6823 *** | −0.2953 * | |
| Resistant C (Cr) | 0.3001 * | −0.1369 | −0.4906 *** |
| Measurement | STN | Nmin | Na+s |
|---|---|---|---|
| 2008 | |||
| 0–5 cm | |||
| Soil Total N (STN) | |||
| Mineralizable N (Nmin) | 0.8177 *** | ||
| Active + Slow N (Na+s) | 0.9649 *** | ||
| Resistant N (Nr) | 0.2442 | 0.2880 | −0.0189 |
| 5–10 cm | |||
| Soil Total N (STN) | |||
| Mineralizable N (Nmin) | 0.5895 *** | ||
| Active + Slow N (Na+s) | 0.9697 *** | ||
| Resistant N (Nr) | 0.2710 | 0.1672 | 0.0278 |
| 2009 | |||
| 0–5 cm | |||
| Soil Total N (STN) | |||
| Mineralizable N (Nmin) | 0.3249 ** | ||
| Active + Slow N (Na+s) | 0.9114 *** | ||
| Resistant N (Nr) | 0.1559 | 0.0680 | −0.2645 * |
| 5–10 cm | |||
| Soil Total N (STN) | |||
| Mineralizable N (Nmin) | 0.6122 *** | ||
| Active + Slow N (Na+s) | 0.9166 *** | ||
| Resistant N (Nr) | 0.2141 | 0.2752 * | −0.1942 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikha, M.M.; Widiastuti, D.P.; Hurisso, T.T.; Brummer, J.E.; Davis, J.G. Influence of Composted Dairy Manure and Perennial Forage on Soil Carbon and Nitrogen Fractions during Transition into Organic Management. Agriculture 2017, 7, 37. https://doi.org/10.3390/agriculture7050037
Mikha MM, Widiastuti DP, Hurisso TT, Brummer JE, Davis JG. Influence of Composted Dairy Manure and Perennial Forage on Soil Carbon and Nitrogen Fractions during Transition into Organic Management. Agriculture. 2017; 7(5):37. https://doi.org/10.3390/agriculture7050037
Chicago/Turabian StyleMikha, Maysoon M., Dwi P. Widiastuti, Tunsisa T. Hurisso, Joe E. Brummer, and Jessica G. Davis. 2017. "Influence of Composted Dairy Manure and Perennial Forage on Soil Carbon and Nitrogen Fractions during Transition into Organic Management" Agriculture 7, no. 5: 37. https://doi.org/10.3390/agriculture7050037






