Low-Input Maize-Based Cropping Systems Implementing IWM Match Conventional Maize Monoculture Productivity and Weed Control
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Cropping Systems Description and Experimental Design
2.2.1. Conventional Maize Monoculture (MMConv)
2.2.2. IWM Low-Input Maize Monoculture (MMLI)
2.2.3. Conservation Tillage Maize Monoculture (MMCT)
2.2.4. Integrated Maize Rotation (MSW)
2.3. Weed Sampling
2.4. Indicator of Weed-Pressure: The Potential of Infestation (PI)
2.5. Yield Assessment
2.6. Statistical Analysis
3. Results
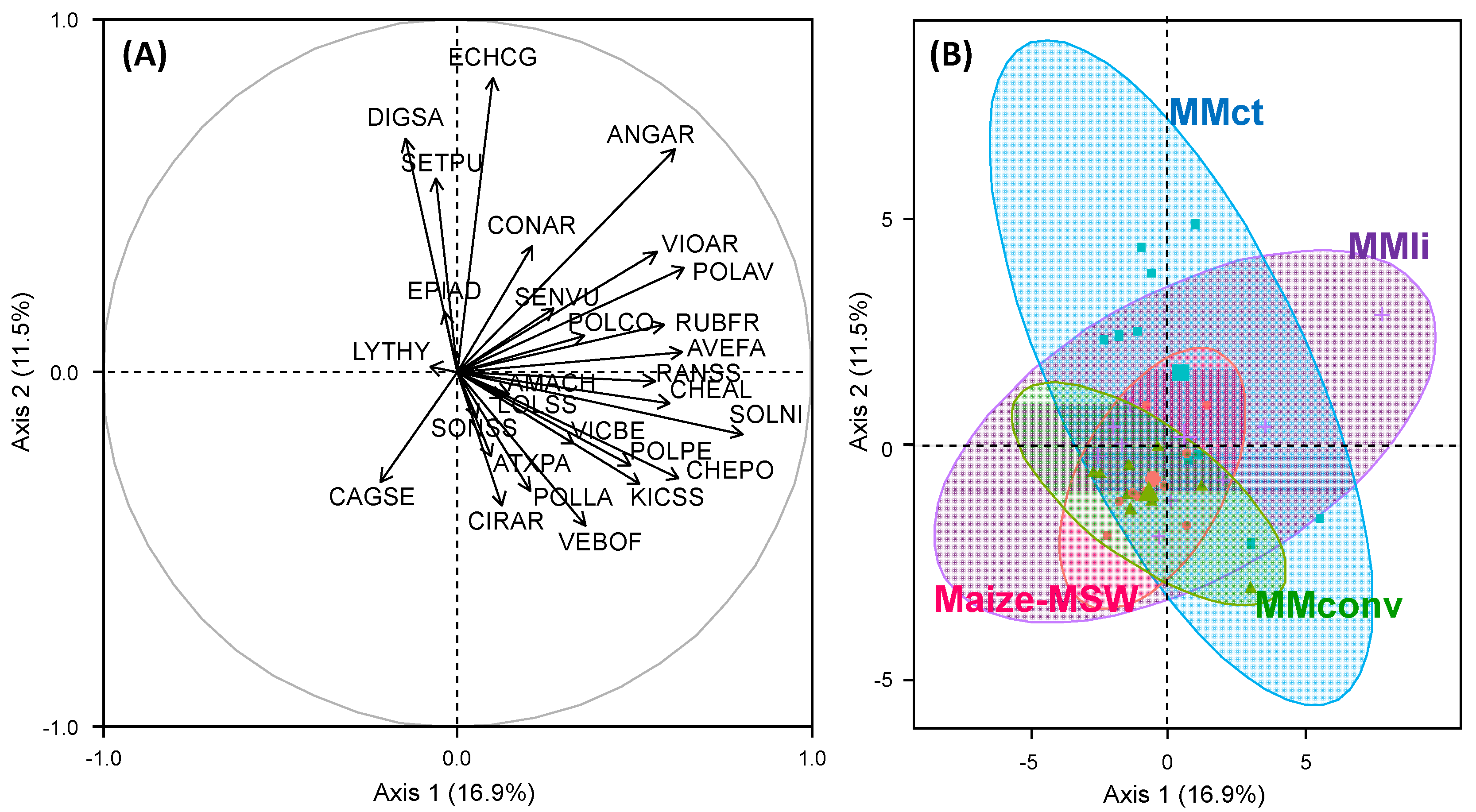
3.1. Effect of Cropping Systems on Weed Communities
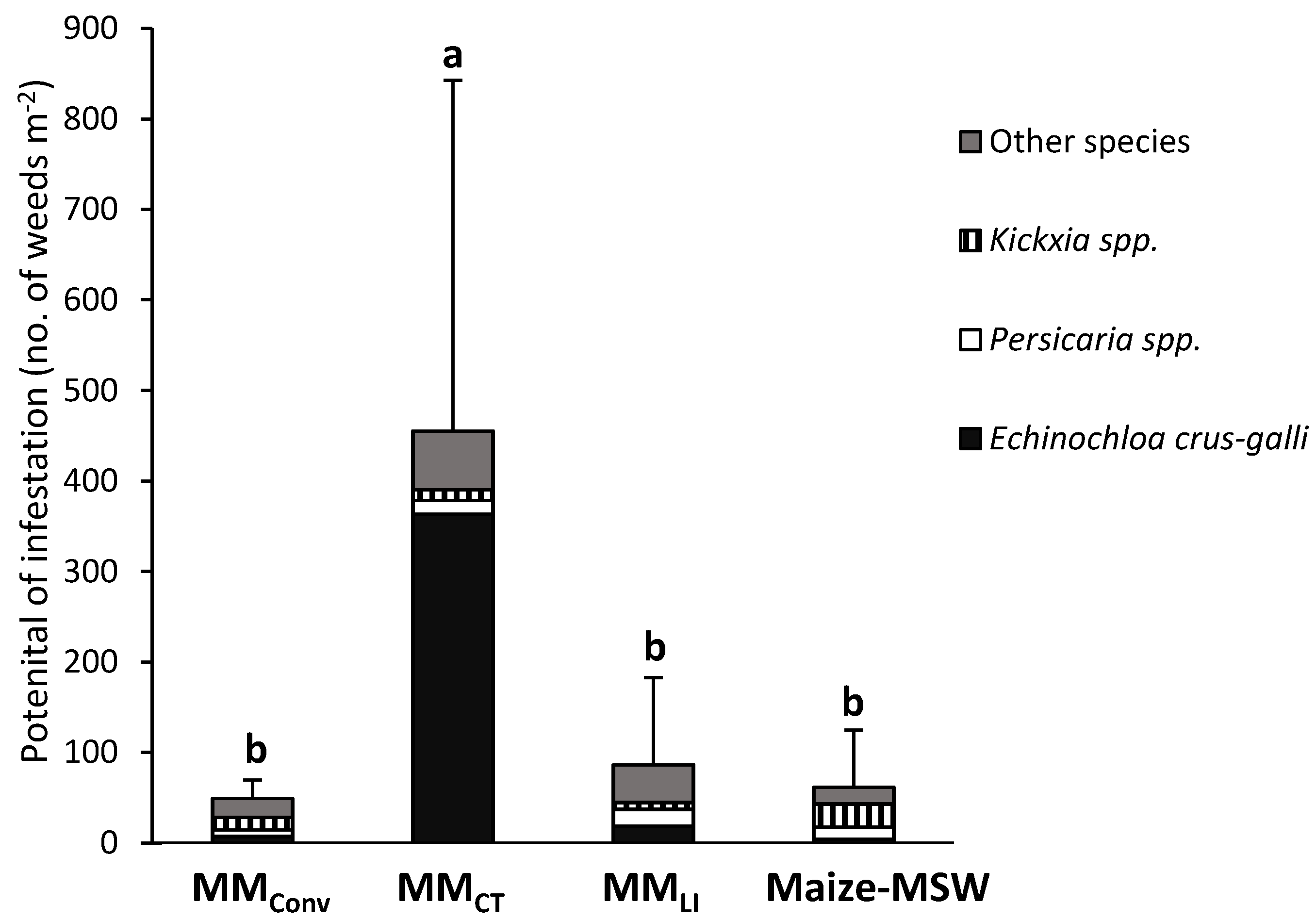
3.2. Potential of Infestation
3.3. Weed Population Dynamics
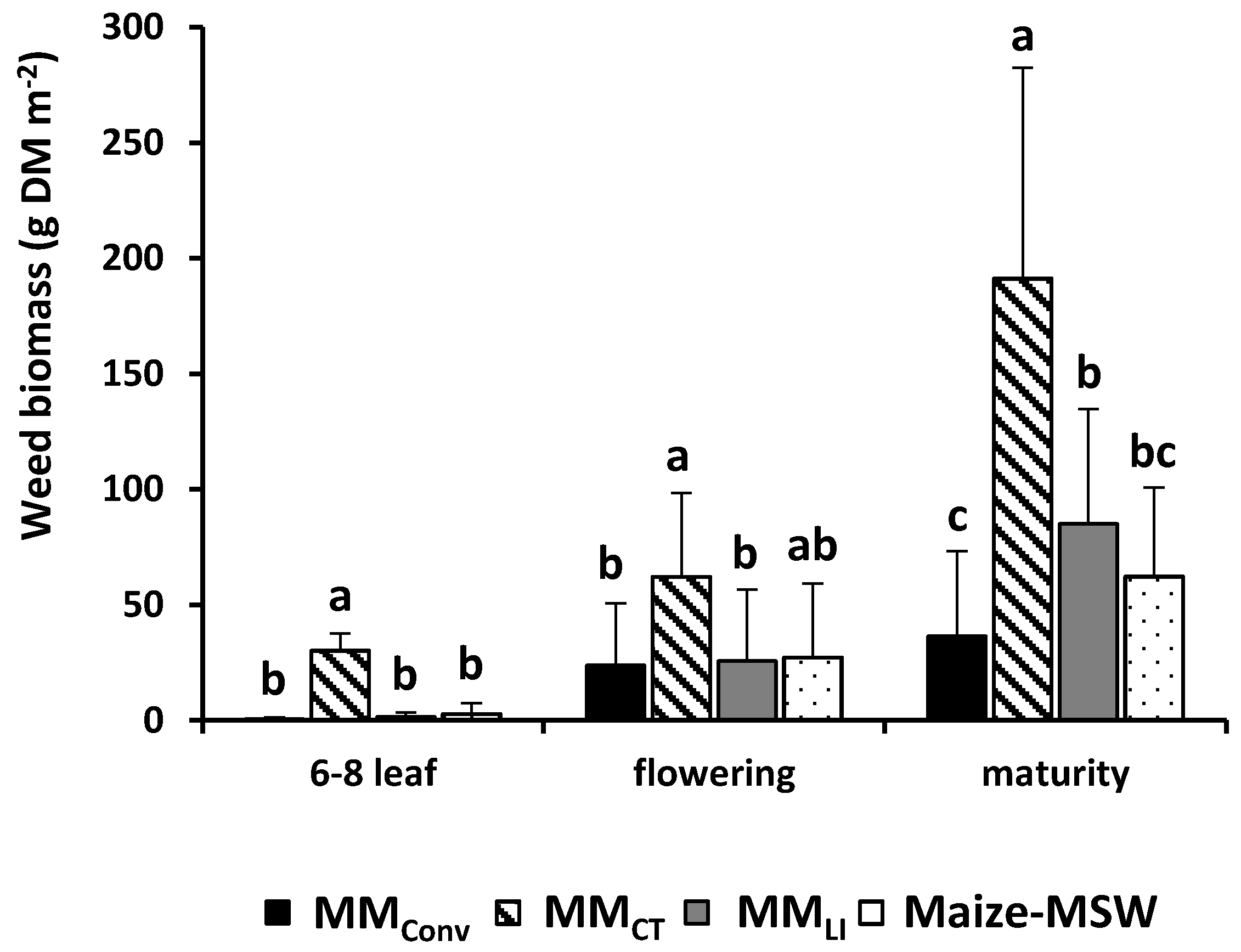
3.4. Weed Biomass: Effect of Cropping Systems at Different Maize Growth Stages
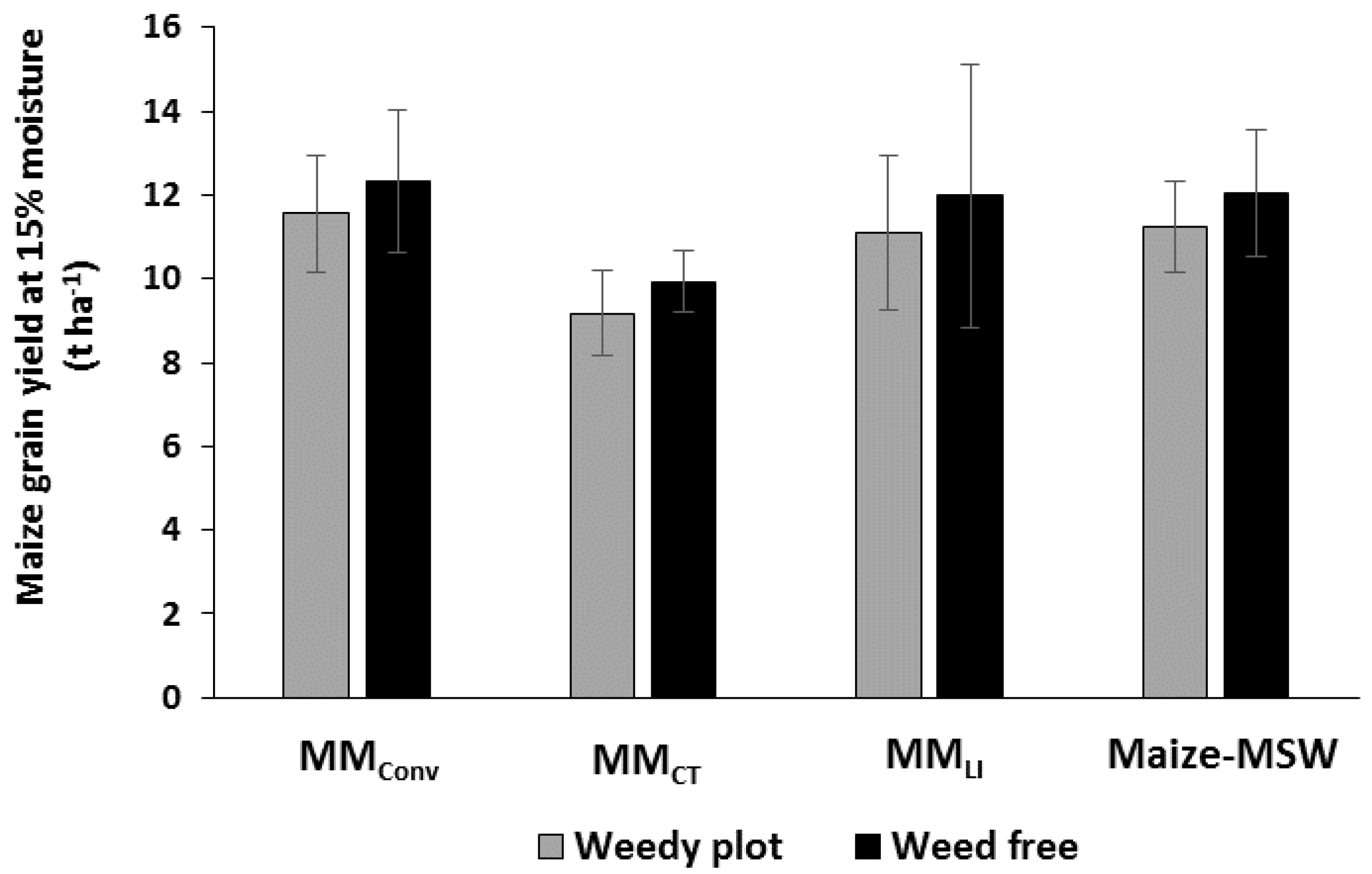
3.5. Impact of Weeds and Cropping Systems on Maize Yields
4. Discussion
4.1. Efficient Weed Management in Low-Input Cropping Systems
4.2. Conservation Tillage
4.3. Rotating Maize
4.4. Weed Biomass at Maturity and Maize Yield
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bosnic, A.C.; Swanton, C.J. Influence of barnyardgrass (Echinochloa. crus-galli) time of emergence and density on corn (Zea mays). Weed Sci. 1997, 45, 276–282. [Google Scholar] [CrossRef]
- Aymard, D.; Cassagne, J.P.; Sablik, M.C. Mémento Agricole du Bassin Adour-Garonne; Ministère de L’agriculture, Agro-Alimentaire et de la Forêt: Paris, France, 2014. [Google Scholar]
- Heap, I. Herbicide resistant weeds. In Integrated Pest Management; Springer: Dordrecht, The Netherlands, 2014; pp. 281–301. [Google Scholar]
- Stoate, C.; Boatman, N.D.; Borralho, R.J.; Carvalho, C.R.; Snoo, G.R.D.; Eden, P. Ecological impacts of arable intensification in Europe. J. Environ. Manag. 2001, 63, 337–365. [Google Scholar] [CrossRef]
- Chikowo, R.; Faloya, V.; Petit, S.; Munier-Jolain, N.M. Integrated weed management systems allow reduced reliance on herbicides and long-term weed control. Agric. Ecosyst. Environ. 2009, 132, 237–242. [Google Scholar] [CrossRef]
- Liebman, M.; Gallandt, E.R. Many little hammers: Ecological management of crop-weed interactions. In Ecology in Agriculture; Academic Press: San Diego, CA, USA, 1997; pp. 291–343. [Google Scholar]
- Fried, G.; Norton, L.R.; Reboud, X. Environmental and management factors determining weed species composition and diversity in France. Agric. Ecosyst. Environ. 2008, 128, 68–76. [Google Scholar] [CrossRef]
- Chauvel, B.; Guillemin, J.; Colbach, N.; Gasquez, J. Evaluation of cropping systems for management of herbicide-resistant populations of blackgrass (Alopecurus. myosuroides Huds.). Crop Prot. 2001, 20, 127–137. [Google Scholar] [CrossRef]
- Booth, B.D.; Swanton, C.J. Assembly theory applied to weed communities. Weed Sci. 2002, 50, 2–13. [Google Scholar] [CrossRef]
- Morris, N.; Miller, P.; Orson, J.; Froud-Williams, R. The adoption of non-inversion tillage systems in the United Kingdom and the agronomic impact on soil, crops and the environment—A review. Soil Tillage Res. 2010, 108, 1–15. [Google Scholar] [CrossRef]
- Zanin, G.; Otto, S.; Riello, L.; Borin, M. Ecological interpretation of weed flora dynamics under different tillage systems. Agric. Ecosyst. Environ. 1997, 66, 177–188. [Google Scholar] [CrossRef]
- Murphy, S.D.; Clements, D.R.; Belaoussoff, S.; Kevan, P.G.; Swanton, C.J. Promotion of weed species diversity and reduction of weed seedbanks with conservation tillage and crop rotation. Weed Sci. 2006, 54, 69–77. [Google Scholar] [CrossRef]
- O’Connell, P.J.; Harms, C.T.; Allen, J.R. Metolachlor, S-metolachlor and their role within sustainable weed-management. Crop Prot. 1998, 17, 207–212. [Google Scholar] [CrossRef]
- Dobbels, A.F.; Kapusta, G. Postemergence weed control in corn (Zea mays) with nicosulfuron combinations. Weed Technol. 1993, 7, 844–850. [Google Scholar] [CrossRef]
- Johnson, B.C.; Young, B.G.; Matthews, J.L. Effect of postemergence application rate and timing of mesotrione on corn (Zea mays) response and weed control. Weed Technol. 2002, 16, 414–420. [Google Scholar] [CrossRef]
- Spaunhorst, D.J.; Bradley, K.W. Influence of dicamba and dicamba plus glyphosate combinations on the control of glyphosate-resistant waterhemp (Amaranthus. rudis). Weed Technol. 2013, 27, 675–681. [Google Scholar] [CrossRef]
- Riemens, M.; Van Der Weide, R.; Bleeker, P.; Lotz, L. Effect of stale seedbed preparations and subsequent weed control in lettuce (cv. Iceboll) on weed densities. Weed Res. 2007, 47, 149–156. [Google Scholar] [CrossRef]
- Leblanc, M.L.; Cloutier, D.C.; Leroux, G.D. Réduction de l’utilisation des herbicides dans le maïs-grain par une application d’herbicides en bandes combinée à des sarclages mécaniques. Weed Res. 1995, 35, 511–522. [Google Scholar] [CrossRef]
- Henson, I. The effects of light, potassium nitrate and temperature on the germination of Chenopodium album. Weed Res. 1970, 10, 27–39. [Google Scholar] [CrossRef]
- Espeby, L. Germination of Weed Seeds and Competition in Stands of Weeds and Barley: Influences of Mineral Nutrients; Department of Crop Production Science, Swedish University of Agricultural Sciences: Uppsala, Sweden, 1989. [Google Scholar]
- Fawcett, R.; Slife, F. Effects of field applications of nitrate on weed seed germination and dormancy. Weed Sci. 1978, 26, 594–596. [Google Scholar] [CrossRef]
- Banks, P.; Santelmann, P.; Tucker, B. Influence of long-term soil fertility treatments on weed species in winter wheat. Agron. J. 1976, 68, 825–827. [Google Scholar] [CrossRef]
- Dyck, E.; Liebman, M. Soil fertility management as a factor in weed control: The effect of crimson clover residue, synthetic nitrogen fertilizer, and their interaction on emergence and early growth of lambsquarters and sweet corn. Plant Soil 1994, 167, 227–237. [Google Scholar] [CrossRef]
- Jørnsgård, B.; Rasmussen, K.; Hill, J.; Christiansen, J.L. Influence of nitrogen on competition between cereals and their natural weed populations. Weed Res. 1996, 36, 461–470. [Google Scholar] [CrossRef]
- Pyšek, P.; Lepš, J. Response of a weed community to nitrogen fertilization: A multivariate analysis. J. Veg. Sci. 1991, 2, 237–244. [Google Scholar] [CrossRef]
- O’Donovan, J.T.; McAndrew, D.W.; Thomas, A.G. Tillage and nitrogen influence weed population dynamics in barley (Hordeum. vulgare). Weed Technol. 1997, 11, 502–509. [Google Scholar] [CrossRef]
- Pawar, L.; Yaduraju, N.; Ahuja, K. Population dynamics of weeds and their growth in tall and dwarf wheat as influenced by sub-optimal levels of irrigation and nitrogen. Indian J. Ecol. 1998, 25, 146–154. [Google Scholar]
- Andersson, T.N.; Milberg, P. Weed Flora and the Relative Importance of Site, Crop, Crop Rotation, and Nitrogen. Weed Sci. 1998, 46, 30–38. Available online: http://www.jstor.org/stable/4046005 (accessed on 8 January 2017).
- Blackshaw, R.E.; Molnar, L.J.; Janzen, H.H. Nitrogen fertilizer timing and application method affect weed growth and competition with spring wheat. Weed Sci. 2004, 52, 614–622. [Google Scholar] [CrossRef]
- Cordeau, S.; Guillemin, J.-P.; Reibel, C.; Chauvel, B. Weed species differ in their ability to emerge in no-till systems that include cover crops. Ann. Appl. Biol. 2015, 166, 444–455. [Google Scholar] [CrossRef]
- Brennan, E.B.; Smith, R.F. Winter cover crop growth and weed suppression on the central coast of California. Weed Technol. 2005, 19, 1017–1024. [Google Scholar] [CrossRef]
- Yeganehpoor, F.; Salmasi, S.Z.; Abedi, G.; Samadiyan, F.; Beyginiya, V. Effects of cover crops and weed management on corn yield. J. Saudi Soc. Agric. Sci. 2015, 14, 178–181. [Google Scholar] [CrossRef]
- Teasdale, J.; Mohler, C. Light transmittance, soil temperature, and soil moisture under residue of hairy vetch and rye. Agron. J. 1993, 85, 673–680. [Google Scholar] [CrossRef]
- Reddy, K.N.; Koger, C.H. Live and killed hairy vetch cover crop effects on weeds and yield in glyphosate-resistant corn. Weed Technol. 2004, 18, 835–840. [Google Scholar] [CrossRef]
- Blackshaw, R.E.; Upadhyaya, M.K. Non-Chemical Weed Management: Principles, Concepts and Technology; Centre for Agriculture and Bioscience International: Oxfordshire, UK, 2007. [Google Scholar]
- Blackshaw, R.E.; Moyer, J.R.; Doram, R.C.; Boswell, A.L. Yellow sweetclover, green manure, and its residues effectively suppress weeds during fallow. Weed Sci. 2001, 49, 406–413. [Google Scholar] [CrossRef]
- Kunz, C.; Sturm, D.; Varnholt, D.; Walker, F.; Gerhards, R. Allelopathic effects and weed suppressive ability of cover crops. Plant Soil Environ. 2016, 62, 60–66. [Google Scholar] [CrossRef]
- Tollenaar, M.; Aguilera, A.; Nissanka, S.P. Grain yield is reduced more by weed interference in an old than in a new maize hybrid. Agron. J. 1997, 89, 239–246. [Google Scholar] [CrossRef]
- Derby, N.E.; Steele, D.D.; Terpstra, J.; Knighton, R.E.; Casey, F.X. Interactions of nitrogen, weather, soil, and irrigation on corn yield. Agron. J. 2005, 97, 1342–1351. [Google Scholar] [CrossRef]
- Gholamhoseini, M.; AghaAlikhani, M.; Modarres Sanavy, S.A.M.; Mirlatifi, S.M. Interactions of irrigation, weed and nitrogen on corn yield, nitrogen use efficiency and nitrate leaching. Agric. Water Manag. 2013, 126, 9–18. [Google Scholar] [CrossRef]
- Das, T.; Yaduraju, N. Effect of weed competition on growth, nutrient uptake and yield of wheat as affected by irrigation and fertilizers. J. Agric. Sci. 1999, 133, 45–51. [Google Scholar] [CrossRef]
- Giuliano, S.; Ryan, M.R.; Véricel, G.; Rametti, G.; Perdrieux, F.; Justes, E.; Alletto, L. Low-input cropping systems to reduce input dependency and environmental impacts in maize production: A multi-criteria assessment. Eur. J. Agron. 2016, 76, 160–175. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2006; First Update 2007; Food and Agriculture Organization (FAO): Rome, Italy, 2007. [Google Scholar]
- Organisation for Economic Co-operation and Development. Environmental indicators for agriculture. In Methods and Results; OECD Publications: Paris, France, 2001; Volume 3. [Google Scholar]
- Cordeau, S.; Dessaint, F.; Munier-Jolain, N.M. Long-term assessment of integrated weed management cropping systems in France. Asp. Appl. Biol. 2015, 128, 275–278. [Google Scholar]
- Stoller, E.W.; Wax, L.M. Periodicity of germination and emergence of some annual weeds. Weed Sci. 1973, 21, 574–580. [Google Scholar] [CrossRef]
- Hughes, G. The problem of weed patchiness. Weed Res. 1990, 30, 223–224. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; The R Foundation for Statistical Computing: Vienna, Austria, 2011. [Google Scholar]
- Roger-Estrade, J.; Colbach, N.; Leterme, P.; Richard, G.; Caneill, J. Modelling vertical and lateral weed seed movements during mouldboard ploughing with a skim-coulter. Soil Tillage Res. 2001, 63, 35–49. [Google Scholar] [CrossRef]
- Pannacci, E.; Graziani, F.; Covarelli, G. Use of herbicide mixtures for pre and post-emergence weed control in sunflower (Helianthus annuus). Crop Prot. 2007, 26, 1150–1157. [Google Scholar] [CrossRef]
- Streit, B.; Rieger, S.B.; Stamp, P.; Richner, W. The effect of tillage intensity and time of herbicide application on weed communities and populations in maize in central Europe. Agric. Ecosyst. Environ. 2002, 92, 221–224. [Google Scholar] [CrossRef]
- Alletto, L.; Giuliano, S.; Perdrieux, F.; Rametti, G.; Benoit, P.; Véricel, G.; Justes, E. Comparison of the environmental performances of four maize monocropping systems: A three years monitoring of pesticides leaching. In Proceedings of the Pesticide Behaviour in Soils, Water and Air Conference, York, North Yorkshire, UK, 2–4 September 2013. [Google Scholar]
- Primot, S.; Valantin-Morison, M.; Makowski, D. Predicting the risk of weed infestation in winter oilseed rape crops. Weed Res. 2006, 46, 22–33. [Google Scholar] [CrossRef]
- Buhler, D.D.; Stoltenberg, D.E.; Becker, R.L.; Gunsolus, J.L. Perennial weed populations after 14 years of variable tillage and cropping practices. Weed Sci. 1994, 42, 205–209. [Google Scholar] [CrossRef]
- Odhiambo, J.A.; Norton, U.; Ashilenje, D.; Omondi, E.C.; Norton, J.B. Weed dynamics during transition to conservation agriculture in western Kenya maize production. PLoS ONE 2015, 10, e0133976. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, B.S.; Singh, R.G.; Mahajan, G. Ecology and management of weeds under conservation agriculture: A review. Crop Prot. 2012, 38, 57–65. [Google Scholar] [CrossRef]
- Van den Putte, A.; Govers, G.; Diels, J.; Gillijns, K.; Demuzere, M. Assessing the effect of soil tillage on crop growth: A meta-regression analysis on European crop yields under conservation agriculture. Eur. J. Agron. 2010, 33, 231–241. [Google Scholar] [CrossRef]
- Pittelkow, C.M.; Liang, X.; Linquist, B.A.; Van Groenigen, K.J.; Lee, J.; Lundy, M.E.; van Gestel, N.; Six, J.; Venterea, R.T.; van Kessel, C. Productivity limits and potentials of the principles of conservation agriculture. Nature 2015, 517, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, B.S.; Johnson, D.E. The role of seed ecology in improving weed management strategies in the tropics. Adv. Agron. 2010, 105, 221–262. [Google Scholar] [CrossRef]
- Bullock, D.G. Crop rotation. Crit. Rev. Plant Sci. 1992, 11, 309–326. [Google Scholar] [CrossRef]
- Van Der Weide, R.Y.; Bleeker, P.O.; Achten, V.T.J.M.; Lotz, L.A.P.; Fogelberg, F.; Melander, B. Innovation in mechanical weed control in crop rows. Weed Res. 2008, 48, 215–224. [Google Scholar] [CrossRef]
- Kropff, M.; Vossen, F.; Spitters, C.; De Groot, W. Competition between a maize crop and a natural population of Echinochloa crus-galli (L.) Pb. Neth. J. Agric. Sci. 1984, 32, 324–327. [Google Scholar]
- Maun, M.; Barrett, S. The biology of Canadian weeds: 77. Echinochloa crus-galli (L.) Beauv. Can. J. Plant Sci. 1986, 66, 739–759. [Google Scholar] [CrossRef]
- Hugo, E.; Morey, L.; Saayman-Du Toit, A.E.; Reinhardt, C.F. Critical periods of weed control for naked crabgrass (Digitaria nuda), a grass weed in corn in South Africa. Weed Sci. 2014, 62, 647–656. [Google Scholar] [CrossRef]
- Hall, M.R.; Swanton, C.J.; Anderson, G.W. The critical period of weed control in grain corn (Zea mays). Weed Sci. 1992, 40, 441–447. [Google Scholar] [CrossRef]
| Cropping System | Weed Species | |||||
|---|---|---|---|---|---|---|
| Convolvulus arvensis L. ‡ | Digitaria sanguinalis (L.) Scop. ‡ | Echinochloa crus-galli (L.) P.Beauv. + | ||||
| MMConv | 2 ± 2 | b | 0 ± 0 | b | 7 ± 7 | b |
| MMCT | 17 ± 7 | a | 13 ± 29 | a | 364 ± 399 | a |
| MMLI | 2 ± 2 | b | 1 ± 1 | ab | 18 ± 26 | ab |
| Maize-MSW | 2 ± 2 | b | 0 ± 0 | b | 4 ± 6 | b |
| Significance p | *** | ** | *** | |||
| Cropping System | Weed Species | |||
|---|---|---|---|---|
| Convolvulus arvensis L. | Digitaria sanguinalis (L.) Scop. | Echinochloa crus-galli (L.) P.Beauv. | Total | |
| MMConv | −0.37 ** | NA | ns | ns |
| MMCT | ns | 0.66 * | 1.37 ** | 0.66 *** |
| MMLI | ns | ns | ns | ns |
| Cropping System | Weed Species | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chenopodium spp. ‡ | Convolvulus arvensis L. ‡ | Digitaria sanguinalis (L) Scop. ‡ | Echinochloa crus-galli (L.) P.Beauv. ° | Persicaria spp. ° | Setaria pumila (Poir.) Roem. & Schult ‡ | |||||||
| MMConv | 1 ± 3 | ab | 1 ± 1 | b | 0 | b | 6 ± 7 | b | 17 ± 35 | b | 0 ± 0 | ab |
| MMCT | 1 ± 3 | ab | 4 ± 3 | a | 4 ± 6 | a | 140 ± 114 | a | 19 ± 2 6 | ab | 3 ± 5 | a |
| MMLI | 4 ± 5 | a | 2 ± 3 | ab | 1 ± 1 | ab | 25 ± 29 | ab | 31 ± 42 | ab | 0 | b |
| Maize-MSW | 0 ± 0 | b | 2 ± 3 | ab | 0 ± 0 | b | 14 ± 21 | b | 35 ± 41 | a | 0 ± 0 | ab |
| Significance p | * | ** | ** | *** | * | * | ||||||
| Cropping System | Year | Mean Yield | |||||
|---|---|---|---|---|---|---|---|
| 2011 | 2012 | 2013 | 2014 | 2015 | |||
| MMConv | 10.8 ± 1.1 | 11.5 ± 1.3 | 10.9 ± 0.2 | 11.7 ± 2.2 | 11.4 ± 1.0 | 11.3 ± 1.1 | a |
| MMCT | 10.0 ± 1.3 | 6.6 ± 0.8 | 5.9 ± 0.4 | 8.5 ± 1.1 | 9.8 ± 0.4 | 8.2 ± 1.8 | b |
| MMLI | 10.0 ± 1.3 | 11.5 ± 1.7 | 7.8 ± 2.8 | 11.8 ± 2.1 | 10.3 ± 1.8 | 10.6 ± 2.3 | a |
| Maize-MSW | 9.4 ± 0.6 | 10.2 ± 1.6 | 6.4 ± 0.5 | 10.3 ± 0.1 | 12.2 ± 0.3 | 9.7 ± 2.1 | ab |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adeux, G.; Giuliano, S.; Cordeau, S.; Savoie, J.-M.; Alletto, L. Low-Input Maize-Based Cropping Systems Implementing IWM Match Conventional Maize Monoculture Productivity and Weed Control. Agriculture 2017, 7, 74. https://doi.org/10.3390/agriculture7090074
Adeux G, Giuliano S, Cordeau S, Savoie J-M, Alletto L. Low-Input Maize-Based Cropping Systems Implementing IWM Match Conventional Maize Monoculture Productivity and Weed Control. Agriculture. 2017; 7(9):74. https://doi.org/10.3390/agriculture7090074
Chicago/Turabian StyleAdeux, Guillaume, Simon Giuliano, Stéphane Cordeau, Jean-Marie Savoie, and Lionel Alletto. 2017. "Low-Input Maize-Based Cropping Systems Implementing IWM Match Conventional Maize Monoculture Productivity and Weed Control" Agriculture 7, no. 9: 74. https://doi.org/10.3390/agriculture7090074






