Isolation of Mercury-Resistant Fungi from Mercury-Contaminated Agricultural Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation, Selection, and Identification of Hg-Resistant Indigenous Fungus
2.2. Bioassay on Chinese Cabbage (Brassica rapa L.)
3. Results
3.1. Tailing Characterization
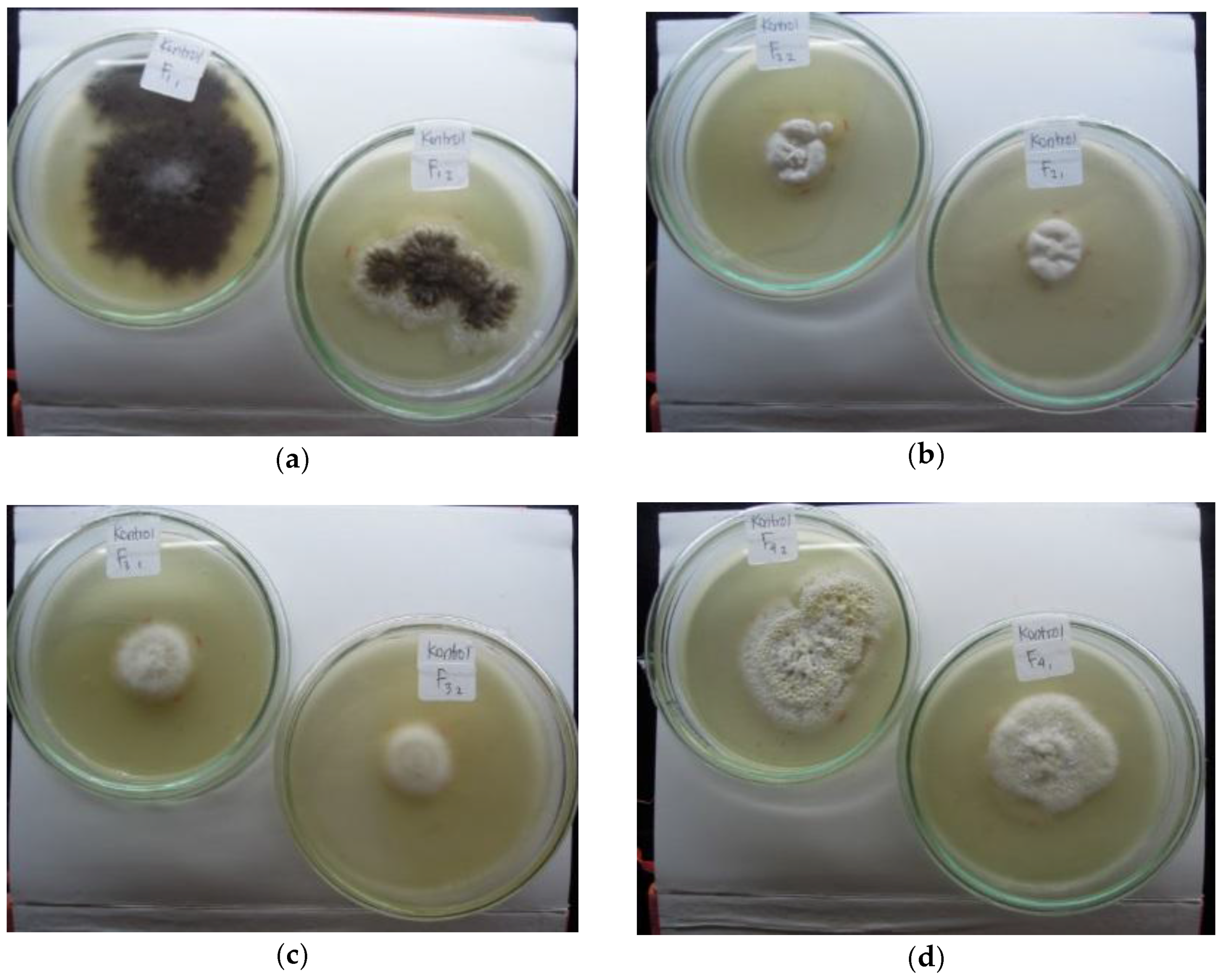
3.2. Indigenous Fungal Isolates
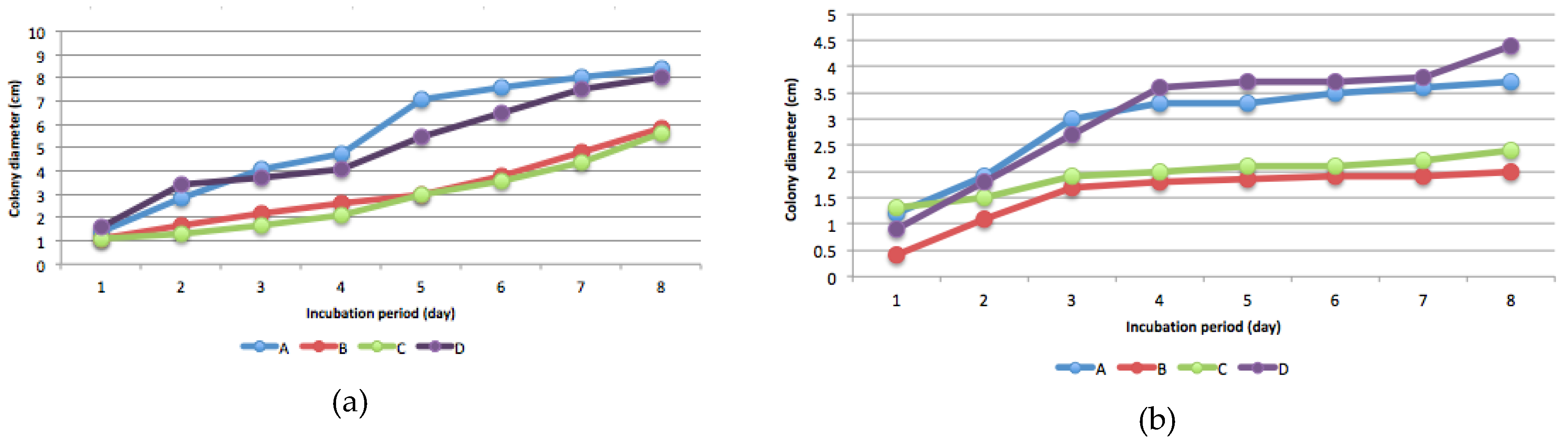
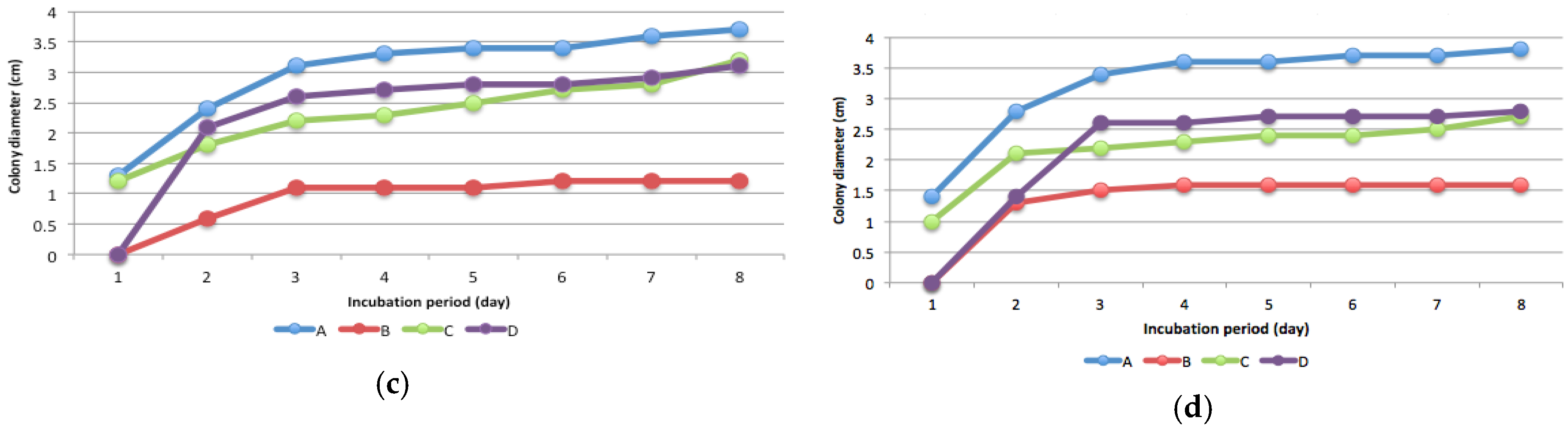
3.3. Mercury Resistant Fungus
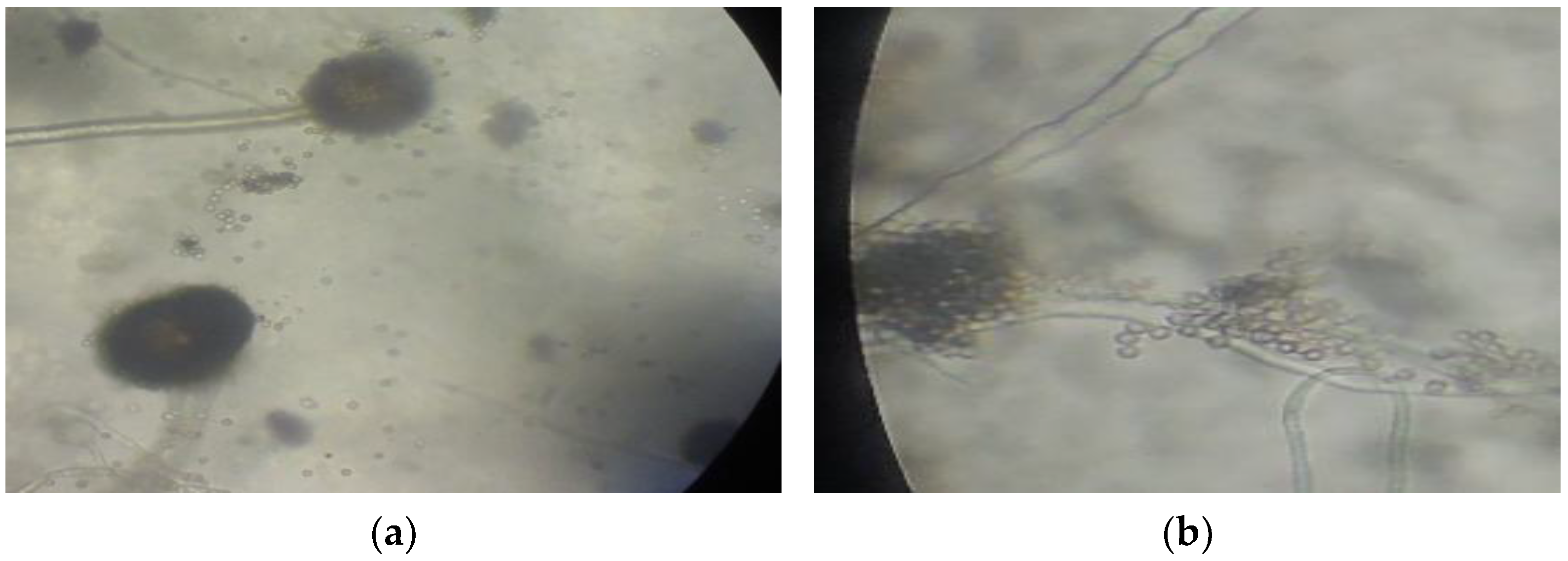
3.4. Fungal Species
3.5. Effect of Fungal Inoculation on Hg in Soil and Biomass
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Porcella, D.B.; Ramel, C.; Jernelov, A. Global mercury pollution and the role of gold mining. Water Air Soil Pollut. 1997, 97, 205–207. [Google Scholar] [CrossRef]
- Wang, Y. Phytoremediation of Mercury by Terrestrial Plants. Ph.D. Thesis, Stockholm University, Stockholm, Sweden, 2004. [Google Scholar]
- Singh, R.; Gautam, N.; Mishra, A.; Gupta, R. Heavy metals and living systems: An overview. Indian J. Pharmacol. 2011, 43, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Steiness, E. Heavy Metals in Soils; Alloway, B.J., Ed.; Blackie Academic & Professional: London, UK, 1995. [Google Scholar]
- Steinnes, E.; Rühling, Å.; Lippo, H.; Mäkinen, A. Reference materials for large-scale metal deposition surveys. Accredit. Qual. Assur. 1997, 2, 243–249. [Google Scholar] [CrossRef]
- Bradl, H.B. Heavy Metal in Environment; Elsevier Academic Press: London, UK, 2005. [Google Scholar]
- Lasat, M.M. Phytoextraction of Toxic Metals. J. Environ. Qual. 2002, 31, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-P.; Shi, J.-Y.; Lin, Q.; Chen, X.-C.; Chen, Y.-X. Heavy metal availability and impact on activity of soil microorganisms along a Cu/Zn contamination gradient. J. Environ. Sci. 2007, 19, 848–853. [Google Scholar] [CrossRef]
- Kurniati, E.; Arfarita, N.; Imai, T.; Higuchi, T.; Kanno, A.; Yamamoto, K.; Sekine, M. Potential bioremediation of mercury-contaminated substrate using filamentous fungi isolated from forest soil. J. Environ. Sci. 2014, 26, 1223–1231. [Google Scholar] [CrossRef]
- Ul-Haq, I.; Javed, M.; Khan, T. Sugar cane bagasse pretreatment: An attempt to enhance the production potential of cellulases by Humicola insolensTAS-13. Biokemistri 2006, 18, 83–88. [Google Scholar] [CrossRef]
- Akinpelu, E.A. Bioremediation of Gold Mine Wastewater Using Fusarium oxysporum. Master’s Thesis, Cape Paninsula University of Technology, Cape Town, South Africa, 2014. [Google Scholar]
- Dave, S.R. Microbial Interactions with Inorganic Pollutants: Acid Mine Drainage, Microbial Accumulation of Heavy Metals and Radionuclides. Applied Microbiology, E-Book Chapter. 2008. Available online: http://nsdl.res.in/handle/123456789/646 (accessed on 27 March 2017).
- Awasthi, A.K.; Pandey, A.K.; Khan, J. A preliminary report of indigenous fungal isolates from contaminated municipal solid waste site in India. Environ. Sci. Pollut. Res. 2017, 24, 8880–8888. [Google Scholar] [CrossRef] [PubMed]
- Samson, A.R.; van Reenen Hoekstra, E.S. Introduction to Food Borne Fungi; Centralbureau Voor Schimmelcultures: Delft, The Netherlands, 1988. [Google Scholar]
- Alloway, B.J. The Origin of heavy metals in soils. In Heavy Metals in Soils; Alloway, B.J., Ed.; Blackie Academic & Professional: Glasgow, Scotland, 1995. [Google Scholar]
- Baldrian, P.; Wiesche, C.I.D.; Gabriel, J.; Nerud, F.; Zadrazil, F. Influence of Cadmium and Mercury on Activities of Ligninolytic Enzymes and Degradation of Polycyclic Aromatic Hydrocarbons by Pleurotus ostreatus in Soil. Appl. Environ. Microbiol. 2000, 66, 2471–2478. [Google Scholar] [CrossRef] [PubMed]
- Jean-Philippe, S.R.; Franklin, J.A.; Buckley, D.S.; Hughes, K. The Effect of Mercury on Trees and Their Mycorrhizal Fungi. Ph.D. Thesis, University of Tennessee, Knoxville, TN, USA, 2010. [Google Scholar]
- Dixit, R.; Wasiullah, E.; Malaviya, D.; Pandiyan, K.; Singh, U.; Sahu, A.; Shukla, R.; Singh, B.; Rai, J.; Sharma, P.; et al. Bioremediation of Heavy Metals from Soil and Aquatic Environment: An Overview of Principles and Criteria of Fundamental Processes. Sustainability 2015, 7, 2189–2212. [Google Scholar] [CrossRef]
- Hepper, C.M.; Smith, G.A. Observation’s on the germination of Endogone spore. Trans. Br. Mycol. Soc. 1976, 66, 189–194. [Google Scholar] [CrossRef]
- McDonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [PubMed]
- Pawlowska, T.E.; Charvat, I. Heavy-Metal Stress and Developmental Patterns of Arbuscular Mycorrhizal Fungi. Appl. Environ. Microbiol. 2004, 70, 6643–6649. [Google Scholar] [CrossRef] [PubMed]
- Moat, A.G.; Foster, J.W.; Spector, M.P. Microbial Physiology, 4th ed.; Wiley-Liss Inc.: New York, NY, USA, 2002. [Google Scholar]
- Raspanti, E.; Cacciola, S.O.; Gotor, C.; Romero, L.C.; García, I. Implications of cysteine metabolism in the heavy metal response in Trichoderma harzianum and in three Fusarium species. Chemosphere 2009, 76, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Babich, H.; Gamba-Vitalo, C.; Stotzky, G. Comparative toxicity of nickel to mycelial proliferation and spore formation of selected fungi. Arch. Environ. Contam. Toxicol. 1982, 11, 465–468. [Google Scholar] [CrossRef]
- Lanfranco, L. Differential Expression of a Metallothionein Gene during the Presymbiotic versus the Symbiotic Phase of an Arbuscular Mycorrhizal Fungus. Plant Physiol. 2002, 130, 58–67. [Google Scholar] [CrossRef] [PubMed]
- De Almeida Paula Figueira, E.M.; Lima, A.I.G.; Pereira, S.I.A. Cadmium tolerance plasticity in Rhizobium leguminosarum bv. viciae: Glutathione as a detoxifying agent. Can. J. Microbiol. 2005, 51, 7–14. [Google Scholar]
- Magnuson, J.K.; Lasure, L.L. Advances in Fungal Biotechnology for Industry, Agriculture, and Medicine; Tkacz, J.S., Lange, L., Eds.; Springer: Boston, MA, USA, 2004. [Google Scholar]

| Treatments | Plant Height ** (cm) | Dry Weight ** (g) | Available Hg in Soil ** (µg kg−1) | Hg Content in Biomass ** (mg kg−1) |
|---|---|---|---|---|
| Without fungi and OM * | 10.50 | 0.23 | 125.86 | 1.35 |
| Isolate A; 1.5% OM | 11.07 | 0.30 | 115.22 | 3.63 |
| Isolate D, 1.5% OM | 11.43 | 0.23 | 137.13 | 7.48 |
| Isolate A + D; 1.5% OM | 13.43 | 0.33 | 162.81 | 6.20 |
| Without fungi; 3% OM | 11.53 | 0.26 | 121.48 | 1.93 |
| Isolate A; 3% OM | 12.07 | 0.44 | 120.23 | 3.63 |
| Isolate D, 3% OM | 12.07 | 0.33 | 14.,27 | 0.63 |
| Isolate A + D; 3% OM | 10.97 | 0.15 | 170.95 | 2.04 |
| Without fungi; 4.5% OM | 14.00 | 0.47 | 110.21 | 1.75 |
| Isolate A; 4.5% OM | 11.27 | 0.28 | 130.87 | 4.51 |
| Isolate D, 4.5% OM | 10.43 | 0.15 | 15717 | 8.85 |
| Isolate A + D; 3% OM | 10.57 | 0.27 | 181.56 | 0.40 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hindersah, R.; Asda, K.R.; Herdiyantoro, D.; Kamaluddin, N.N. Isolation of Mercury-Resistant Fungi from Mercury-Contaminated Agricultural Soil. Agriculture 2018, 8, 33. https://doi.org/10.3390/agriculture8030033
Hindersah R, Asda KR, Herdiyantoro D, Kamaluddin NN. Isolation of Mercury-Resistant Fungi from Mercury-Contaminated Agricultural Soil. Agriculture. 2018; 8(3):33. https://doi.org/10.3390/agriculture8030033
Chicago/Turabian StyleHindersah, Reginawanti, Khainur Rasyid Asda, Diyan Herdiyantoro, and Nadia Nuraniya Kamaluddin. 2018. "Isolation of Mercury-Resistant Fungi from Mercury-Contaminated Agricultural Soil" Agriculture 8, no. 3: 33. https://doi.org/10.3390/agriculture8030033





