Enhanced Plant Performance in Cicer arietinum L. Due to the Addition of a Combination of Plant Growth-Promoting Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Experimental Setting
2.3. Statistical Analysis
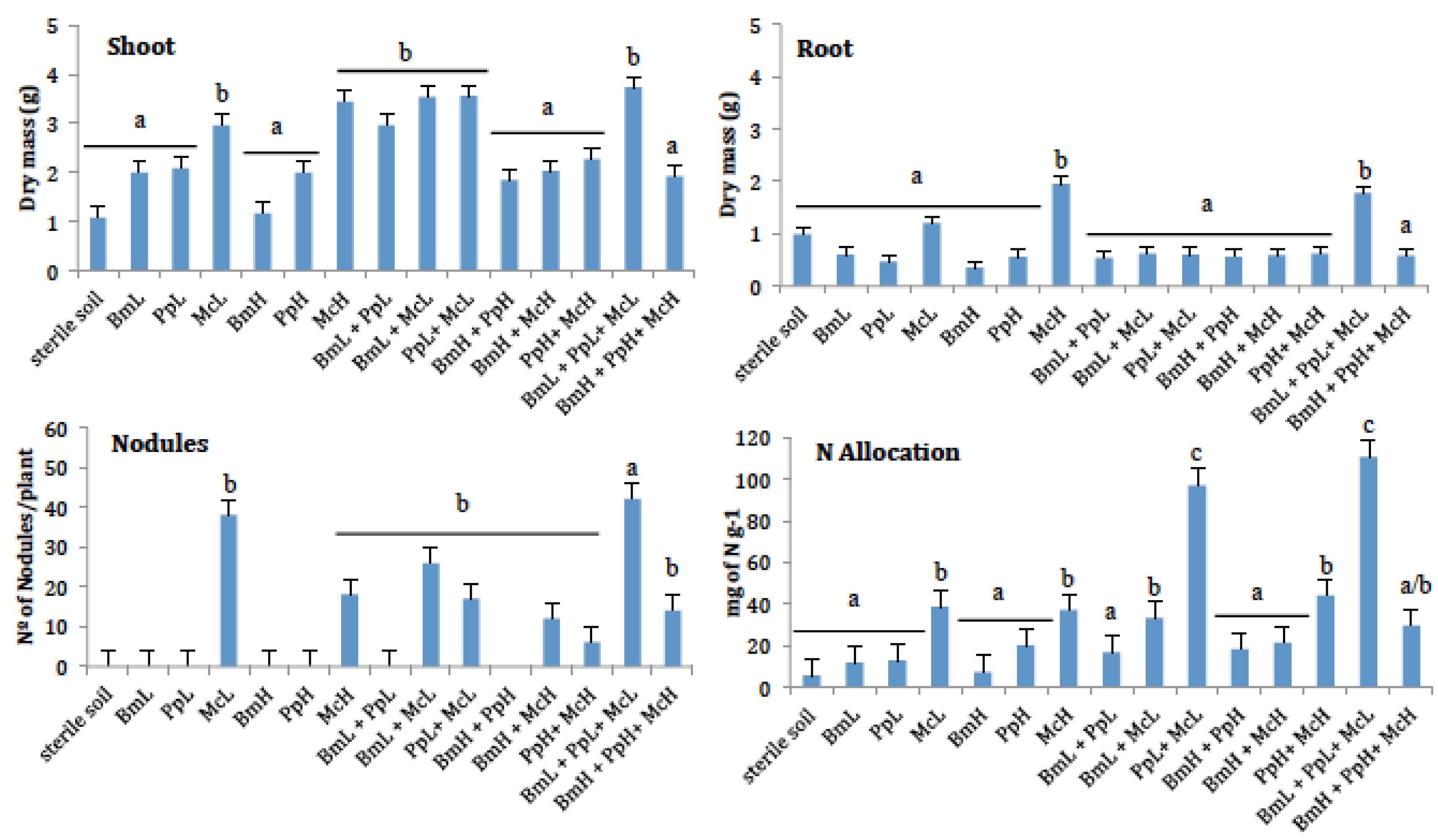
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siddiqui, A.Z.; Mahmood, I. Effects of rhizobacteria and root symbionts on the reproduction of Meloidogyne javanica and growth of chickpea. Bioresour. Technol. 2001, 79, 41–45. [Google Scholar] [CrossRef]
- Jukanti, A.K.; Gaur, P.M.; Gowda, C.L.; Chibbar, R.N. Nutritional quality and health benefits of chickpea (Cicer arietinum L.): A review. Br. J. Nutr. 2012, 108, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Vassilev, N.; Vassilev, M. Biotechnological solubilization of rock phosphate on media containing agro-industrial wastes. Appl. Microbiol. Biotechnol. 2003, 61, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, Y.; Xie, X.; Kim, M.S.; Dowd, S.E.; Paré, P.W. A soil bacterium regulates plant acquisition of iron via deficiency inducible mechanisms. Plant J. 2009, 58, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Ingham, R.E.; Trofymow, J.A.; Ingham, E.R.; Coleman, D.V. Interactions of bacteria, fungi, and their nematode grazers: Effects on nutrient cycling and plant growth. Ecol. Monogr. 1985, 55, 119–140. [Google Scholar] [CrossRef]
- Rodríguez, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef]
- Pérez-Fernández, M.A.; Hill, Y.J.; Calvo-Magro, E.; Valentine, A. Competing Bradyrhizobia strains determine niche occupancy by two native legumes in the Iberian Peninsula. Plant Ecol. 2015, 216, 1537–1549. [Google Scholar] [CrossRef]
- Vesquez, P.; Holguin, G.; Puente, M.E.; López-Cortes, A.; Bashan, Y. Phosphate-solubilizing microorganisms associated with the rhizosphere of mangroves in a semiarid coastal lagoon. Biol. Fertil. Soils 2000, 30, 460–468. [Google Scholar] [CrossRef]
- Pii, Y.; Mimmo, T.; Tomasi, N.; Terzano, R. Cesco and Crecchio C Microbial interactions in the rhizosphere: Beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A Rev. Biol. Fertil. Soils 2015, 51, 403–415. [Google Scholar] [CrossRef]
- Pérez-Fernánde, M.A.; Calvo-Magro, E.; Valentine, A. Benefits of the symbiotic association of shrubby legumes to re-vegetate heavily damaged soils. Land Degrad. Dev. 2016, 27, 395–405. [Google Scholar]
- Omar, S.A. The role of rock phosphate solubilizing fungi and vesicular arbuscular mycorrhiza (VAM) in growth of wheat plants fertilized with rock phosphate. World J. Microbiol. Biotechnol. 1998, 14, 211–219. [Google Scholar] [CrossRef]
- Santi, C.; Bogusz, D.; Franche, C. Nitrogen fixation in non-legumes. Ann. Bot. 2013, 111, 743–767. [Google Scholar] [CrossRef] [PubMed]
- Palacios, O.; Bashan, Y.; de-Bashan, L. Proven and potential involvement of vitamins in interactions of plants with plant growth-promoting bacteria—An overview. Biol. Fertil. Soils 2014, 50, 415–432. [Google Scholar] [CrossRef]
- Mehnaz, S.; Lazarovits, G. Inoculation effects of Pseudomonas putida, Gluconacetobacter azotocaptans, and Azospirillum lipoferum on corn plant growth under greenhouse conditions. Microb. Ecol. 2006, 51, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Ahmad, I.; Kjan, M.S. Screening of free-living rhizospheric bacteria for their multiple plant growth promotion activities. Microbiol. Res. 2008, 163, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Mittal, V.; Singh, O.; Nayyar, H.; Kaur, J.; Tewari, R. Simulatory effect of phosphate-solubilizing fungal strains (Aspergillus awamori and Penicillium citrinum) on the yield of chickpea (Cicer arietinun L. cv GPF2). Soil Biol. Bichem. 2008, 40, 718–727. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information (NCBI). Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information 1988. Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 6 April 2017).
- Cappuccino, J.C.; Sherman, N. Microbiology: A Laboratory Manual, 3rd ed; Benjamin Cummings Pub Co.: New York, NY, USA, 1992; pp. 125–179. [Google Scholar]
- Angulo, V.C.; Sanfuentes, E.A.; Rodríguez, F.; Sossa, K.E. Caracterización de rhizobacterias promotoras de crecimiento en plántulas de Eucalyptus nitens. Revista Argentina de Microbiologia 2014, 46, 338–347. [Google Scholar] [CrossRef]
- Schwyn, B.; Neilands, J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Vincent, J.M. A Manual for the Practical Study of Root-Nodule Bacteria; Blackwell Scientific Publications Ltd.: Oxford, UK, 1970. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method of Growing Plants without Soil; California Agricultural Experimental Station: Berkeley, CA, USA, 1950; p. 347. [Google Scholar]
- Sarruge, J.R.; Haag, H.P. Análises Químicas em Plantas; ESALQ/USP: Piracicaba, Brazil, 1979; p. 27. [Google Scholar]
- Woomer, P.L.; Huising, J.; Giller, K.E. N2 Africa Final Report of the First Phase 2009–2013. Available online: http://www. 2014, N2Africa.org 138 (accessed on 6 April 2017).
- Asei, R.; Ewusi-Mensah, N.; Abaidoo, R.C. Response of Soybean (Glycine max L.) to rhizobia inoculation and molybdenum application in the Northern savannah zones of Ghana. J. Plant Sci. 2015, 3, 64–70. [Google Scholar]
- Mayak, S.; Tirosh, T.; Glick, B. Plant growth-promoting bacteria that confer resistance to water stress in tomatoes and peppers. Plant Sci. 2004, 166, 525–530. [Google Scholar] [CrossRef]
- Mathu, S.; Herrmann, L.; Pypers, P.; Matiru, V.; Mwirichia, R.; Lesueur, D. Potential of indigenous bradyrhizobia versus commercial inoculants to improve cowpea (Vigna unguiculata L. walp) and green gram (Vigna radiate L. wilczek.) yields in Kenya. Soil Sci. Plant Nutr. 2012, 58, 750–763. [Google Scholar] [CrossRef]
- Nieto-Jacobo, M.F.; Steyaert, J.M.; Salazar-Badillo, F.B.; Nguyen, D.V.; Rostás, M.; Braithwaite, M.; De Souza, J.T.; Jimenez-Bremont, J.F.; Ohkura, M.; Stweart, A.; et al. Environmental growth conditions of Trichoderma spp affects indole acetic adic derivates, volatile organic compounds, and plant growth promotion. Front. Plant Sci. 2017, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Lamont, B.; Pérez-Fernández, M.A. Soil bacteria control plant growth and root-cluster production by species from three continents. Ann. Bot. 2016, 118, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Read, T.D.; Salzberg, S.L.; Pop, M.; Shumway, M.; Umayam, L.; Jiang, L.; Holtzapple, E.; Busch, J.D.; Smith, K.L.; Schupp, J.M.; et al. Comparative genome sequencing for discovery of novel polymorphisms in Bacillus anthracis. Science 2002, 296, 2028–2033. [Google Scholar] [CrossRef] [PubMed]
- Ulzen, J.; Abaidoo, R.C.; Mensah, N.A.; Masso, C.; AbdelGadir, A.H. Bradyrhizobium inoculants enhance grain yields of soybean and cowpea in Northern Ghana. Front. Plant Sci. 2016, 7, 1770. [Google Scholar] [CrossRef] [PubMed]
- Albareda, M.; Rodríguez, D.N.; Temprano, F.J. Soybean incoulation: Dose, N fertilizer supplementation and rhizobia persistence in soils. Field Crops Res. 2009, 113, 352–356. [Google Scholar] [CrossRef]
- Wright, S.; Zumoff, C.; Scheider, L.; Beer, S. Pantoea agglomerans EH318 producer two antibiotics that inhibit Erwinia amylovora in vitro. Appl. Environ. Microbiol. 2001, 67, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.; Valencia, S.; Bernal, J.; Martínez, P. Isolation of Enterobacteria, Azotobacter sp. and Pseudomonas spp. producers of 71 Indole-3-Acetic Acid and Siderophores, from Colombian Rice Rhizosphere. Revista Latinoamericana de Microbiología 2000, 42, 171–176. [Google Scholar]
- Bever, J.D. Soil commynity feedback and the coexistence of competitors: Conceptual framework and empirical tests. New Phytol. 2003, 157, 465–473. [Google Scholar] [CrossRef]
- He, W.M.; Zhang, H.; Dong, M. Plasticity in fitness and fitness-related traits at ramet and genet levels in a tillering grass Panicum miiaceum under patchy soil nutrients. Plant Ecol. 2004, 172, 1–10. [Google Scholar] [CrossRef]
- Hawkes, C.V.; Belnap, J.; D’Antonio, C.; Firestone, M.K. Arbuscular mycorrhizal assemblages in native plant roots change in the presence of exotic grasses. Plant Soil 2006, 281, 369–380. [Google Scholar] [CrossRef]
- Hodge, A.; Stewart, J.; Robinson, D.; Griffiths, B.S.; Fitter, A.H. Competition between roots and soil-microorganisms for nutrients from nitrogen-rich patches of varying complexity. J. Ecol. 2000, 88, 150–164. [Google Scholar] [CrossRef]
- Gubry-Rangin, C.; Garcia, M.; Béna, G. Partner choice in Medicago truncatula—Sinorhizobium symbiosis. Proc. R. Soc. 2010, 277, 1947–1951. [Google Scholar] [CrossRef] [PubMed]
| Strain | Accession Numbers | IAA (µg m−1) | Deaminase ACC Activity | Siderophores |
|---|---|---|---|---|
| BmL | DDBJ_58fe2926b6e6bc04ce0040f4 | 132 b | − | − |
| PpL | DDBJ_58fe25e1b6e6bcfeb1005c86 | 176 a | − | − |
| McL | USDA 3383T | 121 b | + | − |
| BmH | JSB 31 | 62 b | + | + |
| PpH | DDBJ_58fe02d7b6e6bcfeb1005be4 | 99 b | + | + |
| McH | WSM1271 | 86 b | − | − |
| Treatment | N° Flowers | N° Pods | N° Seed | N in Seed (mg N. g plant−1) |
|---|---|---|---|---|
| sterile soil | 36 a | 25 a | 2 a | 1.49 a |
| BmL | 132 b | 90 c | 90 b | 2.46 a |
| PpL | 176 b | 148 d | 162 c | 2.72 a |
| McL | 121 b | 86 c | 100 b | 5.06 a,b |
| BmH | 62 b | 49 b | 46 b | 2.57 a |
| PpH | 99 b | 78 c | 82 b | 4.28 a,b |
| McH | 86 b | 66 b | 62 b | 3.72 a |
| BmL + PpL | 49 a | 37 a | 39 b | 2.62 a |
| BmL + McL | 98 b | 74 c | 78 b | 4.33 a,b |
| PpL + McL | 252 c | 213 d | 267 c | 12.67 c |
| BmH + PpH | 139 b | 109 d | 104 c | 4.14 a,b |
| BmH + McH | 77 b | 70 c | 77 b | 4.43 a,b |
| PpH + McH | 163 b | 135 d | 143 c | 8.32c |
| BmL + PpL+ McL | 149 c | 119 d | 115 | 10.93 c |
| BmH + PpH+ McH | 73 b | 53 c | 56 | 6.40 a,b |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Fernández, M.; Alexander, V. Enhanced Plant Performance in Cicer arietinum L. Due to the Addition of a Combination of Plant Growth-Promoting Bacteria. Agriculture 2017, 7, 40. https://doi.org/10.3390/agriculture7050040
Pérez-Fernández M, Alexander V. Enhanced Plant Performance in Cicer arietinum L. Due to the Addition of a Combination of Plant Growth-Promoting Bacteria. Agriculture. 2017; 7(5):40. https://doi.org/10.3390/agriculture7050040
Chicago/Turabian StylePérez-Fernández, María, and Valentine Alexander. 2017. "Enhanced Plant Performance in Cicer arietinum L. Due to the Addition of a Combination of Plant Growth-Promoting Bacteria" Agriculture 7, no. 5: 40. https://doi.org/10.3390/agriculture7050040






