Exploiting Waste Heat from Combine Harvesters to Damage Harvested Weed Seeds and Reduce Weed Infestation
Abstract
:1. Introduction
2. Methods
2.1. Weed Seeds
2.2. Heat Treatment
2.3. Temperature Monitoring
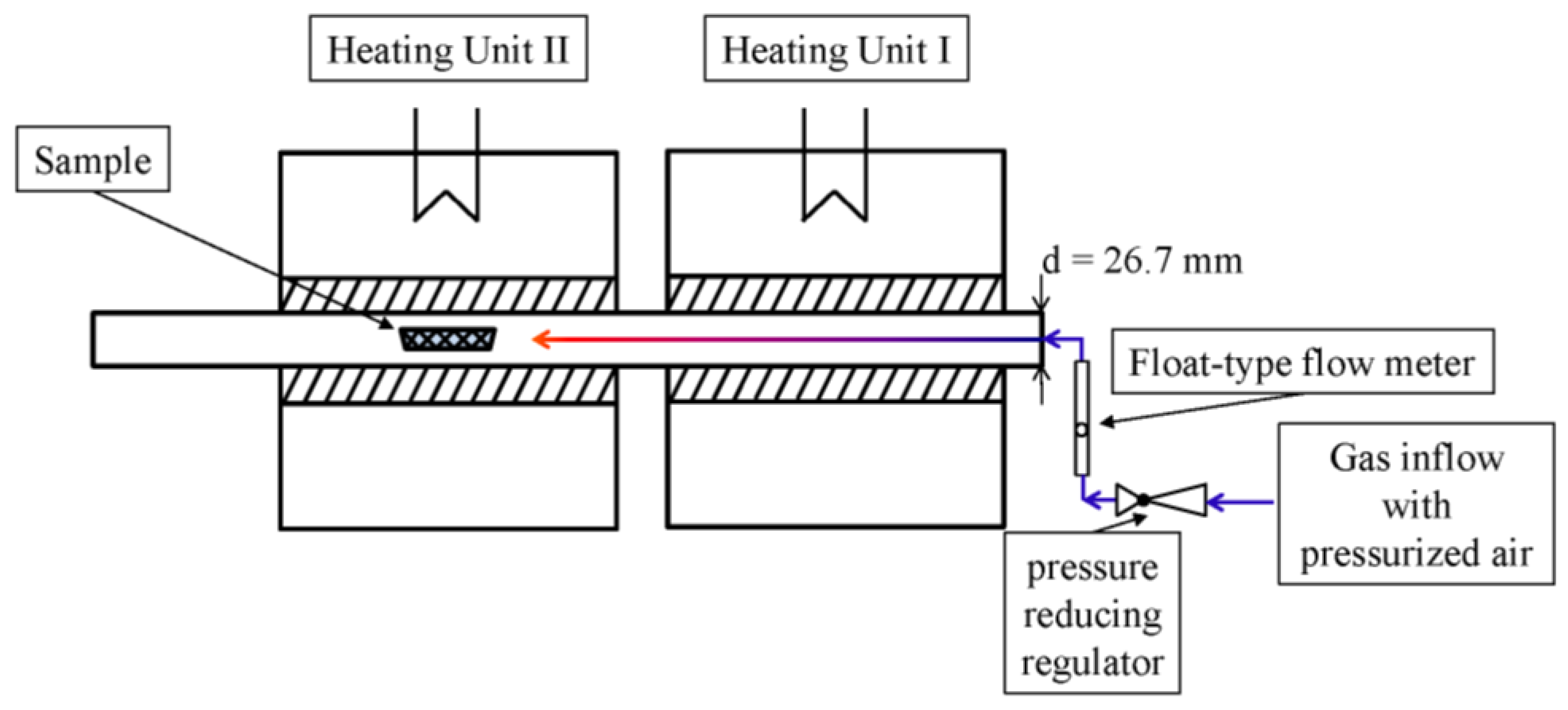
2.4. Velocity of the Gas Flow
2.5. Vessels
2.6. Test Execution
2.7. Germination Experiments
2.8. Statistical Analysis
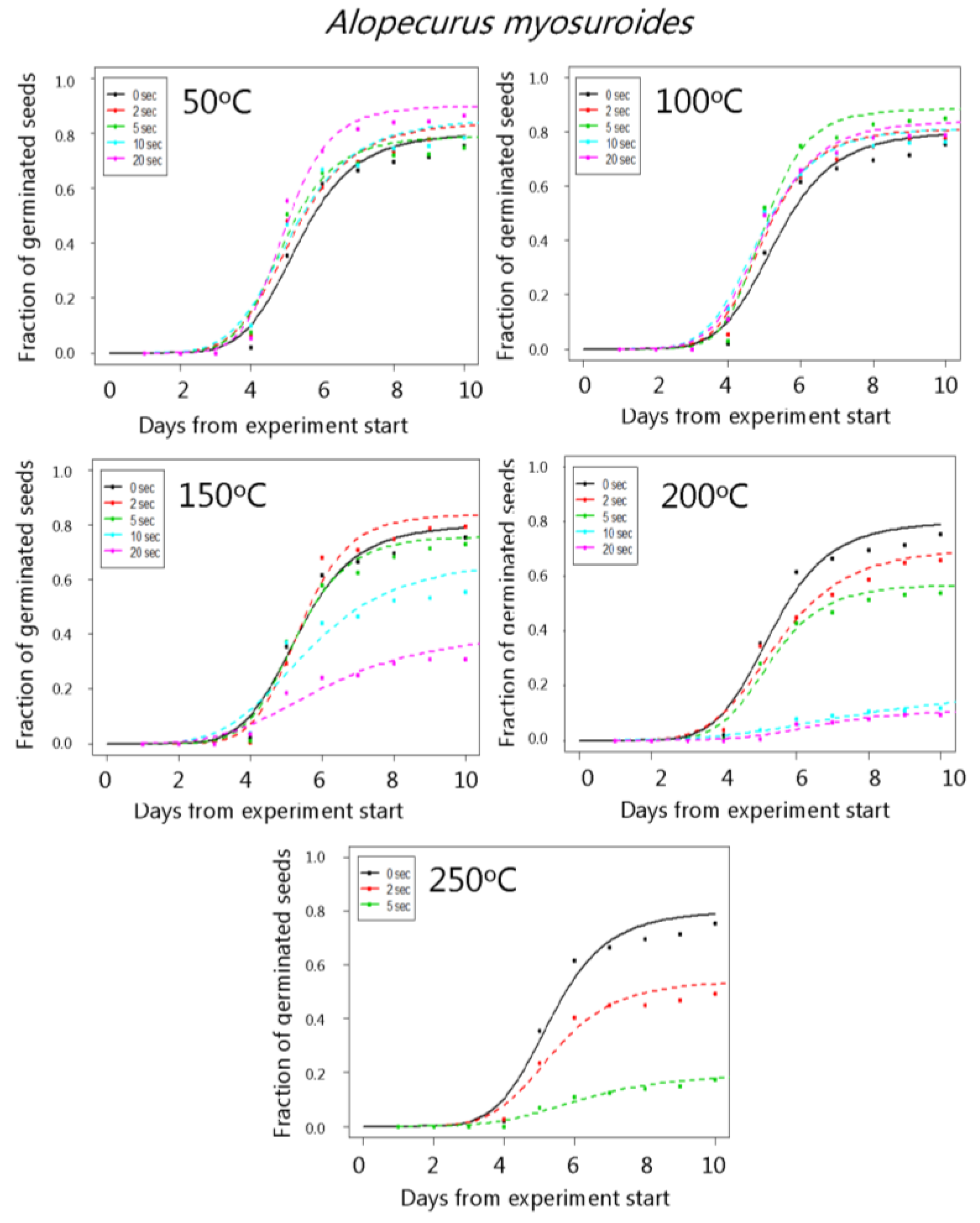
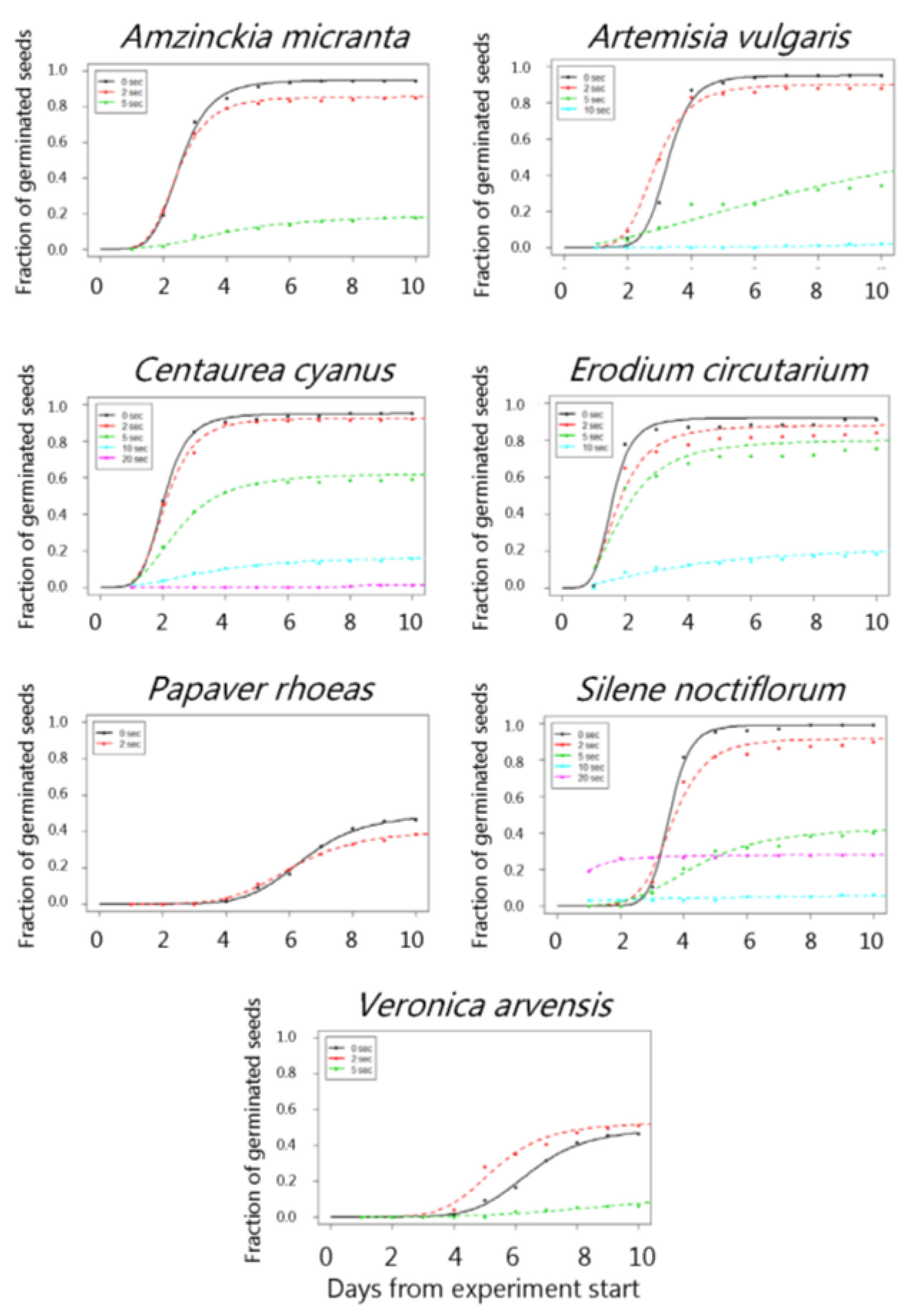
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Heap, I. Herbicide resistant weeds. In Integrated Weed Management; Pimental, D., Peshin, E., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 281–301. Available online: https://doi.org/10.1007/978-94-007-7796-5 (accessed on 17 January 2018).
- Duke, S. Why have no new herbicide modes of action appeared in recent years? Pest Manag. Sci. 2011, 68, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Chauvel, B.; Guillemin, J.-P.; Gasquez, J.; Gauvrit, C. History of chemical weeding from 1944 to 2011 in France: Changes and evolution of herbicide molecules. Crop Prot. 2012, 42, 320–326. [Google Scholar] [CrossRef]
- Funk, C.; Kennedy, B. The New Food Fights: U.S. Public Divides over Food Science, Differing Views on Benefits and Risks of Organic Foods, GMOs as Americans Report Higher Priority for Healthy Eating. Pew Research Center, 1 December 2016. Available online: http://www.pewinternet.org/2016/12/01/the-new-food-fights/ (accessed on 17 January 2018).
- Liebmann, M.; Dyck, E. Crop rotation and intercropping strategies for weed management. Ecol. Appl. 1993, 3, 92–122. [Google Scholar] [CrossRef] [PubMed]
- Melander, B.; Liebman, M.; Davis, A.S.; Gallandt, E.R.; Bàrberi, P.; Moon, A.; Rasmussen, J.; van der Weide, R.; Vidotto, F. Non-chemical weed management. In Weed Research: Expanding Horizons, 1st ed.; Hatcher, P.E., Froud-Williams, R.J., Eds.; John Wiley and Sons, Ltd.: Chichester, UK, 2017; pp. 245–270. [Google Scholar]
- Andreasen, C.; Jensen, H.A.; Jensen, S.M. Decreasing diversity in the soil seed bank after 50 years in Danish arable fields. Agric. Ecosyst. Environ. 2018, 259, 61–71. [Google Scholar] [CrossRef]
- Roberts, H.A.; Feast, P.M. Fate of seeds of some annual weeds in different depths of cultivated and undisturbed soil. Weed Res. 1972, 12, 316–324. [Google Scholar] [CrossRef]
- Roberts, H.A.; Feast, P.M. Emergence and longevity of seeds of annual weeds in cultivated and undisturbed soil. J. Appl. Ecol. 1973, 10, 133–143. [Google Scholar] [CrossRef]
- Walsh, M.J.; Harrington, R.B.; Powles, S.B. Harrington seed destructor: A new nonchemical weed control tool for global grain crops. Crop Sci. 2012, 52, 1343–1347. [Google Scholar] [CrossRef]
- Walsh, M.; Newman, P.; Powles, S. Targeting weed seeds in-crop: A new weed control paradigm for global agriculture. Weed Technol. 2013, 27, 431–436. [Google Scholar] [CrossRef]
- Jakobsen, K.; Jensen, J.A.; Bitarafan, Z.; Andreasen, C. Killing weed seeds with exhaust gas from a combine harvester. Crop Prot. 2017; submitted. [Google Scholar]
- Vieregge, C. Personal information. In CLAAS Selbstfahrende Erntemaschinen GmbH; Vorentwicklung-Funktionstechnik: Harsewinkel, Germany, 2018. [Google Scholar]
- MCC-Instruments. Skalen Umrechnung. 2016. Available online: http://www.mcc-instruments.com (accessed on 30 January 2018).
- VDI-Wärmeatlas, Eleventh, Bearbeitete und Erweiterte Auflage; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 9783642199806/3642199801.
- Ritz, C.; Streibig, J.C. Bioassay analysis using R. J. Stat. Softw. 2005, 12, 1–22. [Google Scholar] [CrossRef]
- Ritz, C.; Pipper, C.B.; Streibig, J.C. Analysis of germination data from agricultural experiments. Eur. J. Agron. 2013, 45, 1–6. [Google Scholar] [CrossRef]
- ISTA. International Rules for Seed Testing; The International Seed Testing Association (ISTA): Bassersdorf, Switzerland, 2011. [Google Scholar]
- Yasin, M.; Andreasen, C. Breaking seed dormancy of Alliaria petiolata with phytohormones. Plant Growth Regul. 2015, 77, 307–315. [Google Scholar] [CrossRef]
- Copeland, L.O.; McDonald, M.B. Principles of Seed Science and Technology, 4th ed.; Kluwer Academic Publishers: Norwell, MA, USA, 2001; p. 488. [Google Scholar]
- Martin, R.E.; Miller, R.L.; Cushwa, C.T. Germination response of legume seeds subjected to moist and dry heat. Ecology 1975, 56, 1441–1445. [Google Scholar] [CrossRef]
- Glasner, C.; Andreasen, C.; Vieregge, C.; Dikiy, A.; Robert, J.; Bölling, R.; Shumilina, E. Sweedhart—Separation of Weeds during Harvesting and Hygienisation. Poster. FACCE Mid-Term Meeting: Paris, France. Available online: https://www.sweedhart.eu/index.php/resources/publications-and-deliverables (accessed on 17 January 2018).
- Walsh, M.J.; Powles, S.B. High seed retention at maturity of annual weeds infesting crop fields highlights the potential for harvest weed seed control. Weed Technol. 2014, 28, 486–493. [Google Scholar] [CrossRef]
- Yasin, M.; Rosenqvist, E.; Andreasen, C. The effect of reduced light intensities on grass weeds species. Weed Sci. 2017, 65, 603–613. [Google Scholar] [CrossRef]

| Weed Species | Short Descriptions | Thousand Seed Weight (g) | Average Size (mm) | Vessel Type |
|---|---|---|---|---|
| Alopecurus myosuroides Huds. | Spikelet with one seed and with a 5–6 mm awn | 2.0 | 5.7 × 1.9 × 0.9 | A |
| Amzinckia micranta (Suksd.) Druce | Surface uneven rough | 1.3 | 2.2 × 1.4 × 1.3 | A |
| Artemisia vulgaris L. | With longitudinal ribs | 0.1 | 1.5 × 0.5 × 0.5 | B |
| Centaurea cyanus L. | Seeds with stiff hairs (4 mm) at the apex | 4.5 | 2.4 × 1.7 × 1.2 | A |
| Erodium cicutarium (L.) L’Hér. | Seeds with spiral shaped awn | 2.7 | 5.4 × 1.1 × 1.1 | A |
| Papaver rhoeas L. | Seeds net meshed in curved row pattern | 0.1 | 0.8 × 0.5 × 0.6 | B |
| Silene noctiflorum (L.) Fr. | Kidney-shaped seeds, dark brownish to black seed | 1.0 | 1.4 × 1.2 × 0.9 | A |
| Tripleurospermum inodorum (L.) Sch. Bip. | Cross-section triangular to square | 0.4 | 1.8 × 0.7 × 0.7 | A |
| Veronica arvensis L. | Orange to yellow-brown | 0.1 | 1.0 × 0.7 × 0.3 | A |
| Temperature (°C) | Volume Flow (L hour−1) | Velocity (m s−1) |
|---|---|---|
| 50 | 2983 | 1.48 |
| 100 | 3445 | 1.71 |
| 150 | 3906 | 1.94 |
| 200 | 4368 | 2.17 |
| 250 | 4829 | 2.40 |
| Species | Temperature | 0 s | 2 s | 5 s | 10 s | 20 s |
|---|---|---|---|---|---|---|
| Alopecurus myosuroides | 50 °C | 80.1 (2.3) | 84.1 (2.5) | 79.1 (2.9) | 85.2 (2.5) | 90.0 (2.1) |
| 100 °C | 80.1 (2.3) | 84.2 (2.6) | 88.5 (2.3) | 81.6 (2.7) | 84.0 (2.6) | |
| 150 °C | 80.1 (2.3) | 84.0 (2.6) | 76.1 (3.0) | 67.5 (3.4) | 41.1 (3.7) | |
| 200 °C | 80.1 (2.3) | 71.3 (3.2) | 57.0 (3.6) | 18.5 (3.2) | 11.1 (2.2) | |
| 250 °C | 80.1 (2.3) | 54.2 (3.5) | 19.3 (2.8) | - | - | |
| Amzinckia micranta | 50 °C | 95.0 (1.5) | 84.5 (2.6) | 90.6 (2.0) | 90.3 (2.1) | 94.0 (1.7) |
| 100 °C | 95.0 (1.5) | 84.5 (2.6) | 93.0 (1.8) | 89.0 (2.2) | 90.5 (2.1) | |
| 150 °C | 95.0 (1.5) | 95.5 (1.5) | 86.6 (2.4) | 80.6 (2.8) | 45.7 (3.5) | |
| 200 °C | 95.0 (1.5) | 92.0 (1.9) | 70.6 (3.2) | 22.2 (3.0) | - | |
| 250 °C | 95.0 (1.5) | 85.5 (2.5) | 19.0 (2.8) | - | - | |
| Artemisia vulgaris | 200 °C | 95.0 (2.1) | 98.0 (13) | 81.3 (3.9) | 77.9 (37.9) | - |
| 250 °C | 95.0 (2.1) | 90.0 (3.0) | 81.9 (23.9) | 53.3 (2.7) | - | |
| Centaurea cyanus | 50 °C | 95.0 (1.5) | 88.5 (2.3) | 100.0 (0.0) | 96.0 (1.3) | 86.6 (2.4) |
| 100 °C | 95.0 (1.5) | 88.5 (2.3) | 88.5 (2.3) | 96.0 (1.3) | 96.0 (0.6) | |
| 150 °C | 95.0 (1.5) | 95.6 (1.4) | 93.5 (1.7) | 93.0 (1.8) | 83.0 (2.6) | |
| 200 °C | 95.0 (1.5) | 81.2 (2.8) | 94.0 (1.6) | 63.8 (3.4) | 27.3 (3.1) | |
| 250 °C | 95.0 (1.5) | 92.5 (1.9) | 62.1 (3.4) | 17.1 (2.7) | 1.0 (1.0) | |
| Erodium cicutarium | 50 °C | 92.0 (1.9) | 82.0 (2.7) | 82.3 (2.7) | 87.9 (2.3) | 86.6 (2.4) |
| 100 °C | 92.0 (1.9) | 82.0 (2.7) | 86.6 (2.4) | 91.0 (2.0) | 89.5 (2.2) | |
| 150 °C | 92.0 (1.9) | 91.7 (2.0) | 92.5 (1.9) | 88.6 (2.2) | 73.7 (3.1) | |
| 200 °C | 92.0 (1.9) | 85.5 (2.4) | 78.7 (2.8) | 70.1 (3.2) | 11.0 (2.3) | |
| 250 °C | 92.0 (1.9) | 88.1 (2.2) | 80.2 (2.8) | 23.2 (3.4) | - | |
| Papaver rhoeas | 50 °C | 49.2 (3.6) | 58.6 (3.5) | 60.3 (3.5) | 64.3 (3.5) | 59.3 (3.5) |
| 100 °C | 49.2 (3.6) | 58.6 (3.5) | 51.8 (3.7) | 59.2 (3.5) | 55.1 (3.5) | |
| 150 °C | 49.2 (3.6) | 58.7 (3.5) | 59.7 (3.4) | 34.8 (3.4) | - | |
| 200 °C | 49.2 (3.6) | 52.6 (3.5) | 15.3 (4.1) | - | - | |
| 250 °C | 49.2 (3.6) | 40.3 (3.5) | - | - | - | |
| Silene noctiflorum | 50 °C | 99.0 (0.6) | 100 (0.0) | 99.5 (0.5) | 99.4 (0.5) | 100.0 (0.0 |
| 100 °C | 99.0 (0.6) | 100.(0.0) | 100.0 (0.0) | 100.0 (0.0) | 99.0 (0.7) | |
| 150 °C | 99.0 (0.6) | 100.0 (0.0) | 90.5 (2.0) | 76.6 (3.0) | 37.1 (3.4) | |
| 200 °C | 99.0 (0.6) | 100.0 (0.0) | 73.5 (3.1) | 20.1 (2.9) | 2.0 (0.1) | |
| 250 °C | 99.0 (0.6) | 91.5 (2.0) | 43.0 (3.5) | 18.7 (17.0) | 28.1 (3.1) | |
| Tripleurospermum inodorum | 50 °C | 76.6 (3.0) | 83.0 (2.7) | 79.6 (2.9) | 80.2 (2.8) | 86.6 (2.4) |
| 100 °C | 76.6 (3.0) | 83.0 (2.6) | 88.5 (2.3) | 85.0 (2.5) | 79.1 (2.9) | |
| 150 °C | 76.6 (3.0) | 82.5 (2.6) | 89.6 (2.1) | 70.5 (3.4) | 1.5 (0.9) | |
| 200 °C | 76.6 (3.0) | 81.2 (2.7) | 24.1 (4.0) | 18.4 (3.2) | 11.1 (2.2) | |
| 250 °C | 76.6 (3.0) | 50.0 (3.5) | 19.3 (2.8) | - | - | |
| Veronica arvensis | 50 °C | 92.0 (1.8) | 94.0 (1.7) | 95.4 (1.5) | 97.0 (1.2) | 92.4 (1.8) |
| 100 °C | 92.0 (1.8) | 94.0 (1.7) | 95.5 (1.5) | 96.5 (1.3) | 94.5 (1.6) | |
| 150 °C | 92.0 (1.8) | 88.5 (2.3) | 96.0 (1.4) | 81.0 (2.8) | 5.8 (1.8) | |
| 200 °C | 92.0 (1.8) | 86.0 (2.5) | 58.6 (3.5) | 0.5 (0.5) | - | |
| 250 °C | 92.0 (1.8) | 62.5 (3.4) | 14.0 (0.0) | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreasen, C.; Bitarafan, Z.; Fenselau, J.; Glasner, C. Exploiting Waste Heat from Combine Harvesters to Damage Harvested Weed Seeds and Reduce Weed Infestation. Agriculture 2018, 8, 42. https://doi.org/10.3390/agriculture8030042
Andreasen C, Bitarafan Z, Fenselau J, Glasner C. Exploiting Waste Heat from Combine Harvesters to Damage Harvested Weed Seeds and Reduce Weed Infestation. Agriculture. 2018; 8(3):42. https://doi.org/10.3390/agriculture8030042
Chicago/Turabian StyleAndreasen, Christian, Zahra Bitarafan, Johanna Fenselau, and Christoph Glasner. 2018. "Exploiting Waste Heat from Combine Harvesters to Damage Harvested Weed Seeds and Reduce Weed Infestation" Agriculture 8, no. 3: 42. https://doi.org/10.3390/agriculture8030042





