1. Introduction
Sugarcane belongs to the genus
Saccharum L., of the tribe
Andropogoneae in the family of
Poaceae (
Gramineae), which is grown widely in tropical and subtropical regions across the world [
1].
In Iran, there is a Cane Development and Sidelong Industrial Research and Education Institute located in Khuzestan province that was officially founded in 1981. The sugarcane germplasm collection located at this institute includes approximately 340 accessions from
Saccharum officinarum L. and interspecific hybrids. This set has been collected from different continents, such as the USA, Cuba, Brazil, Argentina, South Africa, India, and Australia, and has been maintained over the years in Iran. It seems that present accessions are well adapted to the climate of Iran, and the collection probably contains genotypes carrying genes for cold tolerance. The sugarcane collection in Khuzestan (primary collection), which has been selected manually from whole accessions across the world, is actually a core collection (CC). Due to the large size of the collection (340 accessions), there are problems concerning the management and use of this genetic resource. Hence, a diverse mini-core collection or breeding collection could be a beneficial resource for sugarcane breeders [
2]. Formation of a reduced subset of sugarcane genetic resources in Khuzestan is very important for assessing the population structure and diversity and for identifying sources of variation in breeding programs. So far, there has been no research to assess the morphological diversity of the entire sugarcane germplasm in Iran, indicating the necessity of a comprehensive evaluation of the accessions in the mentioned collection.
Despite the diversity of resources and genotypes, one of the major problems faced during plant breeding programs is insufficient use of the whole germplasm, because it is difficult to manage and utilize a large number of genotypes in germplasm collections. It is accepted that recognition and use of diversity in germplasm is very important prior to starting a breeding program [
3]. There is a growing body of literature that recognizes the importance of constructing a core or mini-core collection for conservation of novel variations in genetic resources [
2,
4,
5]. A core collection is defined as a limited set of accessions representing the minimum of repetition and the maximum of the genetic diversity of a crop species and its wild relatives [
6]. Similar to the definition of a core collection [
6], a mini-core collection is defined as a reduced subset of a core collection with minimum repetitiveness and maximum genetic diversity of the source germplasm [
7]. Previous studies have reported that a core collection would be better if it contains 10% of the entire collection and a mini-core collection contains 10% of the core collection or 1% of the entire collection [
8]. A mini-core collection is important in order to identify sources of resistance to biotic and/or abiotic stresses and agronomic and nutritional traits. In sugarcane, it is arguable that the accessions with high sucrose content can be selected for sugar production. More importantly, such a subset of germplasm is also useful for recognition of superior energy cane cultivars, i.e., with high biomass and lignocellulosic compounds and fiber, which are appropriate for lignocellulosic ethanol production [
9]. Additionally, a core or mini-core collection plays an important role in assessing allelic richness and association genetics, especially for identifying important QTLs (quantitative trait locus) controlling desirable traits, such as yield and fiber content.
Over the years, phenotypic traits have played an important role in genetic diversity analysis and in discriminating genotypes in order to form a core and/or mini-core collection [
10,
11,
12]. Similarly, previous studies have reported establishing a core collection of germplasm in sugarcane based on morphological traits [
2,
13,
14,
15]. Phenotypic characterization of a germplasm collection is an increasingly important step in plant breeding programs since breeders can evaluate variations and select high performance accessions more efficiently. Although several studies have been carried out on core collection construction in sugarcane [
2], no single study has established the breeding collection (BC), actually named mini-core collection, of sugarcane germplasm.
The goals of our work were (1) comprehensive evaluation of sugarcane genetic resources in Iran and measuring important phenotypic traits on four dates over two growing seasons and (2) to create a mini-core collection or breeding collection that captures most of the phenotypic diversity of the primary collection. The present research attempts, for the first time, to establish a mini-core collection in sugarcane germplasm. This study provides an exciting opportunity to advance our knowledge of germplasm characterization for discovering new sources of variations in order to enhance the use of germplasm for cultivar improvement.
4. Discussion
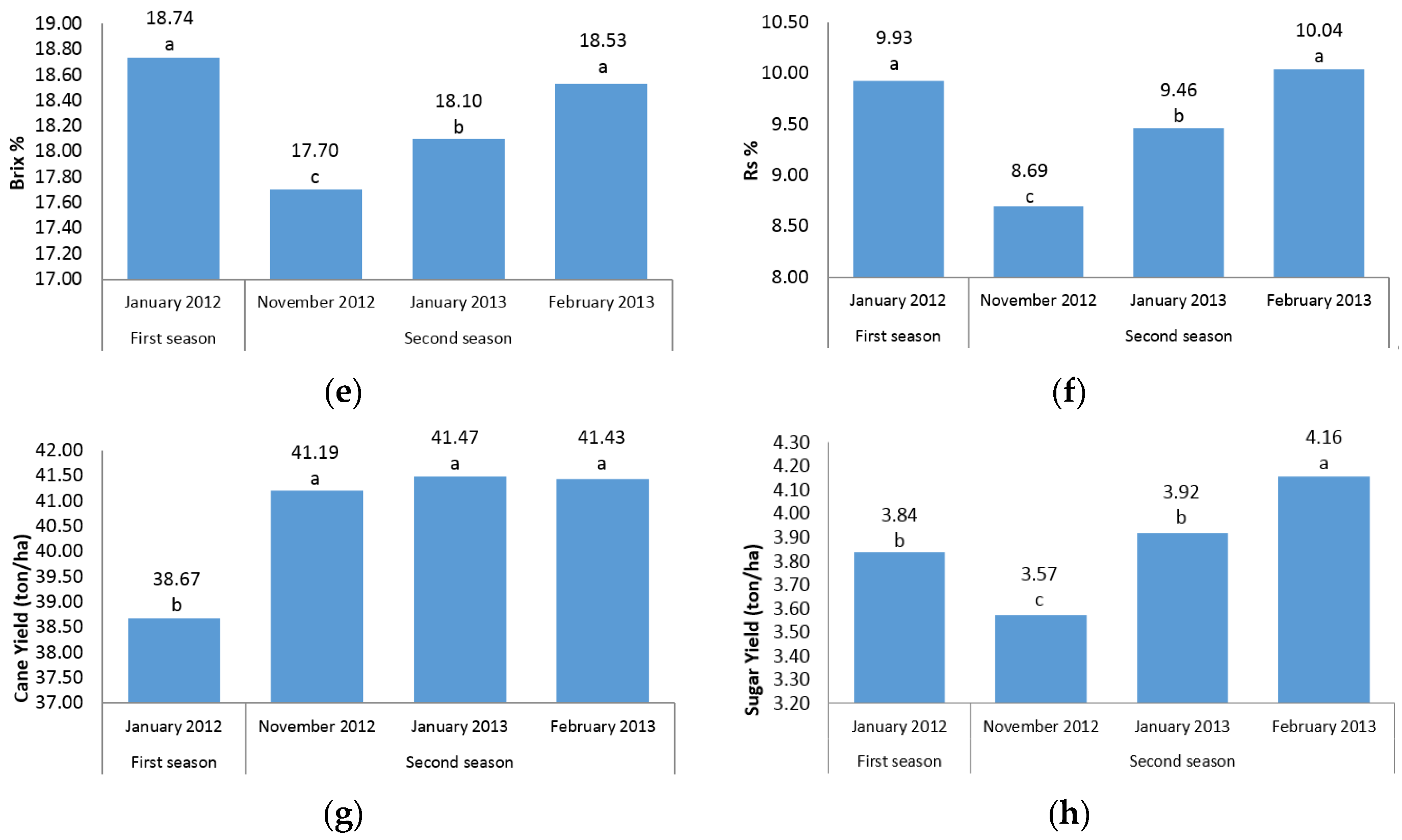
The first question of this study sought to characterize the sugarcane germplasm with the assessment of phenotypic traits. The mean analysis showed that no differences were found among three harvest time points of the second season for stalk height, internode length, stalk diameter, and cane yield (
Figure 1a–c,g). A possible explanation for this result might be that there is cold weather and short daylight in November, January, and February in the second season in Iran, which may have led to a lack of adequate photosynthesis and no differences between the time points were found. Another reason could probably be due to the short time intervals between three harvests; hence, plants did not have growth opportunities. However, for other traits there were significant differences between the three time points in the second season (
Figure 1d–f,h). In fact, for morphological traits, the harvest time differences were not significant; while for sugar-related traits significant differences between the three time points of the second season were observed. A significant increase in height from one season to the next was observed (
Figure 1a). These results are consistent with those reported by Todd et al. [
2], who showed a significant increase in height in the
Saccharum officinarum species at different measurement times.
The present experiment was conducted during four harvest times (in two seasons) to determine the effect of growth periods to select the best harvest time in order to achieve the highest cane and sugar yield. It is interesting to note that in all sugar-related traits, significant differences between two seasons and/or different time points were observed (
Figure 1d–f,h). This finding showed that the best time point with the maximum sugar yield is February in the second season. The same trend was observed for cane yield in which second season values were significantly (
p ≤ 0.05) higher than first season records. Due to the significant increase in cane yield in the second season, these findings suggest the best time for sugarcane harvest to be the second season. Also, it could conceivably be hypothesized that energy-cane cultivars/accessions are preferably harvested in the second year (ratoon stage) due to the importance of cane yield in the production of high biomass and lingo-cellulosic compounds (
Figure 1g).
As mentioned earlier, the greatest magnitudes of correlations were observed between January 2013 and February 2013 for stalk height, internode length, stalk diameter and cane yield (
Table 2). The observed correlation may be due to the short time interval between January and February harvest times and subsequently, a lack of plant growth. These results are in agreement with those reported by Todd et al., [
2] in which there were high correlations for stalk height, stalk diameter, and internode length between two time points. In correlation analysis among traits, positive association was seen between cane yield with stalk height and internode length, at α = 0.01. Cane yield is one of the major parameters for assessment of sugarcane performance [
24]. Hence, stalk height and internode length are two effective traits to improve and to recognize the high cane yield genotype. These results are in agreement with recent studies indicating that cane yield was associated positively with stalk height, internode length and cane thickness [
25]. Another important significant positive correlation was found between sugar-related factors such as RS%, Pol%, Brix%, PTY%, with sugar yield. The present results are significant in at least two major respects. On the one hand, for sugar yield improvement in sugarcane accessions, it is suggested to focus and to manipulate the factors such as RS%, Pol%, Brix%, PTY%. On the other hand, for increase in cane yield, it is recommended to focus on stalk height and internode length.
In current research, Brix% and PTY% were identified as effective traits on RS%, according to multiple stepwise regression analysis results. Positive regression coefficients of these variables indicated that these variables can be mentioned as logical and reasonable indices for selection in order to improve recoverable sucrose in sugarcane. This result confirms the strong association between RS% with two variables of Brix% and PTY%, (
Table 3). The question that arises here is that in the study of correlation analysis (
Table 3), positive correlation between RS% and POL% was found while it is missing in the model. In fact, due to high multicollinearity among independent variables scaled by VIF, POL% was omitted from the analysis.
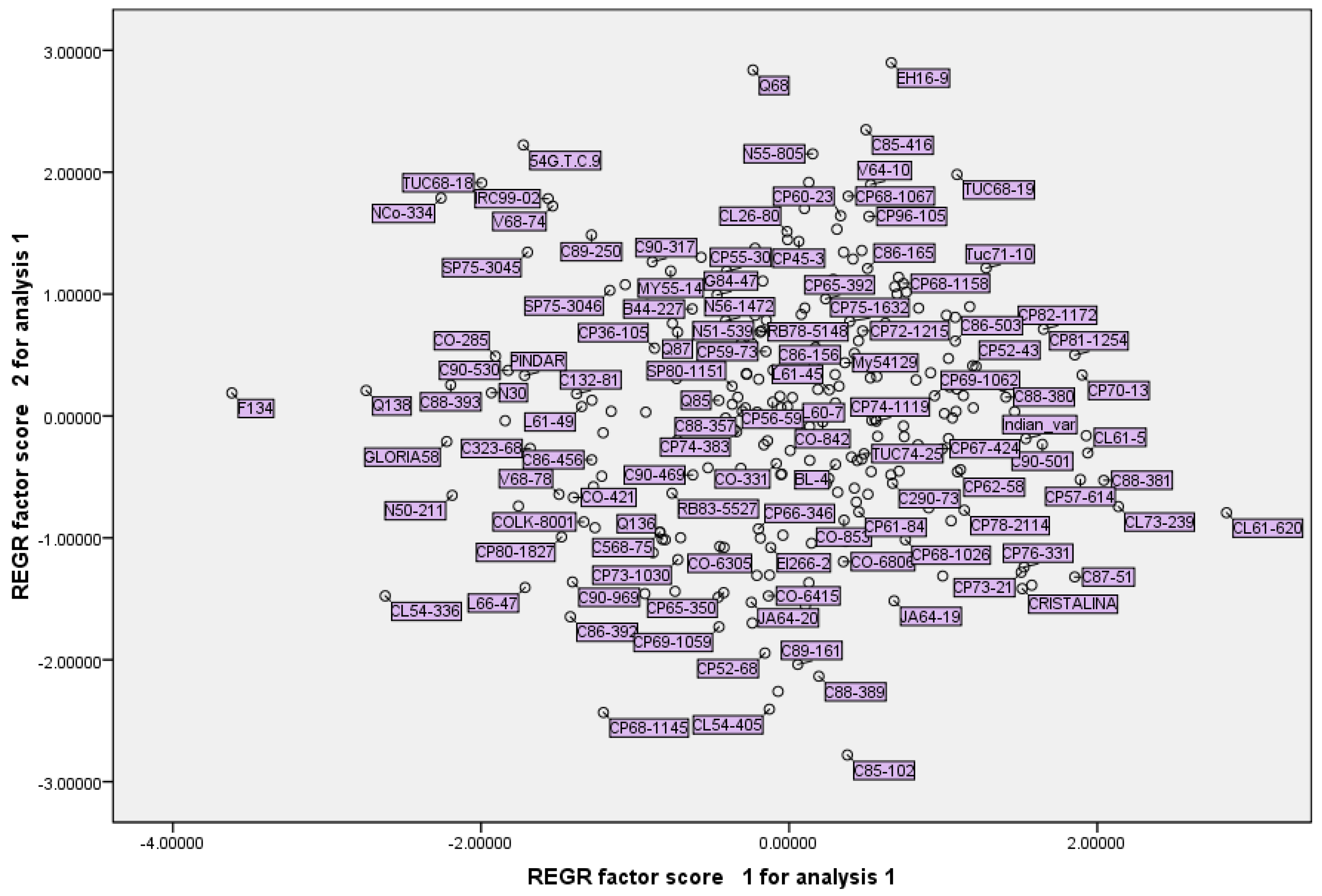
The PCA scatterplot of two principal components axes provided the distribution of primary collection accessions in a biplot. The PCA finding has important implications for introducing accessions with desirable traits, such as accessions with high content of RS% and Brix% or accessions with high/low sugar yield or cane yield (
Figure 2). Arguably, depending on the approach of sugarcane breeding, these traits can be utilized by breeders. Some of the clear findings in the biplot are that CL61-620 and CL73-239 are accessions with the highest content of RS% and Brix% percent, and that CP45-3, Q68 and EH16-9 are accessions with the highest cane yield (
Figure 2). Interestingly these accessions are present in the constructed mini-core collection in this research (
Table 6).
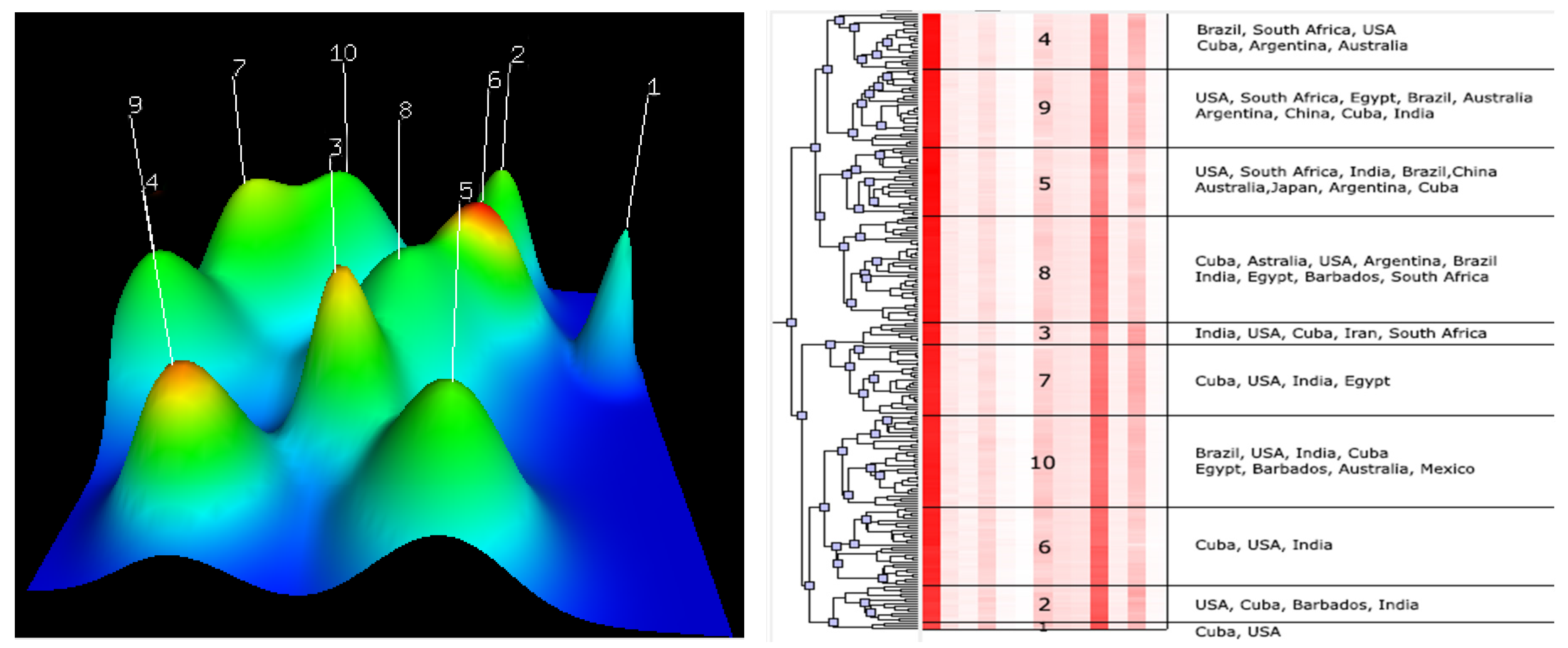
The clustering analysis revealed the morphologic similarity of different accessions from different breeding centres. For example, grouping of the USA and Cuba accessions in the same clusters indicated that there are morphologic similarities between these accessions (
Figure 3). Likewise, the grouping of the Iran–Cuba hybrid (Iran) with the USA, India, Cuba, and South Africa varieties in Cluster 3 demonstrate the morphologic similarities between them (
Figure 3).
The range values of all traits in both collections are shown in
Table 7. As can be seen from the table (above), in the core collection (primary collection), the range value of stalk height and stalk diameter obtained 129.17 (cm) and 15.46 (mm) respectively. Todd et al. showed that range value of stalk height and stalk diameter 304.13 (cm) and 37.79 (mm), respectively, in World Collection of Sugarcane (
Saccharum spp.) and Related Grasses of Sugarcane in Miami, Florida. This is different from the findings presented here about the range value of traits, but the mean value of the mentioned traits in our collection are consistent with data obtained with Todd et al [
2].
With respect to the second research goal, PowerCore software was used to construct a mini-core collection. PowerCore software had been used previously to create core/mini collections for rice (
Oryza sativa L.) [
26,
27], sesame (
Sesamum indicum L.) [
28], and chickpea (
Cicer arietinum L.) [
29]. Using this software, 21 accessions were selected to build a mini-core collection out of 253 accessions of sugarcane. These results confirm the appropriate number of subset collections (10% of the primary collection), as was mentioned in the literature [
8]. A core/mini-core collection with a coincidence rate (CR%) greater than 80% has been recommended as a proper collection for breeding purposes [
30]. In our analysis, the CR was found to be 93.96%, indicating that the mini-core collection selected from sugarcane germplasm is a good representative subset of the phenotypic diversity in the primary collection. Also,
t- and Wilcoxon test results showed no significant difference in means and medians between the mini-core and primary sugarcane collections (
Table 7). Furthermore, high values for VR% and VD% [
31], which are the evaluating factors of core collections, reconfirm that the mini-core collection in this study incorporates the main portion of diversity of the primary sugarcane collection. Additionally, the
F and Ansari–Bradley test results also revealed no significant loss of diversity in mini-core collection compared with the primary collection. Interestingly, for normally distributed traits, the mini-core collection was more diverse than the whole collection. However, for non-normally distributed traits, Ansari–Bradley test results showed non-significant difference between two collections, except for juice weight.
For comparing the Shannon–Weaver diversity indices between the two collections pairwise
t-test was carried out. Accordingly, significant differences were found between the two collections, so that Shannon–Weaver index scores in the mini-core were significantly higher than in the primary collection. Higher variance in the core/mini collection relative to the entire collection has been reported frequently in previous researches [
2,
31,
32].