Phytoaccumulation of Copper from Irrigation Water and Its Effect on the Internal Structure of Lettuce
Abstract
:1. Introduction
2. Materials and Methods
Effect of Copper in Irrigation Water on Plants’ Growth
3. Plant Samples’ Analysis
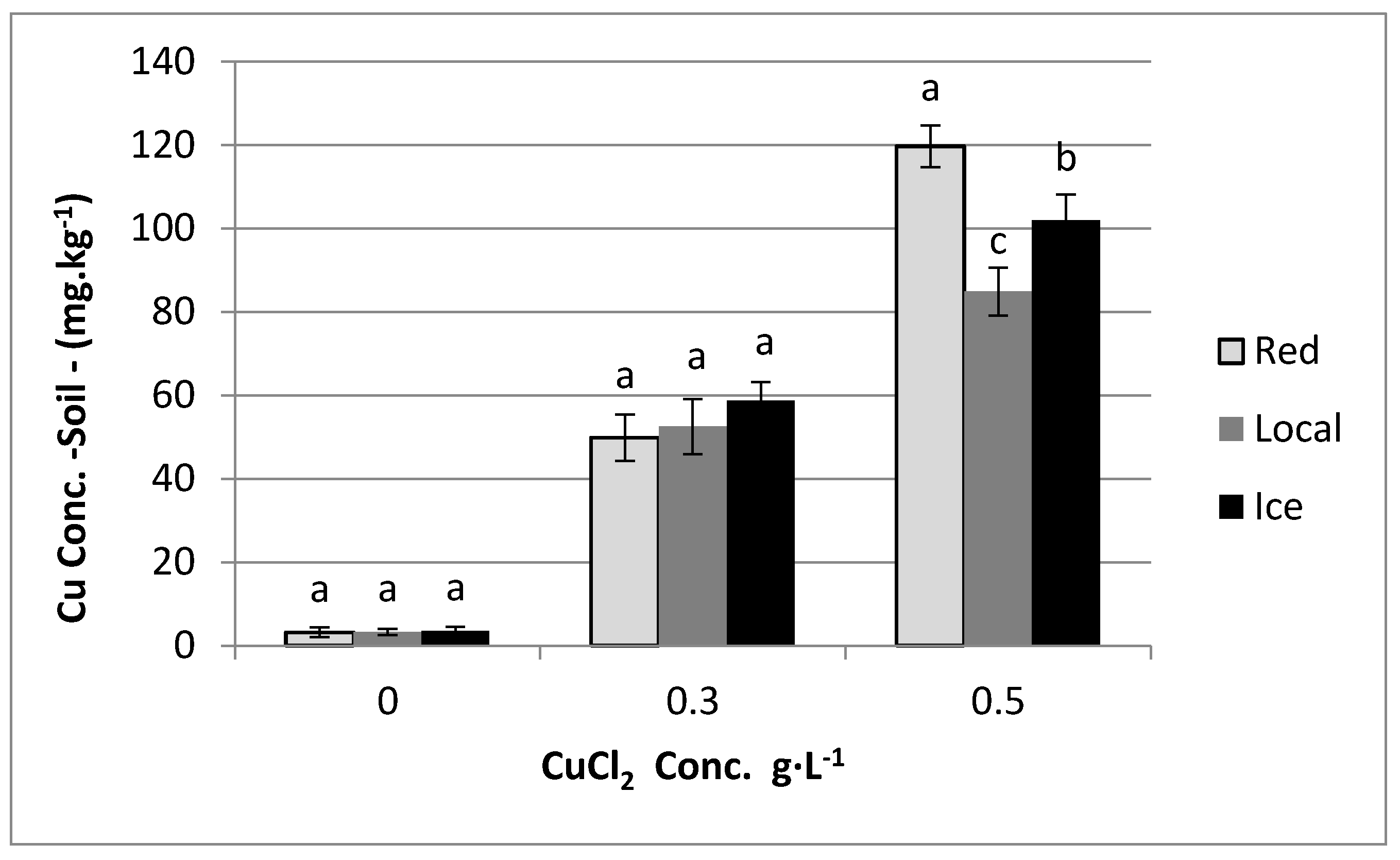
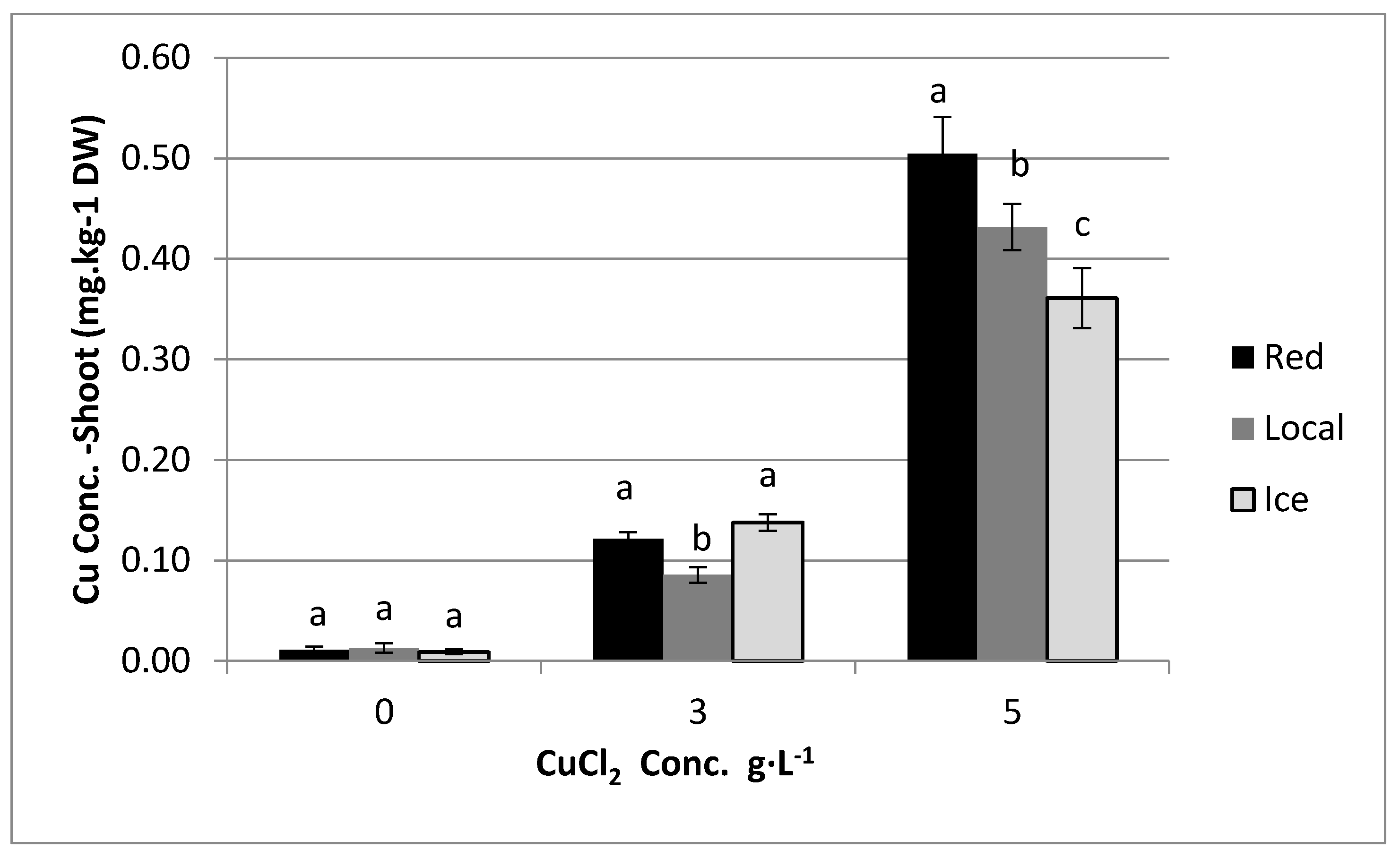
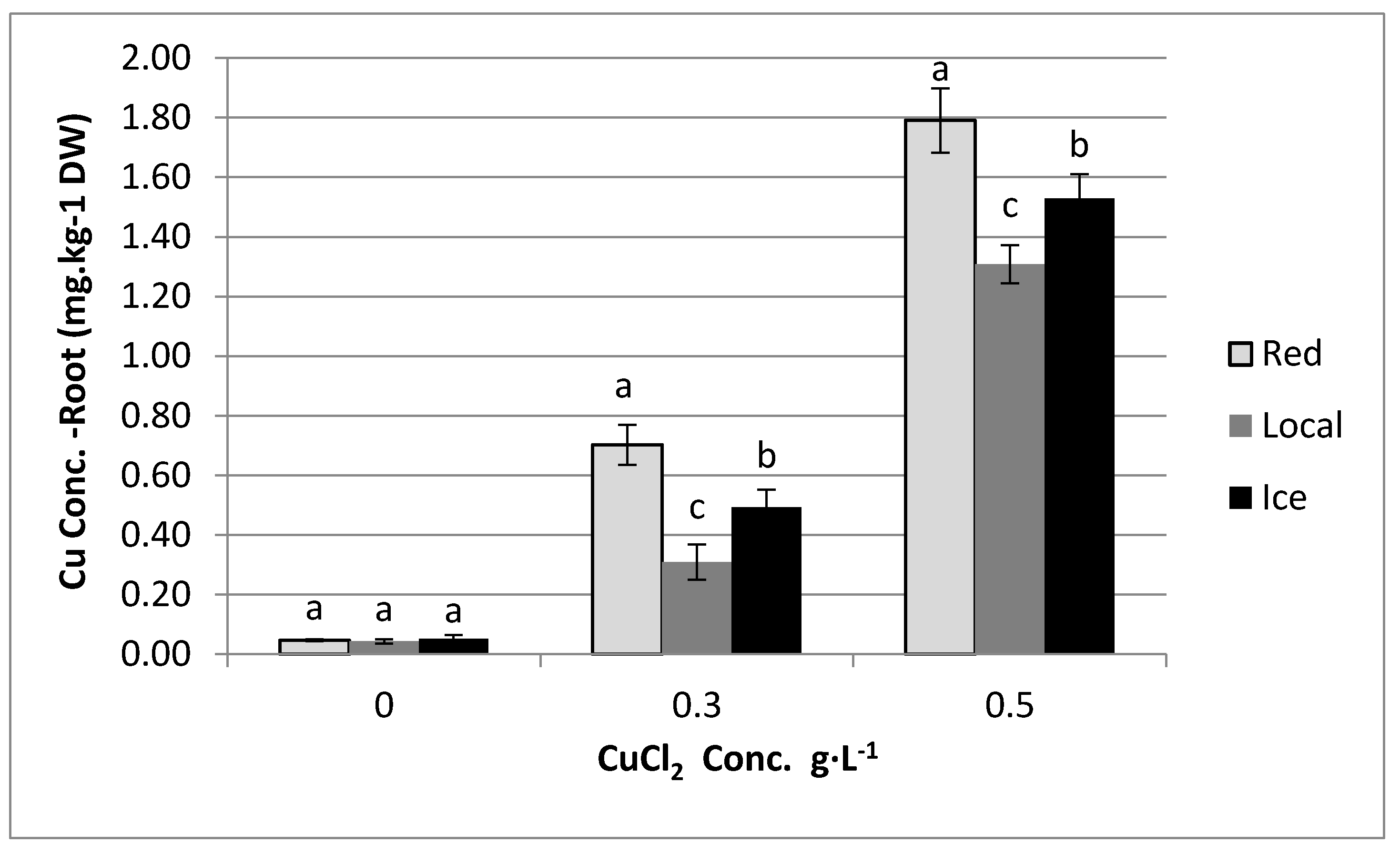
3.1. Effect of Copper in Irrigation Water on Copper Content in Plants
3.2. Determination of Copper in Soil Samples
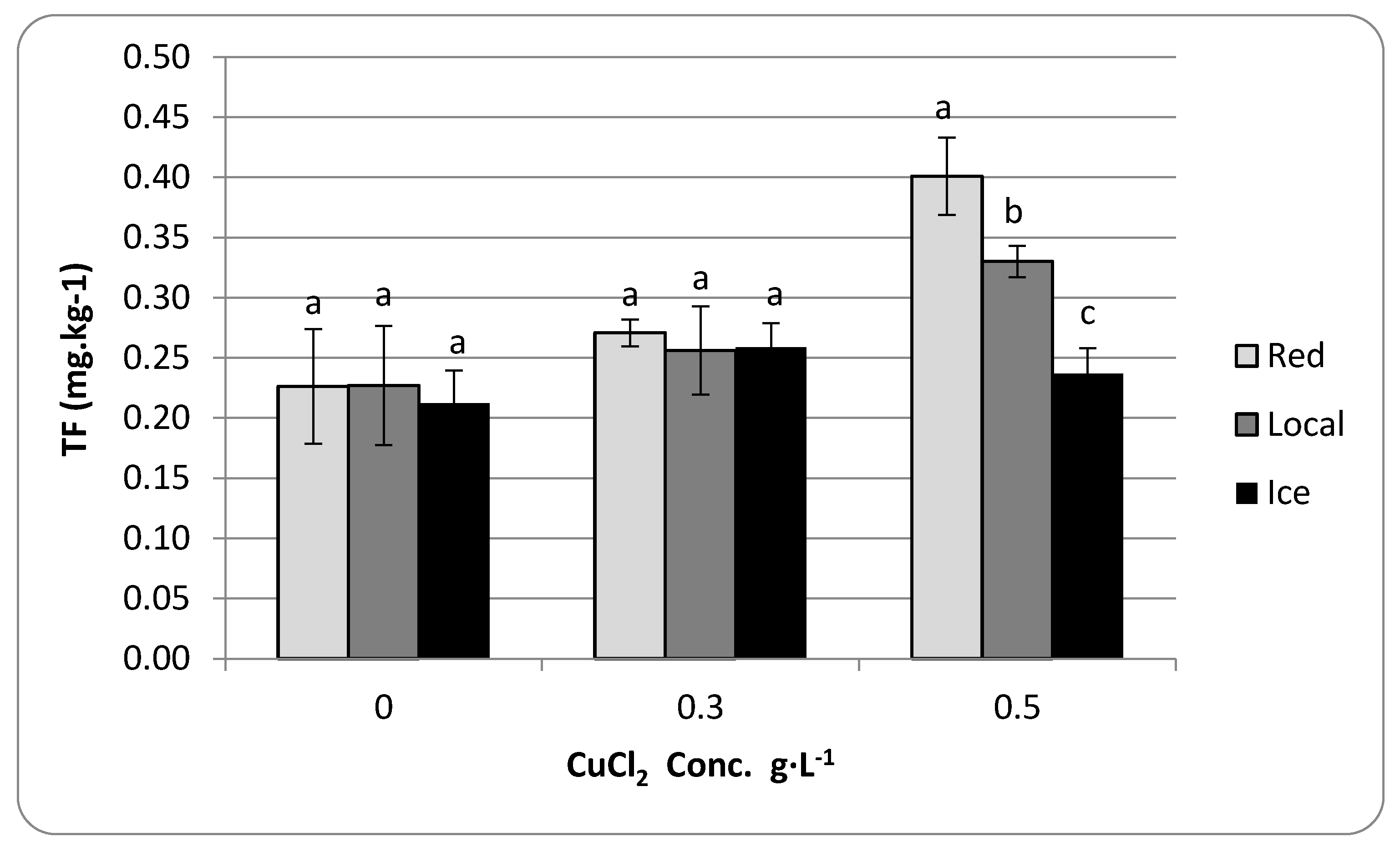
3.3. Translocation Factor
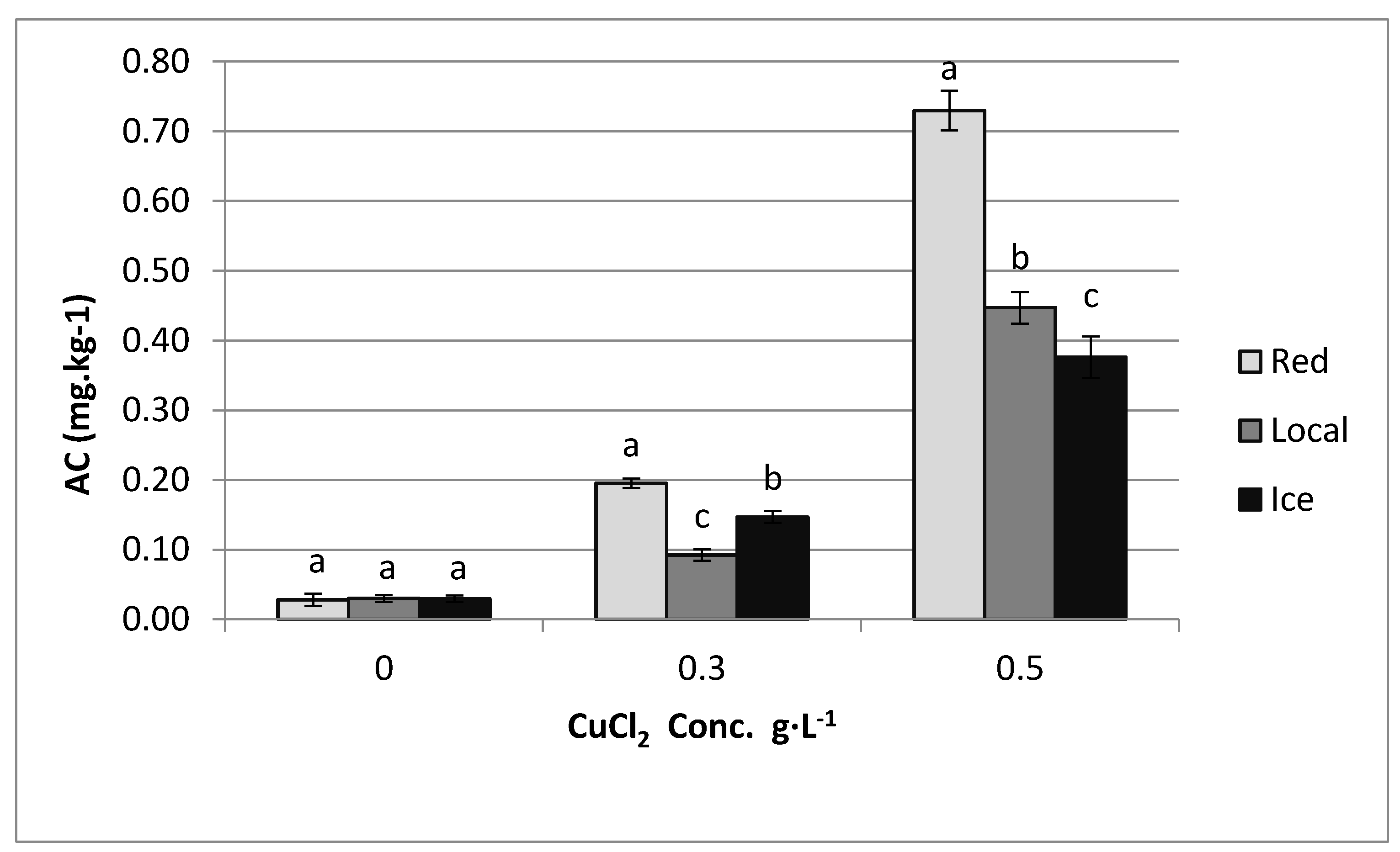
3.4. Accumulation Coefficient
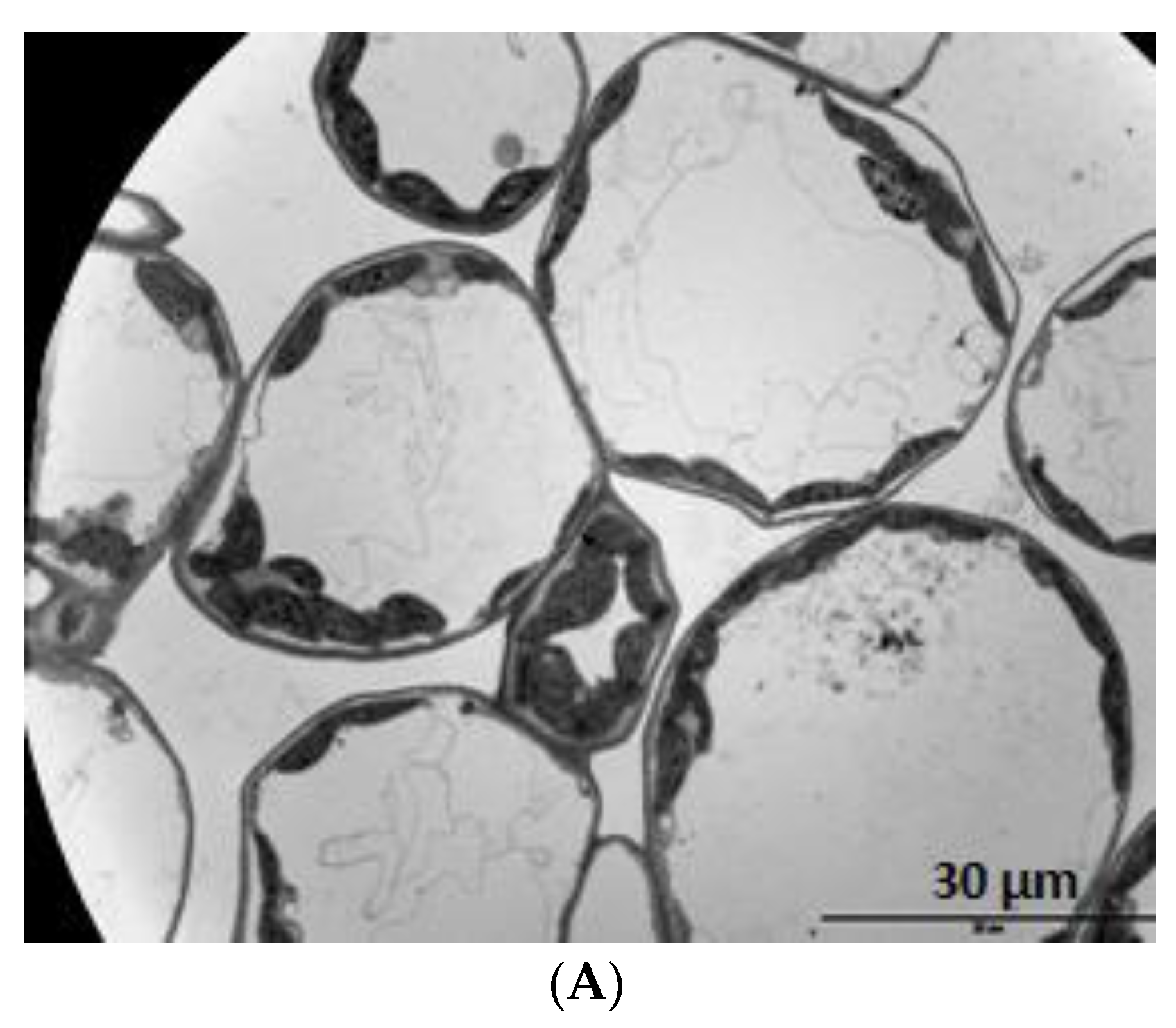
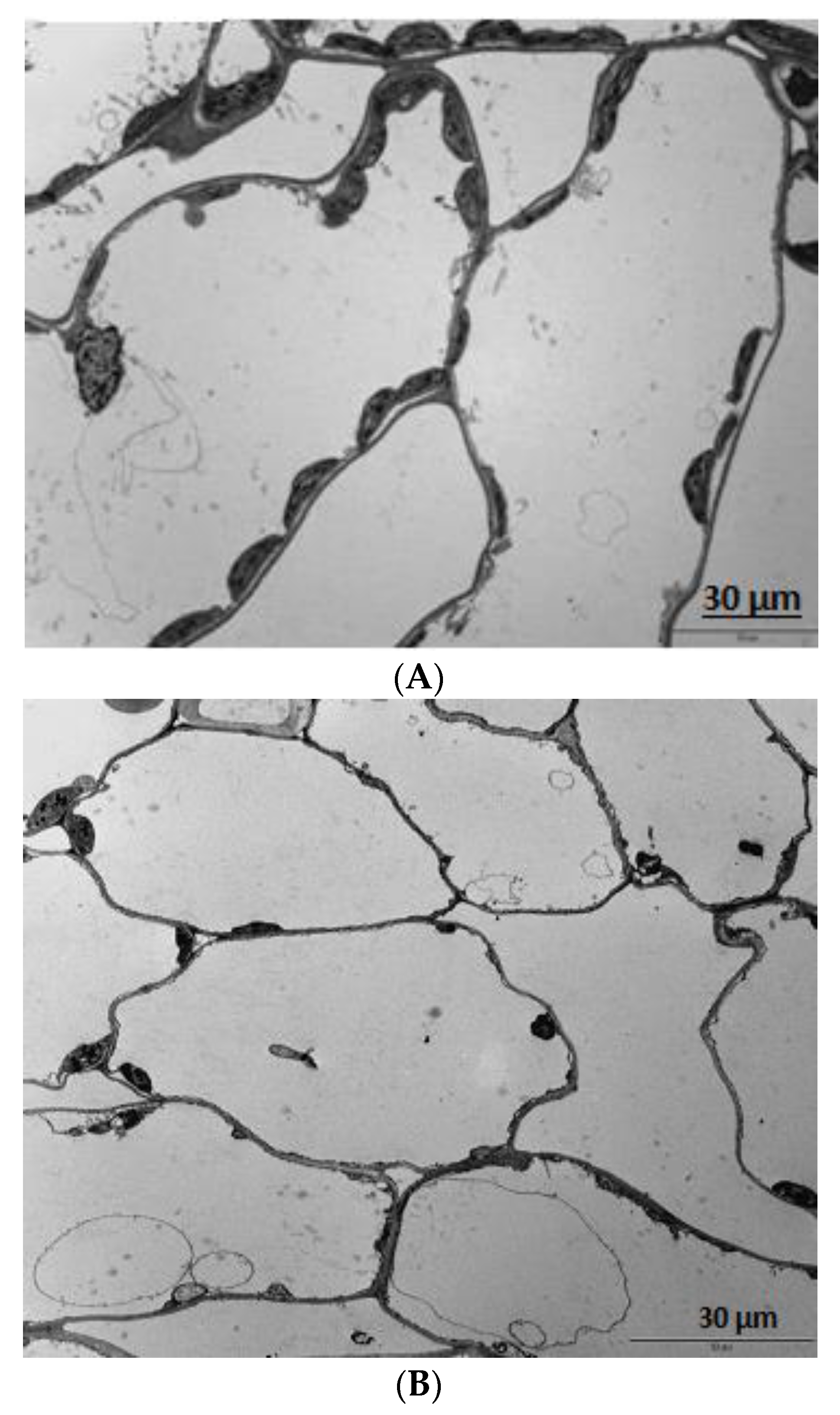
3.5. Microscopy
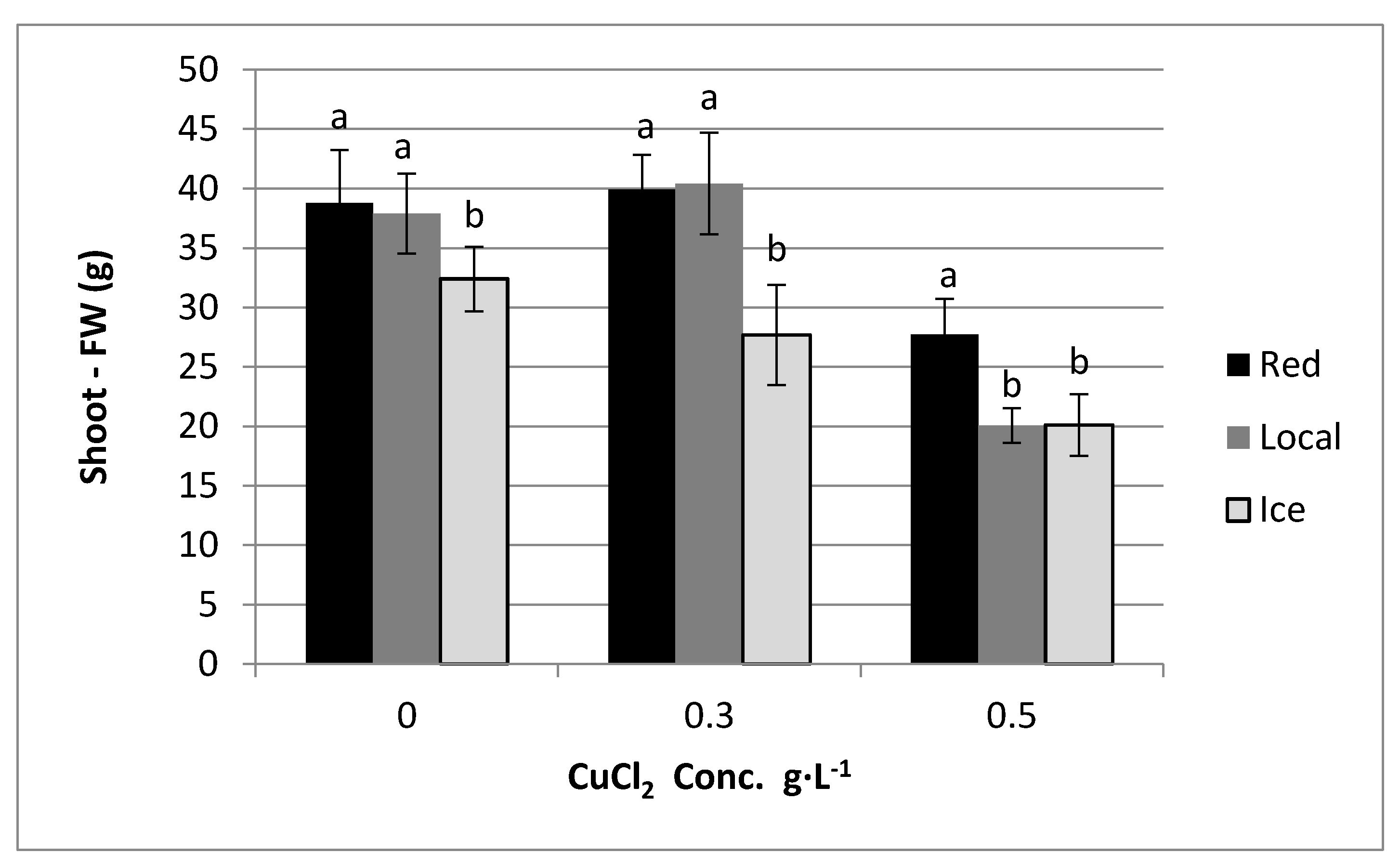
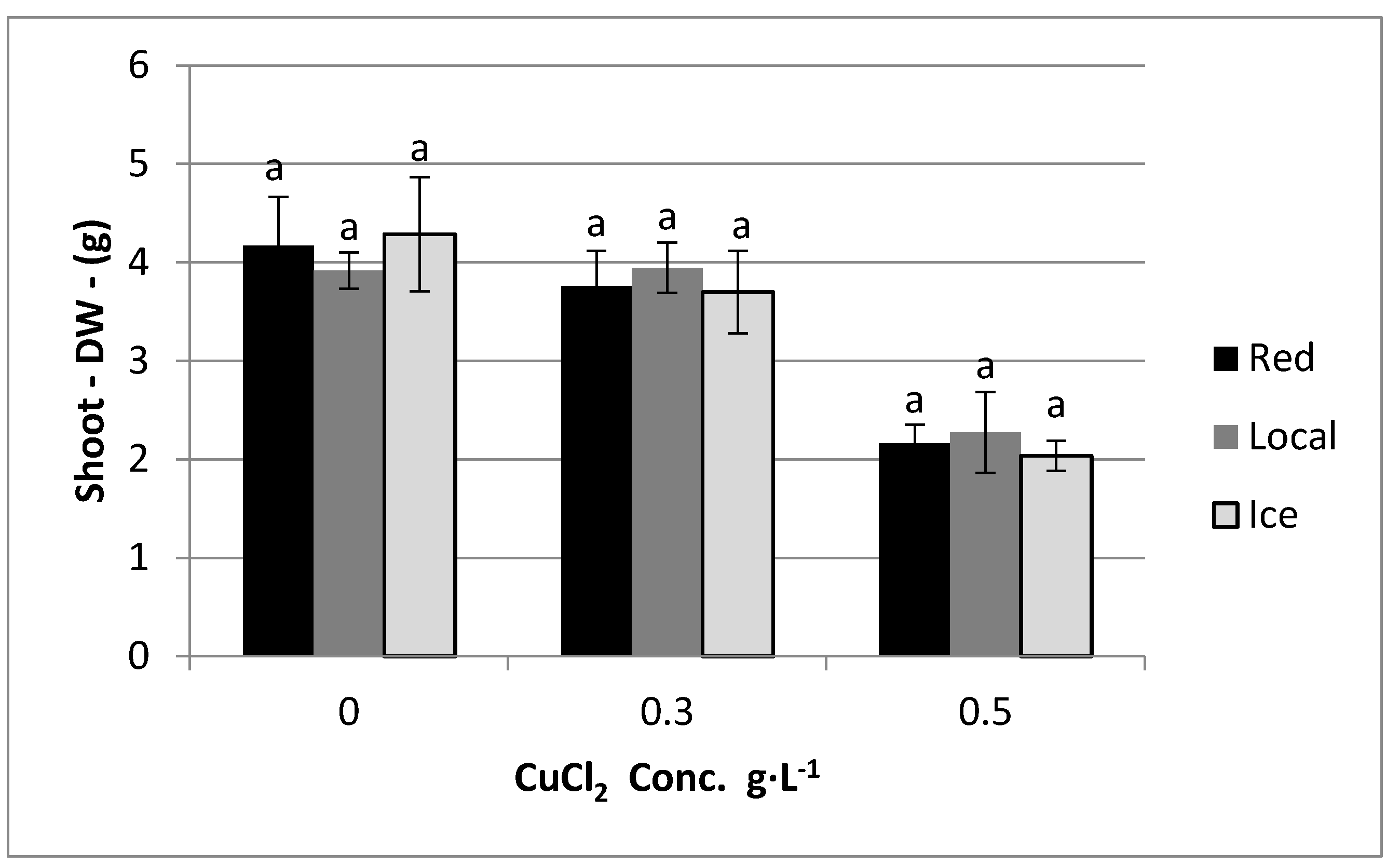
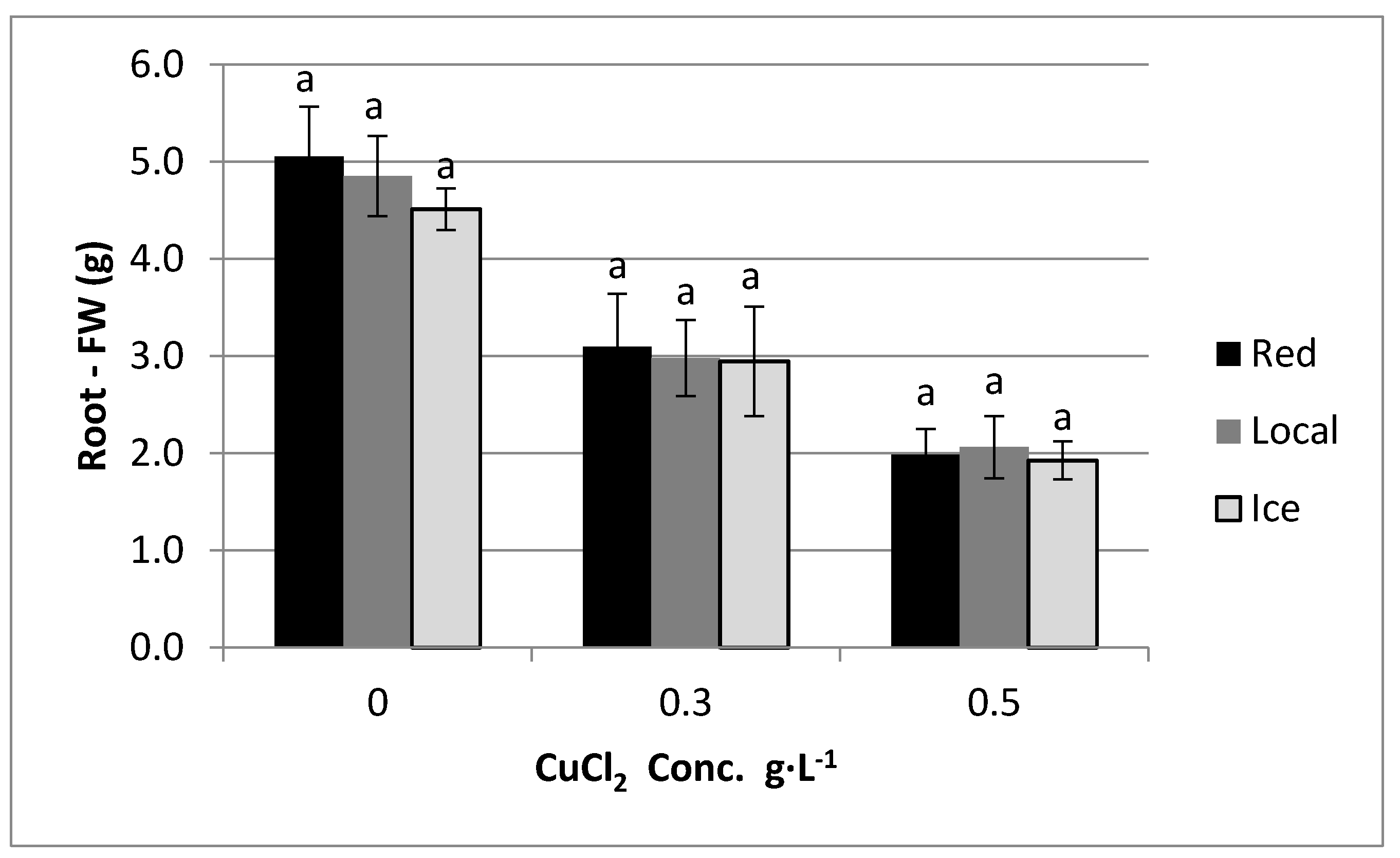
4. Results
5. Discussion
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Naser, H.M.; Sultana, S.; Mahmud, N.; Gomes, R.; Noor, S. Heavy metal levels in vegetables with growth stage and plant species variations. Bangladesh J. Agric. Res. 2011, 36, 563–574. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.K.; Pendias, H. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, USA, 1989; pp. 152–186. [Google Scholar]
- Mashiatullah, A.M.; Riffat Qureshi, M.; Niaz, A.; Javed, T.; Nisar, A. Biological quality of ground water in Rawalpindi/Islamabad. Environ. Monit. 2005, 5, 13–18. [Google Scholar]
- Chehregani, A.; Malayeri, B.; Golmohammad, R. Effect of heavy metals on the developmental stages of ovules and embryonic sac in Euphorbia cheirandenia. Pak. J. Biol. Sci. 2005, 8, 5–622. [Google Scholar]
- Stagnitti, F. A model of the effects of nonuniform soil water distribution on the subsurface migration of bacteria: Implications for land disposal of sewage. Math. Comput. Model. 1999, 29, 41–52. [Google Scholar] [CrossRef]
- Sauve, S.; Cook, N.; Hendershot, W.H.; McBride, M.B. Linking plant tissue concentrations and soil copper pools in urban contaminated soils. Environ. Pollut. 1996, 94, 153–157. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants; Academic Press: London, UK, 1995. [Google Scholar]
- Raven, J.A.; Evans, M.C.W.; Korb, R.E. The role of trace metals in photosynthetic electron transport in O2-evolving organisms. Photosynth. Res. 1999, 60, 111–149. [Google Scholar] [CrossRef]
- Itanna, F. Metals in leafy vegetables grown in Addis Ababa and toxicological amplications. Ethiop. J. Health Dev. 2002, 16, 295–302. [Google Scholar] [CrossRef]
- Alexander, P.D.; Alloway, B.J.; Dourado, A.M. Genotypic variations in the accumulation of Cd, Cu, Pb and Zn exhibited by six commonly grown vegetables. Environ. Pollut. 2006, 144, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Cao, Q.; Zheng, Y.M.; Huang, Y.Z.; Zhu, Y.G. Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ. Pollut. 2008, 15, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Duruibe, J.O.; Ogwuegbu, M.D.O.; Egwurugwu, J.N. Heavy metal pollution and human biotoxic effects. Int. J. Phys. Sci. 2007, 2, 112–118. [Google Scholar]
- Kumar, N.; Dushenkov, V.; Motto, H.; Raskin, I. Phytoextraction: The use of plants to remove heavy metals from soils. Environ. Sci. Technol. 1995, 29, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Behrouz, E.M.; Abdolkarim, C.; Nafiseh, Y.; Bahareh, L. Identification of the hyper accumulator plants in copper and iron mine in Iran. Pak. J. Biol. Sci. 2008, 11, 490–492. [Google Scholar]
- Lasat, M.M. Phytoextraction of toxic metals: A review of biological mechanisms. J. Environ. Qual. 2002, 31, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Nicula, C.; Peter, A.; Mihaly_Cozmuta, A. The uptake of heavy metals in Phaseolus Vulgaris and Zea Mays seeds harvested from polluted and unpolluted areas, Carpathian. J. Food Sci. Technol. 2013, 5, 1–8. [Google Scholar]
- Tijana, M.; Dubravka, R.; Aleksandar, P. Determination of bioavailable macro- and microelements from agricultural soil using different extractants. In Proceedings of the Geophysical Research Abstracts, Vienna, Austria, 12–17 April 2005. [Google Scholar]
- Sass, J.E. Botanical Microtechniques; Iowa St. University Press: Ames, IA, USA, 1958. [Google Scholar]
- Antoniou, V.; Zantopoulos, N.; Tsoukapapadoulou, H. Selected heavy metal concentrations in goat liver and kidney. Vet. Hum. Toxicol. 1995, 37, 20–22. [Google Scholar] [PubMed]
- Cheng, W.D.; Zhang, G.P.; Yao, H.G.; Wu, M.; Xu, M. Genotypic and environmental variation in cadmium, chromium, arsenic, nickel, and lead concentrations in rice grains. J. Zhejiang Univ. 2006, 7, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.R.; Mao, Y.; Cheng, W.D.; Wu, F.B.; Zhang, G.P. Genotypic and environmental variation in chromium, cadmium and lead concentrations in rice. Environ. Pollut. 2008, 153, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kong, G.T.; Jia, Q.Y.; Wang, F.; Xu, R.S.; Li, F.B.; Wang, Y.; Zhou, H.R. Effects of soil properties on heavy metal accumulation in flowering Chinese cabbage (Brassica campestris L. ssp. chinensis var. utilis Tsen et Lee) in Pearl River Delta, China. J. Environ. Sci. Health Part B 2007, 42, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Loneragan, J.F. Distribution and movement of Cu in plants. In Copper in Soils and Plants; Loneragan, J.F., Robson, A.D., Graham, R.D., Eds.; Academic Press: Sydney, Australia, 1981. [Google Scholar]
- Cacador, I.; Vale, C.; Catarino, F. Seasonal variations of heavy metal concentrations in plants and sediments of a Tagus estuary salt-marsh (Portugal). Heavy Met. Environ. 1995, 1, 61. [Google Scholar]
- Lidon, F.C.; Henriques, F.S. Effects of copper on the nitrate to ammonia reduction mechanism in rice plants. Photosynthetica 1992, 26, 371–380. [Google Scholar]
- Achakzai, A.K.K.; Bazai, Z.A. Phytoaccumulation of heavy metals in spinach (Spinacea oleracea L.) irrigated with wastewater of Quetta city. J. Chem. Soc. Pak. 2006, 28, 473–477. [Google Scholar]
- Mohamad, H.H.; Latif, P.A. Uptake of cadmium and zinc from synthetic effluent by water hyacinth (Eichhornia crassipes). Environ. Asia 2010, 3, 36–42. [Google Scholar]
- Liu, D.H.; Jiang, W.S.; Hou, W.Q. Uptake and accumulation of copper by roots and shoots of maize (Zea mays L). J. Environ. Sci. 2001, 13, 228–232. [Google Scholar]
- Cook, C.M.; Vandaka, E.; Lanaras, T. Concentrations of Cu, growth and chlorophyll content of field cultivated wheat growing in naturally enriched Cu soil. Bull. Environ. Contam. Toxicol. 1997, 58, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Dudka, S.M.; Piotrouska, M.; Chlopecka, A. Effect of elevated concentrations of Cd and Zn in soil on spring wheat yield and the metal content of the plants. Water Air Soil Pollut. 1994, 76, 333–341. [Google Scholar] [CrossRef]
- Ramraj, A.; Afshan Gowda, T.P.H.; Karanth, N.G.K. Sorption of copper (II) and Lead (II) by microbial cultures during growth. Indian J. Environ. Health 2000, 42, 95–99. [Google Scholar]
- Demirezen, D.; Aksoy, A. Heavy metal levels in vegetables in Turkey are within safe limits for Cu, Zn, Ni and exceeded for Cd and Pb. J. Food Qual. 2006, 29, 252–265. [Google Scholar] [CrossRef]
- Farooq, M.; Anwar, F.; Rashid, U. Appraisal of heavy metal contents in different vegetables grown in the vicinity of an industrial area. Pak. J. Bot. 2008, 40, 2099–2106. [Google Scholar]
- Zhuang, P.; McBride, M.B.; Xia, H.; Li, N.; Li, Z. Health risk from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine South China. Sci. Total Environ. 2009, 407, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Crowley, D.E.; Wang, Y.V.; Reid, C.P.P.; Szansiszlo, P.J. Mechanism of iron acquisition from siderophores by microorganisms and plants. Plant Soil 1991, 130, 179–198. [Google Scholar] [CrossRef]
- Chlopecka, A.; Bacon, J.R.; Wilson, M.J.; Kay, J. Forms of cadmium, Lead, and Zinc in soils from southwest Potland. J. Environ. Qual. 1996, 25, 6–79. [Google Scholar] [CrossRef]
- Stiborova, M.; Ditrichova, M.; Brezinova, A. Effects of heavy metals ions on growth and biochemical characteristics of photosynthesis of barley and maize Seedlings. Biol. Plant 1987, 29, 453–467. [Google Scholar] [CrossRef]
- Drazkeiwice, M. Chlorophyll-occurrence, function and mechanism of action. Effects of external and internal factors. Photosynthetica 1994, 50, 321–331. [Google Scholar]
- Ohsumi, Y.; Kitamoto, K.; Anraku, Y. Changes induced in the permeability barrier of the yeast plasma membrane by cupric ion. J. Bacteriol. 1988, 170, 2676–2682. [Google Scholar] [CrossRef] [PubMed]




| Soil Factors | Value |
|---|---|
| Soil particle size: Sand (%) | 20 |
| Silt (%) | 38 |
| Clay (%) | 42 |
| Texture class | Clay loam |
| Organic matter | 1.9 |
| Mineral N content: NH4+ N (ppm) | 6.7 |
| NO3− (ppm) | 12.7 |
| pH | 6.3 |
| Electrical conductivity (dS·m−1) | 0.21 |
| Copper concentration (mg·kg−1) | 3.5 |
| Extractable phosphorus (ppm) | 17.1 |
| Extractable potassium (ppm) | 428 |
| Bulk density | 1.02 |
| Water holding capacity | 0.35 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiyab, S. Phytoaccumulation of Copper from Irrigation Water and Its Effect on the Internal Structure of Lettuce. Agriculture 2018, 8, 29. https://doi.org/10.3390/agriculture8020029
Shiyab S. Phytoaccumulation of Copper from Irrigation Water and Its Effect on the Internal Structure of Lettuce. Agriculture. 2018; 8(2):29. https://doi.org/10.3390/agriculture8020029
Chicago/Turabian StyleShiyab, Safwan. 2018. "Phytoaccumulation of Copper from Irrigation Water and Its Effect on the Internal Structure of Lettuce" Agriculture 8, no. 2: 29. https://doi.org/10.3390/agriculture8020029




