Living mulches generated substantial biomass and effectively suppressed weeds in plots where they established well. Where emergence of the living mulch was high and 80% cover was reached within 3 to 4 weeks after planting, weed densities were below 15 plants m
−2 (
Table 2). Numerous weed species occurred at the four trial sites; and no single weed species dominated any site-year, except parthenium (
Parthenium hysterophorus L.) in Site 1-Year 1. Abundant weeds included Indian copperleaf (
Acalypha indica L.), tropical whiteweed (
Ageratum conyzoides L.), sessile joyweed [
Alternanthera sessilis (L.) R. Br. ex DC.], slender amaranth (
Amaranthus viridis L.), spurge [
Chamaesyce hirta (L.) Millsp.], Benghal dayflower (
Commelina benghalensis L.), purple nutsedge (
Cyperus rotundus L.), crowfoot grass [
Dactyloctenium aegyptium (L.) Willd.], viper grass [
Dinebra retroflexa (Vahl) Panzer], Japanese lovegrass [
Eragrostis amabilis (L.) Wight & Arn. ex Nees] and common wireweed (
Sida acuta Burm. f.). Weed species that emerged early in the season included purple nutsedge, Benghal dayflower, spurge and
Amaranthus spp. Benghal dayflower and spurge were particularly prolific alongside drip irrigation lines (located within the cotton rows). Grass weeds such as crowfoot grass, viper grass and Japanese lovegrass occurred much later in the season towards the start of the harvest period.
3.1. Cotton Growth and Yield
Sesbania and sunnhemp were the two living mulch treatments that were successfully evaluated during both Years 1 and 2, at all trial sites. The other species lacked vigor and reliability in establishing. From data pooled across Years 1 and 2, Sites 1 and 2, and sesbania and sunnhemp treatments, there was no treatment by year interaction (
p = 0.9). Cotton yields from sesbania (1540 kg ha
−1) and sunnhemp (1522 kg ha
−1) plots were not statistically different from the hand-weeded control (1809 kg ha
−1) (
p = 0.23) (
Table 3). No treatment by year interaction was found for cotton height (
p = 0.74) and boll number (
p = 0.53). Cotton height and boll number in sesbania (112 cm and 31, respectively) and sunnhemp (119 cm and 32, respectively) were not different from the control (114 cm and 43, respectively) (
p = 0.56 and 0.06, respectively).
Although no treatment by year interaction was found for the sesbania and sunnhemp treatments, all cotton parameters differed in Year 1 and Year 2- yield (1467 and 1780 kg ha
−1, respectively;
p = 0.04), height (92 and 139 cm, respectively;
p < 0.0001) and boll count (27 and 44, respectively;
p = 0.0002). In Year 1, across Sites 1 and 2, cotton yields from all the living mulch treatments (sesbania (1343 kg ha
−1), sunnhemp (1380 kg ha
−1) and sorghum sudan grass (1315 kg ha
−1)) did not differ from each other or the control (1585 kg ha
−1) (
p = 0.19) (
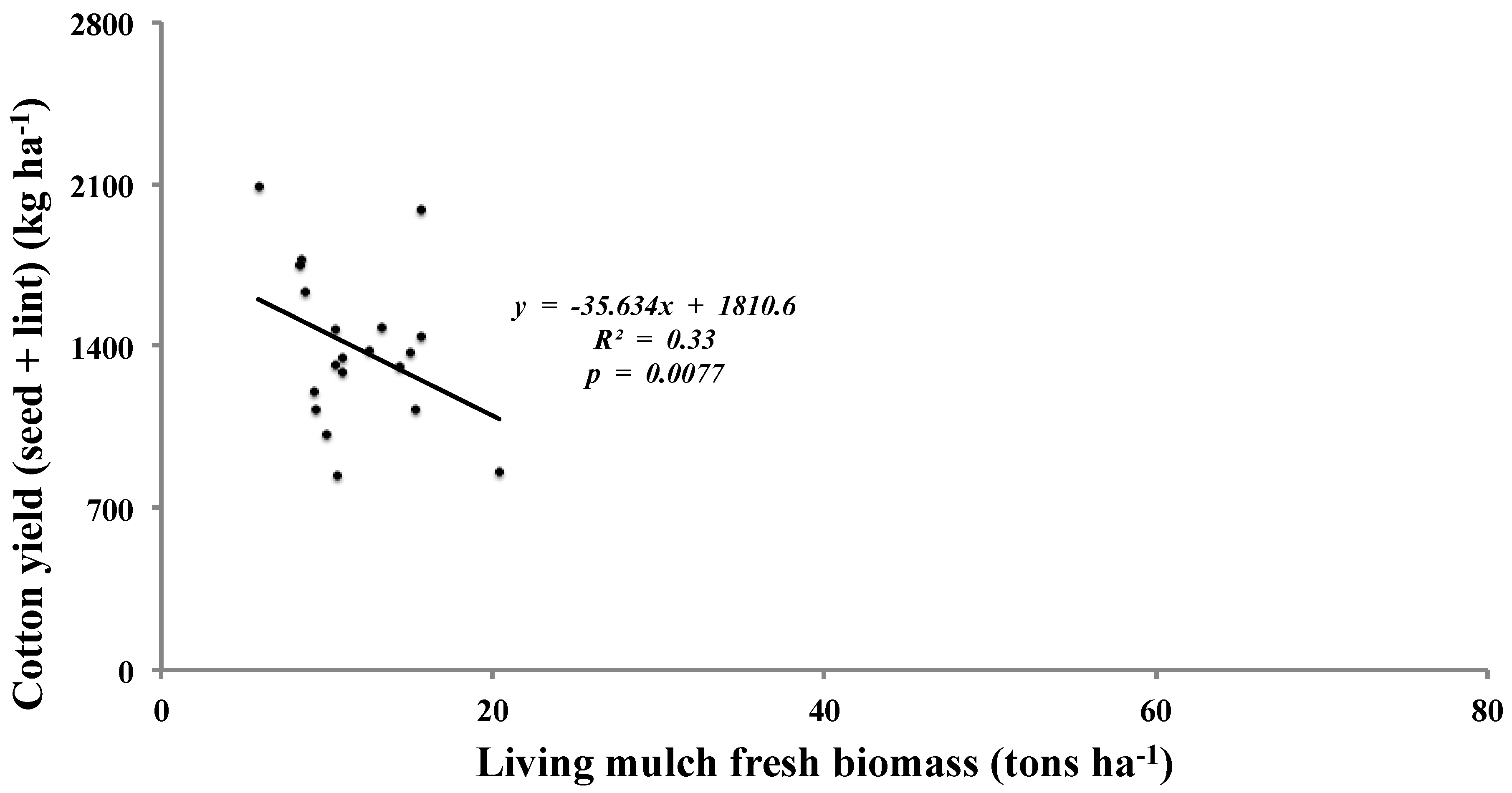
Table 4). Although the treatments did not have any effect on cotton yields, there was a negative correlation (
p = 0.0077) between cotton yield and living mulch biomass (
Figure 1).
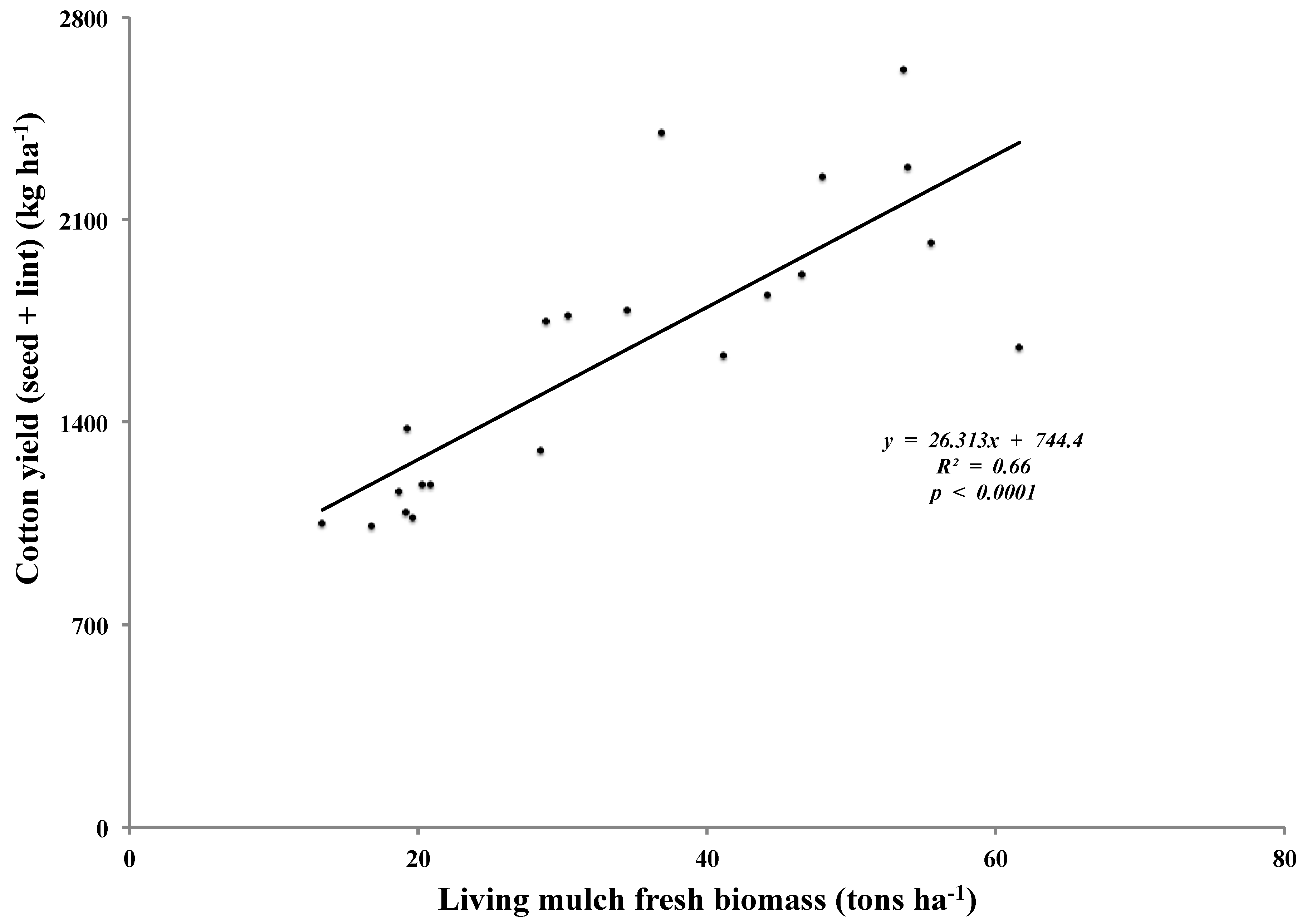
This relationship between cotton yield and living mulch biomass during Year 1 was in contrast to that in Year 2. When sesbania and sunnhemp treatments from Year 2 were considered, there was a strong positive correlation between cotton yield and living mulch biomass (
p < 0.0001) (
Figure 2). Site 3 was excluded from this regression analysis due to overall poor cotton yield at this site, resulting from untimely planting during wet conditions. Gliricidia, sorghum sudan grass and the mixture treatments were also excluded since they were not present at all trial sites. Even though, in 2012, limited overhead irrigation was provided at Site 1 and drip irrigation was provided at Site 2, these contrasting results were probably due to the dry conditions in 2012 relative to 2013 [
22].
Sesbania and sunnhemp data from all trial sites during Year 2 were used to examine treatment effects on cotton (
Table 5). Cotton yields from sesbania (1476 kg ha
−1) or sunnhemp (1462 kg ha
−1) plots were not statistically different (
p = 0.25) from control (1643 kg ha
−1) plots. This suggests that the planting and management techniques used for these living mulch treatments were successful in preventing competition with the cotton crop. Reduced tillage and use of a short duration sunnhemp intercrop has been previously reported to improve cotton yields in the Vidarbha region [
6]. However, in the Blaise [
6] study, sunnhemp was planted late and incorporated into the soil relatively early. This resulted in sunnhemp dry matter production of only about 1 ton ha
−1 and necessitated an inter-row tillage operation for incorporation of the sunnhemp intercrop.
In Year 2-Site 3, sorghum sudan grass plots were associated with the greatest weed cover and least cotton yield (
Table 6). Only at this site and in this treatment, were the effects of competition between living mulch and cotton apparent. Cotton plants in these plots had chlorotic leaves, were severely stunted, had sparse canopies, and small bolls. These bolls only opened partially and dried up prematurely. Such effects were not visible in the legume living mulch plots even though these legumes produced substantially more biomass than the sorghum sudan grass. Therefore, competition between sorghum sudan grass and cotton was more likely for nitrogen than water, especially given that irrigation was provided at this site. It was not clear why, but these visible effects of competition did not reflect on cotton yield (
Table 6); and cotton yield from sorghum sudan grass plots was not different from sunnhemp and sesbania plots. During Year 2, lower cotton yields at Site 1 compared with yields at Sites 2 and 4 (
Table 6) were likely due to a severe outbreak of black root rot [
Thielaviopsis basicola (Berk. And Br.) Ferraris]. Even though only a few cotton plants died from the disease, plants were weakened.
Prolonged nitrogen and water availability due to presence of living mulch and abundant surface mulch could have increased cotton yields in plots with well-established living mulches. Such improved growing conditions can prolong cotton boll development and enhance boll filling [
24]. Earlier in the season, the living mulches likely tied up considerable amounts of fertilizer nitrogen in their tissue, thus minimizing nitrogen losses via leaching and decreasing availability to weeds [
21,
25]. In a long duration crop like cotton, this nitrogen, along with biologically-fixed nitrogen, would likely become available to the crop later in the season [
11]. Leaves of the legume living mulches falling on the soil surface following mowing can also release nitrogen very rapidly [
26].
Boll weight was not measured but bolls associated with more vigorous living mulch stands were observed to be generally larger. This is corroborated by the lower boll count in plots with living mulches compared with the control plots, even though differences in cotton yields between them were absent. In Year 2, when sesbania (34 bolls per plant) and sunnhemp (32) treatments across all trial sites were compared with the control (41), boll counts in these treatments were lower (
p = 0.0005) (
Table 5). And, although living mulch biomass was positively correlated with cotton yield, there was a strong negative correlation (
p < 0.0001) between living mulch biomass and boll count.
However, in case of plant height, cotton plants in the sunnhemp treatment plots were taller (125 cm) than in the control (111 cm) plots (
p = 0.04) (
Table 5). During the wetter Year 2, in plots with vigorous living mulches, new apical shoot growth and new boll formation were observed in cotton plants at the beginning of the harvest period. This was likely due to enhanced soil moisture and nitrogen availability [
27]. Increases in cotton yields from adoption of reduced tillage and cover crops, as a result of higher soil moisture availability, have been previously reported [
8,
10]. This late-season growth extended harvest by more than a month and could have contributed to the increase in cotton yield in these plots. Soil moisture levels during Year 2 were probably more effectively conserved in plots with vigorous living mulch stands as more surface residue was produced from their clipping [
28].
The cut plant material, which was not incorporated but left as surface mulch where it fell, could also have moderated soil surface temperatures. Evapo-transpiration losses of soil moisture from within living mulch stands were likely reduced later in the season because of (1) surface residue and (2) loss of living mulch vigor with both age and stress from multiple clippings. The presence of a mulch layer, the consequent increase in soil moisture and absence of inter-row tillage can also prevent crusting of the soil surface [
29,
30], which is a severe problem in the clayey soils of Vidarbha.
3.2. Living Mulch Performance and Weed Suppression
Overall, living mulches produced 8 to 79 tons ha−1 of fresh biomass corresponding to 1 to 12.7 tons ha−1 of dry matter. During the latter part of the growing season, surface mulch at Sites 2, 3 and 4 were several centimeters thick and completely covered the soil surface. In order to compare living mulch biomass between Year 1 and Year 2, sesbania and sunnhemp data from Sites 1 and 2 were pooled. Average living mulch biomass production in Year 2 (29 tons ha−1) was higher (p = 0.0002) than in Year 1 (12 tons ha−1). One possible contributing factor for low biomass production during Year 1 may have been the use of flail-type blades, which were unable to make clean cuts, for mowing during that year. Hence, during Year 2, garden shears were used to clip the living mulches.
The difference in the amount of rainfall between the two years could also have influenced this outcome. Year 1 was a dry year, while Year 2 was a wet year, facilitating better living mulch recovery during Year 2. Final clippings were done in September during Year 1 and in November during Year 2. Although irrigation was provided at all the on-farm sites, the more precise delivery of water to the cotton plants using drip irrigation likely prevented any considerable impact of irrigation on the living mulches. It is expected that longer lasting living mulch stands, like in Year 2, will generate greater biomass. Previous studies in green manure sesbania have reported that 36-day old crops (DOC) produced 1.5 tons ha
−1 more dry matter than 24 DOC, and 48 DOC produced 3 tons ha
−1 more than 36 DOC [
31], suggestive of a progressively greater rate of biomass accumulation.
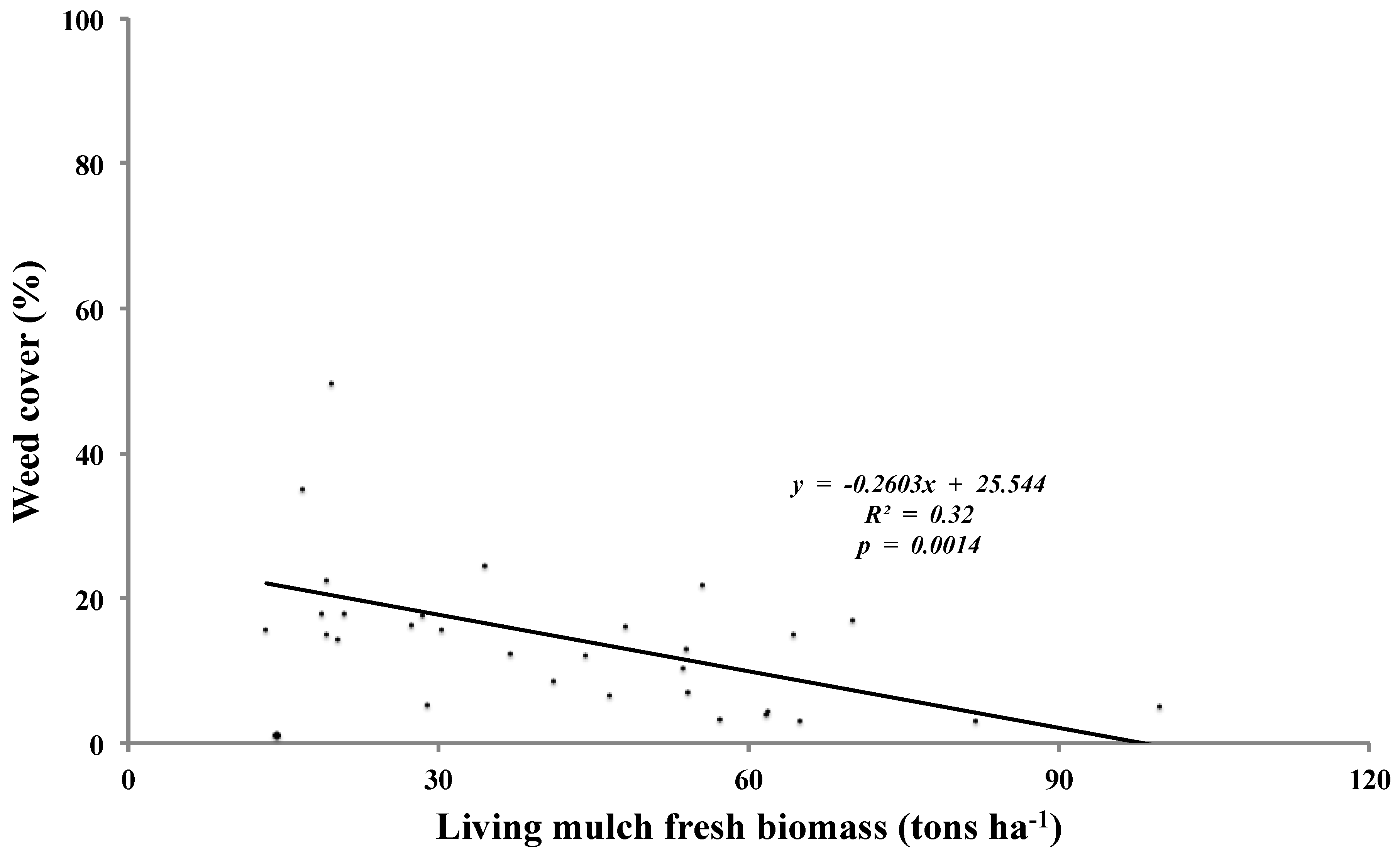
From Year 1 sesbania and sunnhemp data, there was no correlation (
p = 0.84) between weed cover and living mulch biomass. In contrast, Year 2 data from the same treatments showed a negative relationship (
p = 0.0014) between weed cover and living mulch biomass (
Figure 3). Living mulch performance was greatest at Site 3 and here, weed suppression was excellent (
Table 6). At this site, high living mulch vigor and regeneration and healthy, long-lasting stands led to multiple successful mowing operations (
Table 2) and consequently, thick surface mulch. Sesbania and sunnhemp produced an average of 59 and 79 tons ha
−1 of fresh biomass, respectively, the greatest amounts recorded in these trials (
Table 6). Average living mulch ground cover in sesbania and sunnhemp plots at Site 3 were 85 and 97%, respectively. These living mulch stands were so vigorous that average weed cover was only 7% in both treatments. Living mulches at Site 3 remained healthy through cotton harvest in December. At Sites 1 and 2, the living mulches began to dry up and senesce by October.
Vigorous living mulch stands were cut back more often than weaker stands (
Table 2). This could have greatly improved weed control since weed biomass decreases rapidly with increasing frequency of mowing [
32]. In healthy living mulch stands, taller weeds like
Amaranthus spp., Japanese lovegrass, parthenium and pigmy groundcherry (
Physalis minima L.) were more abundant than short or prostrate weeds like sessile joyweed, spurge, Benghal dayflower and common wireweed. The latter weeds were more prominent in weaker, less dense intercrop stands. Such differences in weed growth responses have been previously documented [
32,
33,
34,
35,
36] in environments where light competition occurs. These weed responses could explain how mowing might have controlled weeds that grew quickly towards the top of the living mulch canopy, where light availability increases rapidly [
34]. Competition for light also drives movement of photosynthates from roots to shoots [
37]. Hence, living mulches, in tandem with the mowing operations, can suppress perennial weeds, which are difficult to control in reduced tillage systems [
38]. Stimulation of weed seed germination through primary tillage, followed by subsequent mowing, is also an effective strategy to exhaust the weed seed bank [
39,
40].