Translocation of Endosulfan from Soil to Ginseng (Panax ginseng C. A. Meyer)
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Agrochemical
2.2. Soil of Experimental Field
2.3. Preparation of the Mixture of Endosulfan (Total) and Transplantation of Seedling of Ginseng
2.4. Collection of Specimens of Soil and Ginseng (Leaf, Stem, and Root)
2.5. Pretreatment of Specimens and Instrumental Analysis
2.5.1. Extraction and Refinement of Endosulfan (Total) from Soil Specimens
2.5.2. Extraction and Refinement of Endosulfan (Total) from Specimens (Leaf, Stem, and Root) of Ginseng
2.6. Recovery of Endosulfan (Total) from Specimens of Soil and Ginseng (Leaf, Stem, and Root)
3. Result and Discussions
3.1. Standard Calibration Curve of Endosulfan (Total)
3.2. Limit of Quantitation and Recovery of the Analysis
3.3. Translocation Characteristics of Endosulfan (Total) in Soil to Leaf, Stem, and Root of Ginseng
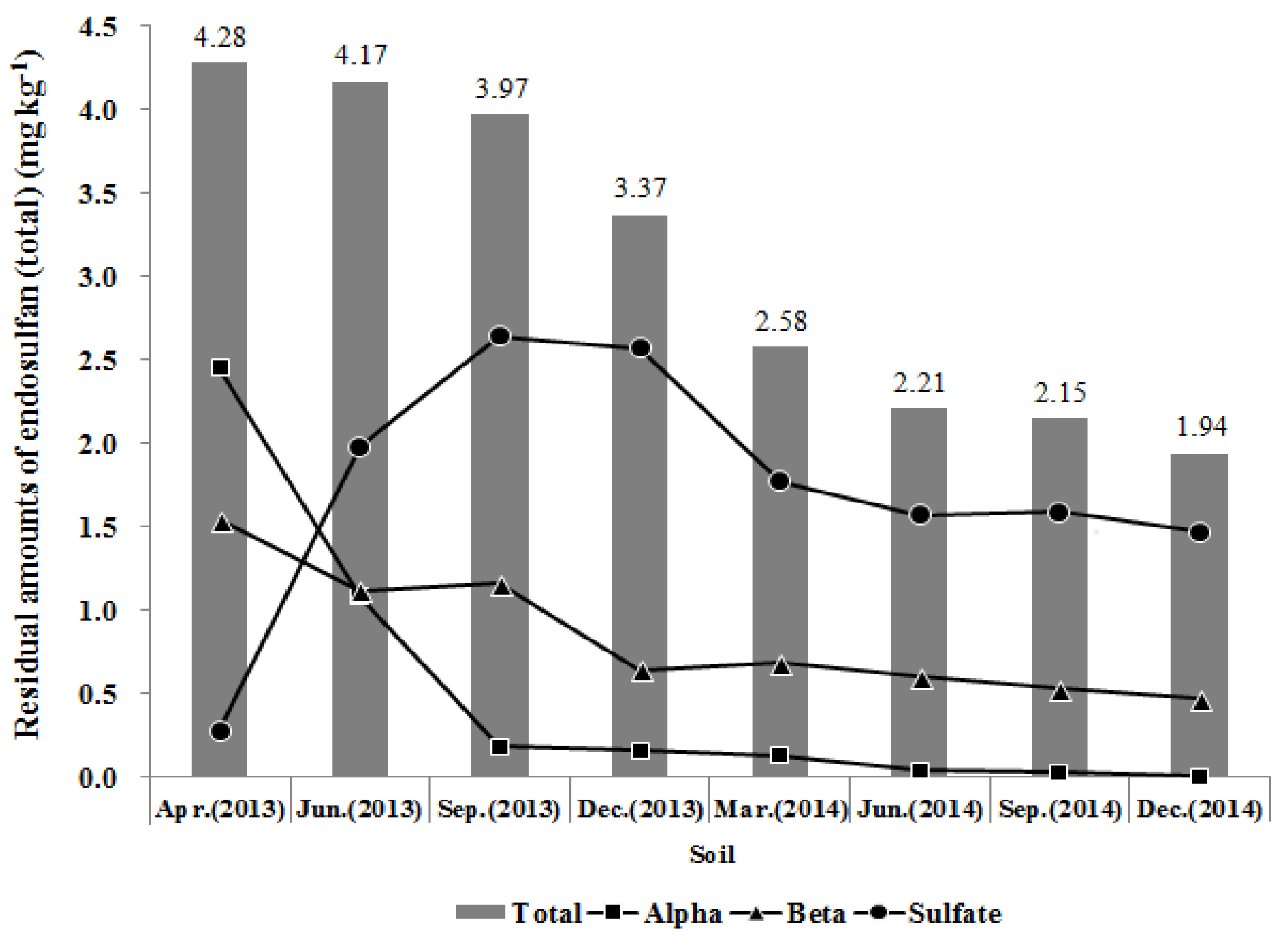
3.3.1. Retention of Endosulfan (Total) in Soil
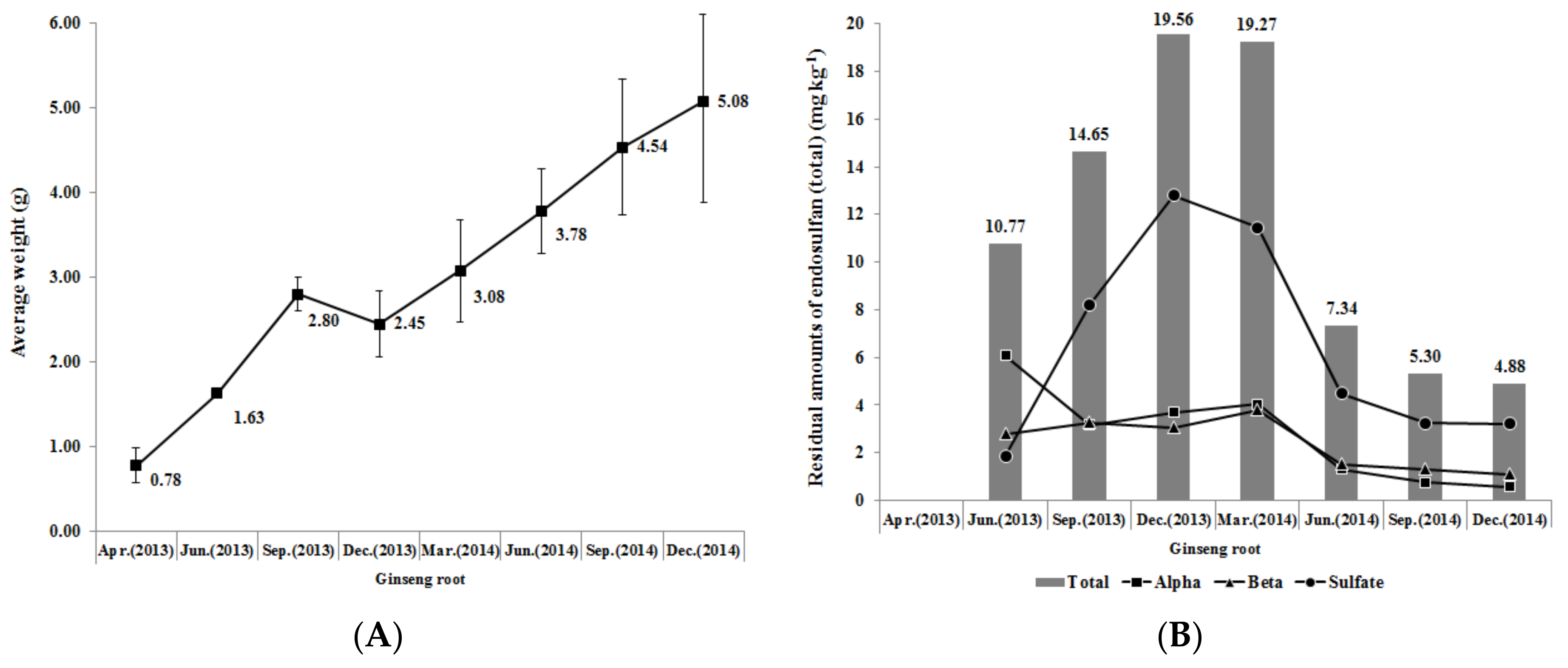
3.3.2. Retention of Endosulfan (Total) in Leaf and Stem of Ginseng
3.3.3. Retention of Endosulfan (Total) in Root of Ginseng
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, H.-J.; Cheong, S.-S.; Kim, D.-W.; Park, J.-S.; Ryu, J.; Bea, Y.-S.; Yoo, S.-J. Investigation into disease and pest incidence of Panax ginseng in Jeonbuk province. Korean J. Med. Crop Sci. 2008, 16, 33–38. [Google Scholar]
- Lee, J.-Y.; Noh, H.-H.; Lee, K.-H.; Park, H.-K.; Oh, J.-H.; Im, M.-H.; Kwon, C.-H.; Lee, J.-K.; Woo, H.-D.; Kwon, K.-S. Processing factors of azoxystrobin in processed ginseng products. Korean J. Pestic. Sci. 2012, 16, 222–229. [Google Scholar] [CrossRef]
- Harris, C. Factors influencing the effectiveness of soil insecticides. Annu. Rev. Entomol. 1972, 17, 177–198. [Google Scholar] [CrossRef]
- In, M.-H.; Kwon, K.-I.; Park, K.-S.; Choi, D.-M.; Chang, M.-I.; Jeong, J.-Y.; Lee, K.-J.; Yun, W.-K.; Hong, M.-K.; Woo, G.-J. Study on reduction factors of residual pesticides in processing of ginseng (I). Korean J. Pestic. Sci. 2006, 10, 22–27. [Google Scholar]
- Noh, H.-H.; Lee, J.-Y.; Park, S.-H.; Lee, K.-H.; Park, H.-K.; Oh, J.-H.; Im, M.-H.; Kwon, C.-H.; Lee, J.-K.; Woo, H.-D.; et al. Residual characteristics of tolclofos-methyl treated by seed dressing in ginseng. Korean J. Pestic. Sci. 2012, 16, 217–221. [Google Scholar] [CrossRef]
- Park, B.-J.; Choi, J.-H.; Lee, B.-M.; Im, G.-J.; Kim, C.-S.; Park, K.-H. Decomposition rate of iprobenfos, isoprothiolane, and diazinon by some environmental factors in aqueous systems. Korean J. Pestic. Sci. 1998, 2, 39–44. [Google Scholar]
- Juraske, R.; Castells, F.; Vijay, A.; Muñoz, P.; Antón, A. Uptake and persistence of pesticides in plants: measurements and model estimates for imidacloprid after foliar and soil application. J. Hazard. Mater. 2009, 165, 683–689. [Google Scholar] [CrossRef] [PubMed]
- National Agricultural Products Quality Management Service. Available online: http://www.naqs.go.kr/eng/contents/Agrifood/Agrifood/B_07.naqs (accessed on 17 June 2017).
- Tellez-Bañuelos, M.C.; Santerre, A.; Casas-Solis, J.; Bravo-Cuellar, A.; Zaitseva, G. Oxidative stress in macrophages from spleen of Nile tilapia (Oreochromis niloticus) exposed to sublethal concentration of endosulfan. Fish Shellfish Immunol. 2009, 27, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Capkin, E.; Altinok, I.; Karahan, S. Water quality and fish size affect toxicity of endosulfan, an organochlorine pesticide, to rainbow trout. Chemosphere 2006, 64, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- Dorval, J.; Leblond, V.; Hontela, A. Oxidative stress and loss of cortisol secretion in adrenocortical cells of rainbow trout (Oncorhynchus mykiss) exposed in vitro to endosulfan, an organochlorine pesticide. Aquat. Toxicol. 2003, 63, 229–241. [Google Scholar] [CrossRef]
- Singh, V.; Singh, N. Uptake and accumulation of endosulfan isomers and its metabolite endosulfan sulfate in naturally growing plants of contaminated area. Ecotoxicol. Environ. Saf. 2014, 104, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.B. Residual Characteristics of Endosulfan in Busan and Gimhae Farmland. Master’s Thesis, Dong-A University, Busan, Korea, 2009. [Google Scholar]
- Choi, O.K.; Kim, K.C.; Kwon, S.M.; Ryu, K.S.; Yun, S.J.; Cho, Y.S.; Kang, H.G.; Kim, C.Y.; Jang, J.H.; Lee, J.B. Study on the Migration of Endosulfan Isomers from Soil to Agricultural Products. Res. Inst. Public Health Environ. 2012, 25, 107–114. [Google Scholar]
- Park, M.E. Bioremediation of Organochlorine Insecticide Endosulfan by Bioaugmentation with a Bacterium. Master’s Thesis, Pu-Kyung National University, Busan, Korea, 2011. [Google Scholar]
- El-Saeid, M.; Selim, M. Multiresidue analysis of 86 pesticides using gas chromatography mass spectrometry: II-Nonleafy vegetables. J. Chem. 2012, 2013, 727149. [Google Scholar] [CrossRef]
- Boes, E.; Rosmalina, R.T.; Ridwan, Y.S.; Nugraha, W.C.; Yusiasih, R. Development of validated method using QuEChERS technique for organochlorine pesticide residues in vegetable. Procedia Chem. 2015, 16, 229–236. [Google Scholar] [CrossRef]
- Jahanmard, E.; Ansari, F.; Feizi, M. Evaluation of Quechers sample preparation and GC mass spectrometry method for the determination of 15 pesticide residues in tomatoes used in salad production plants. Iran. J. Public Health 2016, 45, 230–238. [Google Scholar] [PubMed]
- Jallow, M.F.; Awadh, D.G.; Albaho, M.S.; Devi, V.Y.; Ahmad, N. Monitoring of pesticide residues in commonly used fruits and vegetables in Kuwait. Int. J. Environ. Res. Public Health 2017, 14, 833. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-I.; Zimmerman, A.R.; Kim, J.-E. Bioconcentration factor-based management of soil pesticide residues: Endosulfan uptake by carrot and potato plants. Sci. Total Environ. 2018, 627, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Kullman, S.W.; Matsumura, F. Metabolic pathways utilized by Phanerochaete chrysosporium for degradation of the cyclodiene pesticide endosulfan. Appl. Environ. Microbiol. 1996, 62, 593–600. [Google Scholar] [PubMed]
- Ministry of Food and Drug Safety, Korea (MFDS). What Is Endosulfan in Food. 2008. Available online: http://www.mfds.go.kr (accessed on 8 March 2016).
- Tomlin, C.D. The Pesticide Manual: A World Compendium; British Crop Production Council: Hampshire, UK, 2009. [Google Scholar]
- Sparks, D.L. Elucidating the fundamental chemistry of soils: past and recent achievements and future frontiers. Geoderma 2001, 100, 303–319. [Google Scholar] [CrossRef]
- Park, E.J. Tolerance of Cultivation Period Residue Limit of the Fungicides in Korean Melon under Greenhouse Condition. Master’s Thesis, Kyung-Pook National University, Daegu, Korea, 2010. [Google Scholar]
- Park, H.K. Residual Characteristics of Bifenthrin, Chlorpyrifos and Imidacloprid in Squash and Estimation of Their Residues before Harvest. Master’s Thesis, Chung-buk National University, Cheongju, Korea, 2012. [Google Scholar]
- Choi, C.-M.; Yook, D.-H.; Hong, C.-K.; Kim, T.-R.; Hwang, Y.-S.; Hwang, I.-S.; Kim, J.-H.; Kim, M.-S.; Chae, Y.-Z. Monitoring of residual pesticides in agricultural land from the southern area of Seoul. Korean J. Pestic. Sci. 2011, 15, 160–165. [Google Scholar]
- Kim, C.-S.; Lee, H.-D.; Ihm, Y.-B.; Im, G.-J. Runoff of endosulfan by rainfall simulation and from soybean-grown field lysimeter. Korean J. Environ. Agric. 2007, 26, 343–350. [Google Scholar] [CrossRef]
- McCall, P.; Swann, R.; Laskowski, D.; Unger, S.; Vrona, S.; Dishburger, H. Estimation of chemical mobility in soil from liquid chromatographic retention times. Bull. Environ. Contam. Toxicol. 1980, 24, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Guerin, T. Abiological loss of endosulfan and related chlorinated organic compounds from aqueous systems in the presence and absence of oxygen. Environ. Pollut. 2001, 115, 219–230. [Google Scholar] [CrossRef]
- Australian Government National Registration Authority for Agricultural and Veterinary Chemicals; The NRA ECRP Review of Endosulfan: Commonwealth, Kingston, Australia, 1998; Volume 1.
- Park, H.-J.; Choi, J.-H.; Park, B.-J.; Kim, C.-S.; Ihm, Y.-B.; Ryu, G.-H. Uptake of endosulfan and procymidone from arable soil by several vegetables I (Green House Study). Korean J. Pestic. Sci. 2004, 8, 280–287. [Google Scholar]
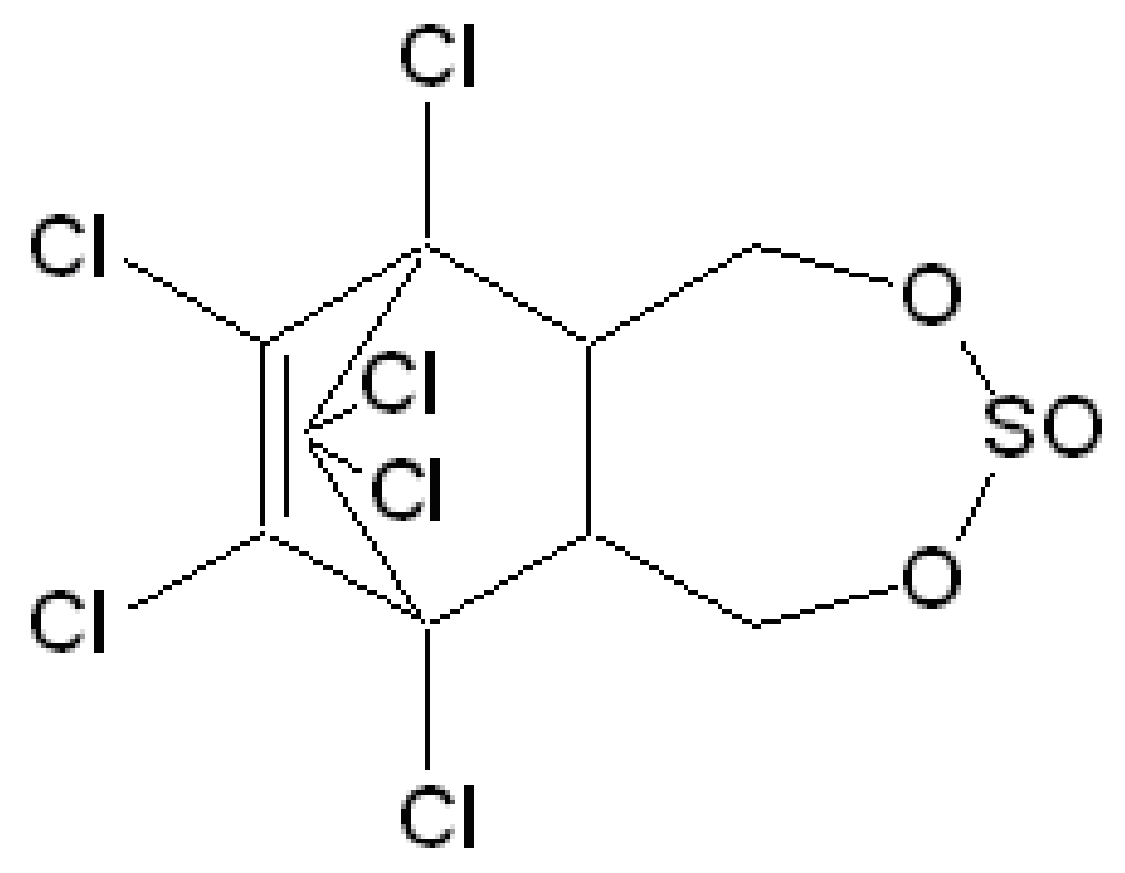

| Formular | Mol.wt. | M.P. | Kow logP | Activity |
|---|---|---|---|---|
| C9H6Cl6O3S | 406.9 | ≥80 °C (tech); α- 109.2 °C; β- 213.3 °C | α- = 4.74; β- = 4.79 (both at pH 5, 22 °C) | Insecticide |
| pH (1:5) | OM1 (g kg−1) | Ca (cmol (+) kg−1) | K (cmol (+) kg−1) | Mg (cmol (+) kg−1) | Na (cmol (+) kg−1) | P2O5 (mg kg−1) | NO3 (mg kg−1) | Sand (%) | Silt (%) | Clay (%) | Soil Texture |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 6.91 | 22.88 | 5.44 | 0.54 | 0.90 | 0.07 | 120.08 | 5.69 | 55.9 | 38.4 | 5.8 | SL2 |
| Instrument | GC-MS 5975C (Agilent, Santa Clara, CA, USA) | ||
|---|---|---|---|
| Column | DB-5MS (30 m × I.D. 0.25 mm, 0.25 μm) | ||
| Temp. | Inlet | 270 °C | |
| Oven | 100 °C (2 min hold) → increased at 20 °C min−1 to 280 °C (19 min hold) | ||
| Detector | 300 °C | ||
| Flow rate | 1.0 mL min−1 (He) | ||
| Injection volume | 1 μL | ||
| Split ratio | Splitless mode | ||
| Mass range (m/z) (SIM1) mode) | α-Endosulfan | 195, 237 | |
| β-Endosulfan | 195, 237 | ||
| Endosulfan-sulfate | 272, 387, 422 | ||
| Matrixes | Agrochemicals | Fortification Level (mg kg−1) | Mean ± CV (%) | LOQ (mg kg−1) |
|---|---|---|---|---|
| Soil | alpha | 0.2 | 95.6 ± 1.7 | 0.02 |
| 1.0 | 90.7 ± 2.3 | |||
| beta | 0.2 | 91.9 ± 2.3 | ||
| 1.0 | 83.3 ± 2.2 | |||
| sulfate | 0.2 | 85.3 ± 4.8 | ||
| 1.0 | 93.4 ± 4.1 | |||
| Ginseng root | alpha | 0.2 | 85.2 ± 3.8 | |
| 1.0 | 79.3 ± 0.5 | |||
| beta | 0.2 | 93.5 ± 0.9 | ||
| 1.0 | 83.4 ± 0.6 | |||
| sulfate | 0.2 | 112.5 ± 0.6 | ||
| 1.0 | 97.1 ± 0.3 | |||
| Ginseng leaf·stem | alpha | 0.2 | 103.1 ± 7.6 | |
| 1.0 | 81.8 ± 2.5 | |||
| beta | 0.2 | 79.3 ± 5.0 | ||
| 1.0 | 75.1 ± 2.7 | |||
| sulfate | 0.2 | 80.4 ± 4.5 | ||
| 1.0 | 74.5 ± 3.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Saravanan, M.; Palansooriya, K.N.; Hur, J.H. Translocation of Endosulfan from Soil to Ginseng (Panax ginseng C. A. Meyer). Agriculture 2018, 8, 52. https://doi.org/10.3390/agriculture8040052
Kim J, Saravanan M, Palansooriya KN, Hur JH. Translocation of Endosulfan from Soil to Ginseng (Panax ginseng C. A. Meyer). Agriculture. 2018; 8(4):52. https://doi.org/10.3390/agriculture8040052
Chicago/Turabian StyleKim, Jiyoon, Manoharan Saravanan, Kumuduni Niroshika Palansooriya, and Jang Hyun Hur. 2018. "Translocation of Endosulfan from Soil to Ginseng (Panax ginseng C. A. Meyer)" Agriculture 8, no. 4: 52. https://doi.org/10.3390/agriculture8040052





