To What Extent Can Existing Research Help Project Climate Change Impacts on Biodiversity in Aquatic Environments? A Review of Methodological Approaches
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol for Literature Search and Extraction of Data
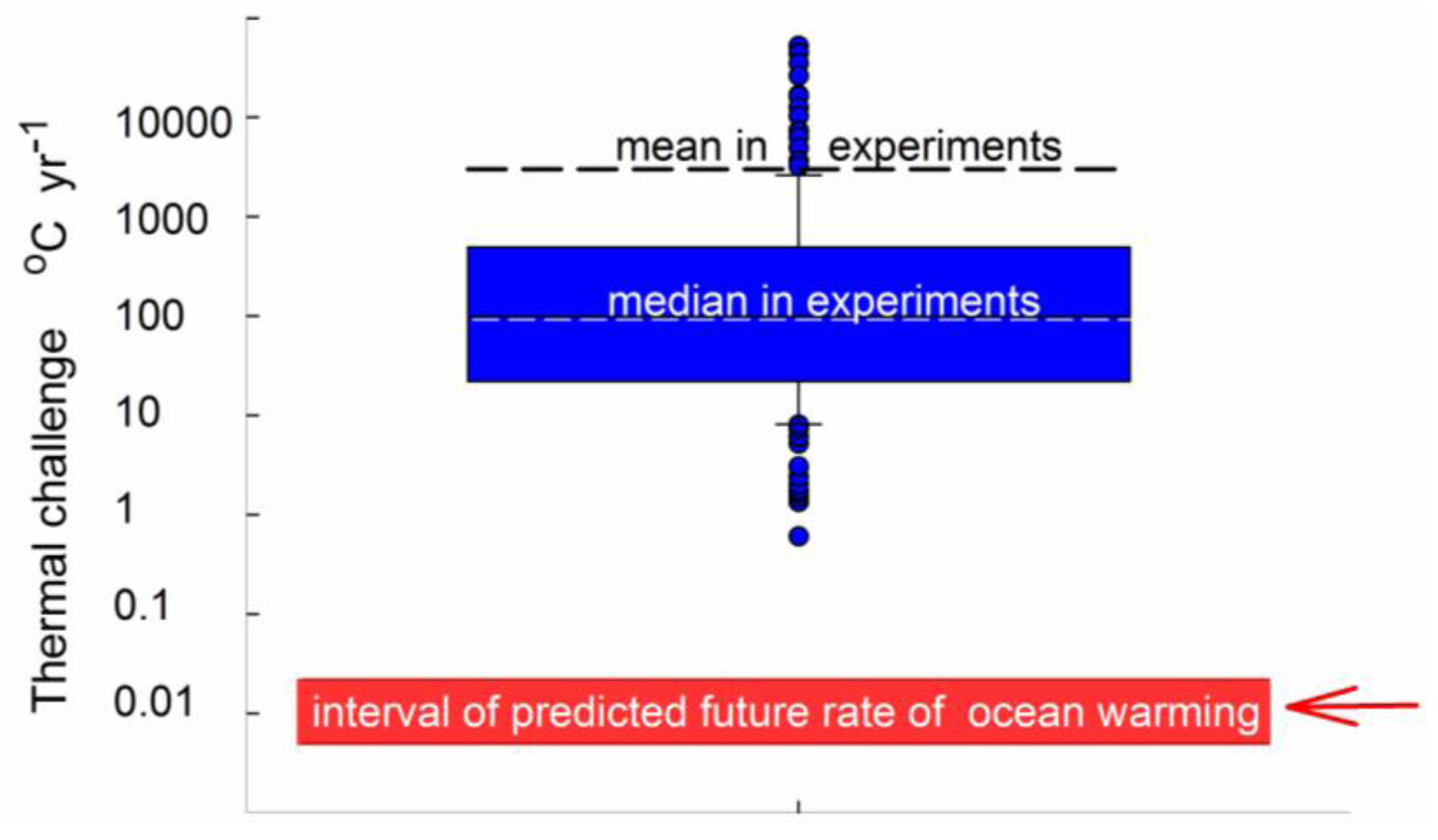
2.2. Estimating Thermal Challenge
3. Review Findings and Discussion
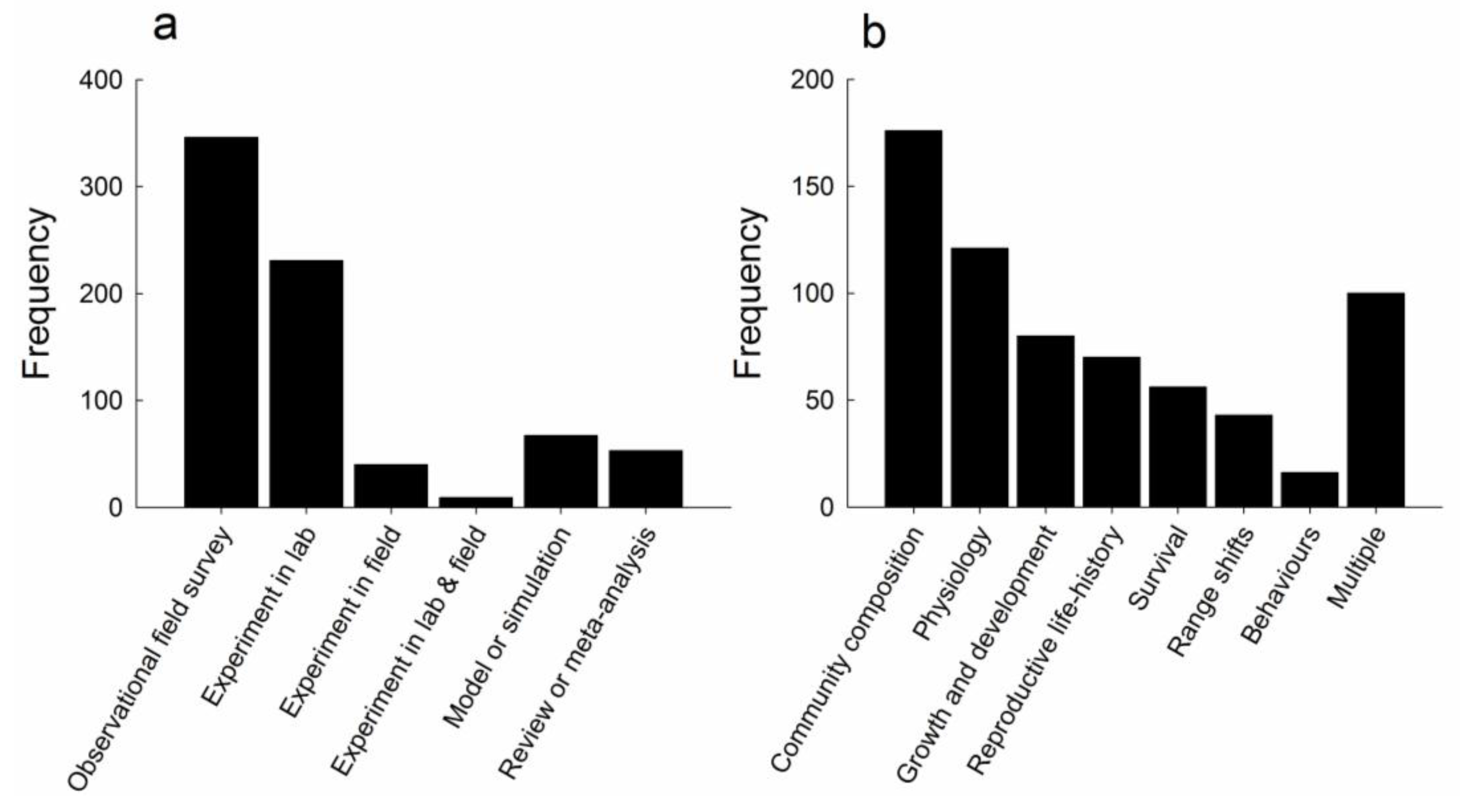
3.1. Frequency of Observational and Experimental Studies in the Literature Search
3.2. Characteristics of Experimental Studies
3.3. Response Variables Investigated
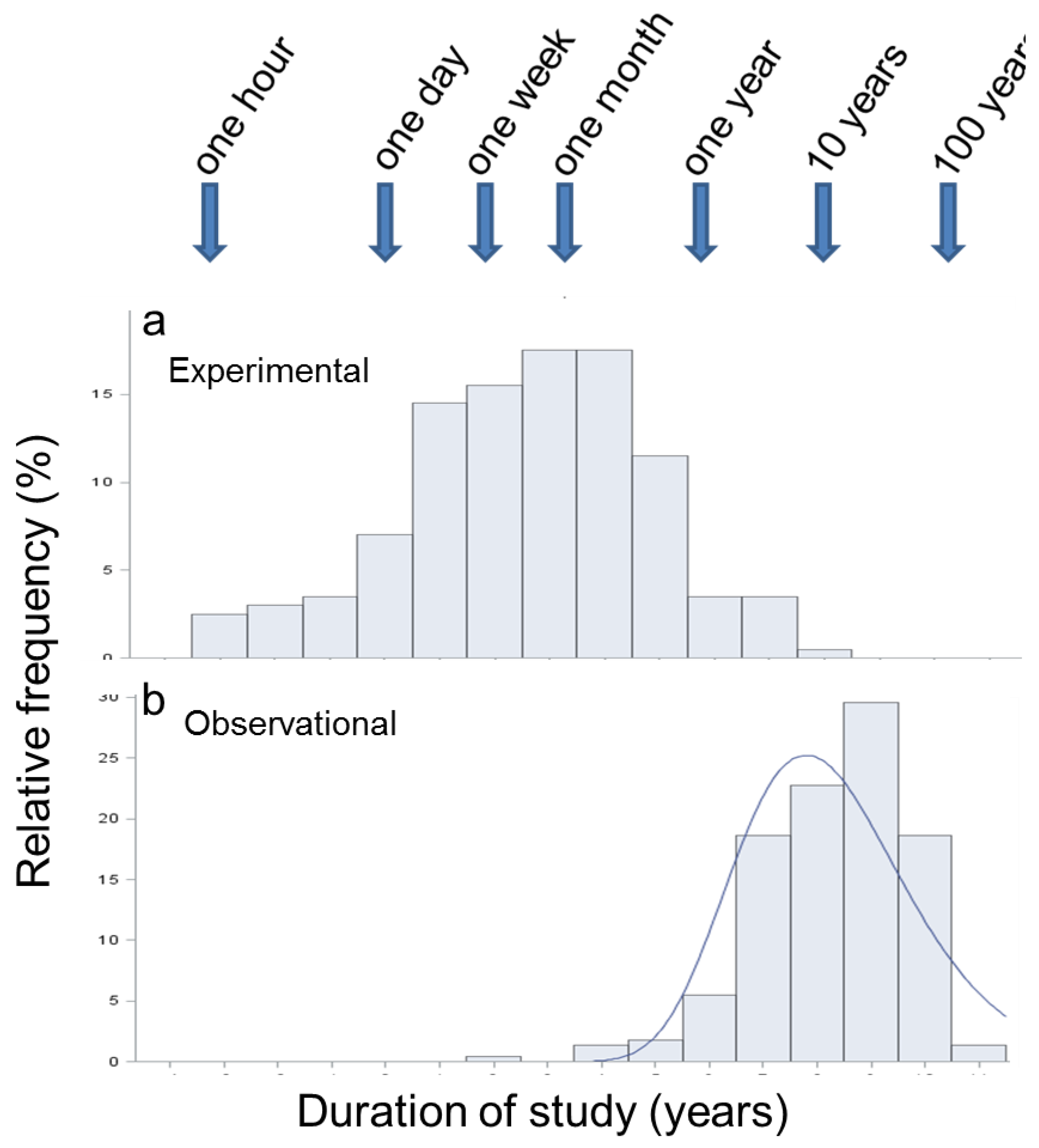
3.4. Duration of Studies
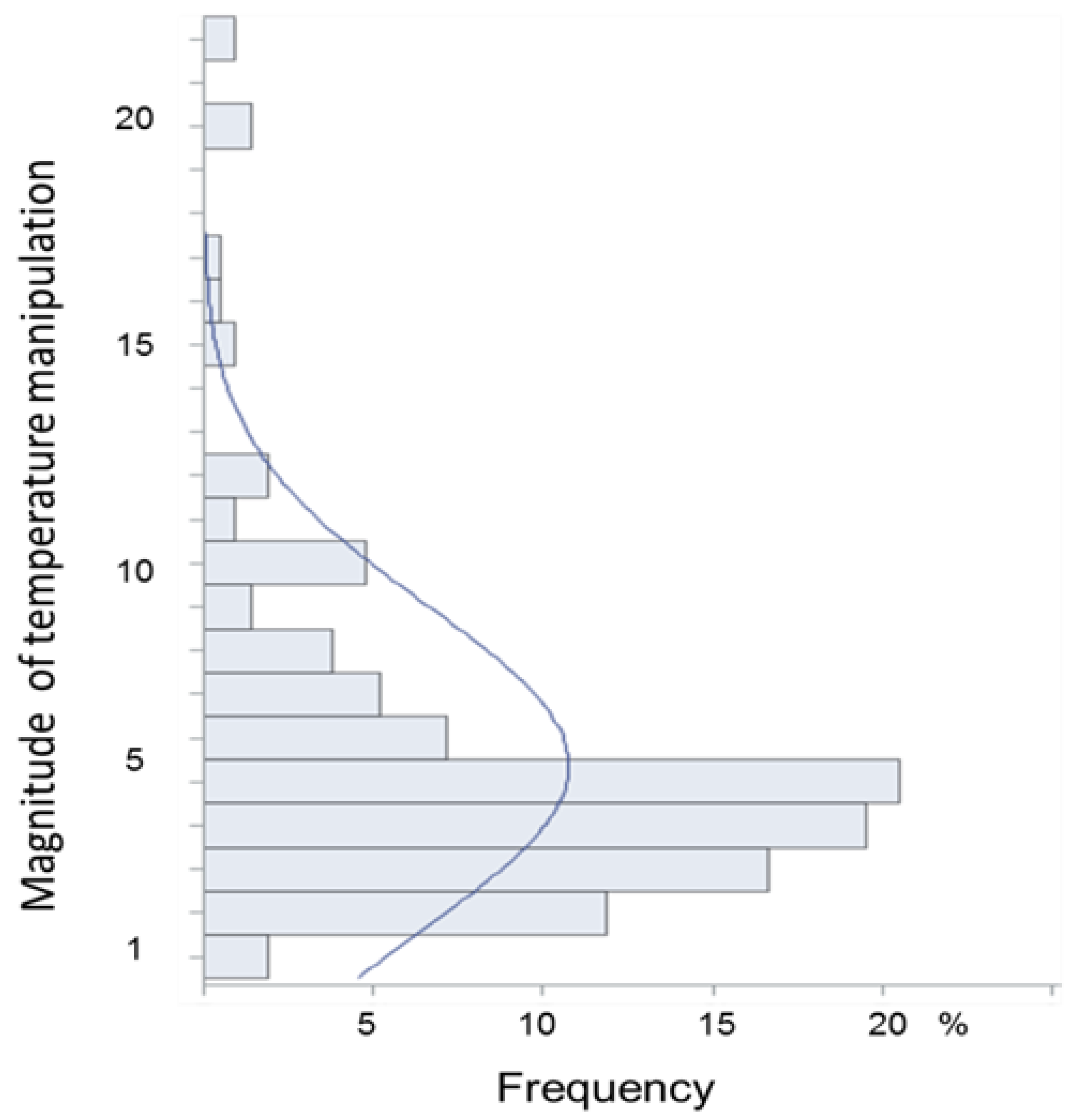
3.5. Nature and Magnitude of Experimental Temperature Manipulations
3.6. General Discussion and Overall Assessment of Approaches Used in the Reviewed Studies
3.7. Finding the Balance between Realism and Methodological Tractability
4. Directions for the Future
4.1. Steps towards Increased Realism
4.1.1. Assess and Identify Causes of Heterogeneity of Biodiversity Responses to Climate Change
4.1.2. Target those Response Variables that are likely to be of Importance for Biodiversity Responses to Climate Change
4.1.3. Use Multi-Species Approaches
4.1.4. Use Temperature Regimes Better Suited for Making Inferences about Climate Change Effects on Biodiversity
4.2. Big Challenges Call for Ambitious Actions and Solutions
4.3. Experimental Temperature Manipulation in the Wild
4.4. Ecologists and Evolutionary Biologists Must Rise to the Challenge
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Intergovernmental Panel on Climate Change. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; p. 1535. [Google Scholar]
- Gleick, P.H.; Adams, R.M.; Amasino, R.M.; Anders, E.; Anderson, D.J.; Anderson, W.W.; Anselin, L.E.; Arroyo, M.K.; Asfaw, B.; Ayala, F.J.; et al. Climate change and the integrity of science. Science 2010, 328, 689–690. [Google Scholar] [CrossRef] [PubMed]
- Botkin, D.B.; Saxe, H.; Araújo, M.B.; Betts, R.; Bradshaw, R.H.W.; Cedhagen, T.; Chesson, P.; Dawson, T.P.; Etterson, J.R.; Faith, D.P.; et al. Forecasting the effects of global warming on biodiversity. BioScience 2007, 57, 227–236. [Google Scholar] [CrossRef]
- Tilman, D.; Fargione, J.; Wolff, B.; D′Antonio, C.; Dobson, A.P.; Howarth, R.; Schindler, D.; Schlesinger, W.H.; Simberloff, D.; Swackhamer, D. Forecasting agriculturally driven global environmental change. Science 2001, 292, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Ash, C.; Culotta, E.; Fahrenkamp-Uppenbrink, J.; Malakoff, D.; Smith, J.; Sugden, A.; Vignieri, S.N. Once and future climate change. Science 2013, 341, 472–473. [Google Scholar] [CrossRef] [PubMed]
- Dawson, T.P.; Jackson, S.T.; House, J.I.; Prentice, I.C.; Mace, G.M. Beyond predictions: Biodiversity conservation in a changing climate. Science 2011, 332, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Shaver, G.R.; Canadell, J.; Chapin, F.S.; Gurevitch, J.; Harte, J.; Henry, G.; Ineson, P.; Jonasson, S.; Melillo, J.; Pitelka, L.; et al. Global warming and terrestrial ecosystems: A conceptual framework for analysis. BioScience 2000, 50, 871–882. [Google Scholar] [CrossRef]
- Hsiang, S.M.; Burke, M.; Miguel, E. Quantifying the influence of climate on human conflict. Science 2013, 341. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.D.; Franco, A.M.A.; Hill, J.K. Range retractions and extinctions in the face of climate warming. Trends Ecol. Evol. 2006, 21, 415–416. [Google Scholar] [CrossRef] [PubMed]
- Walther, G.-R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.-M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Devictor, V.; van Swaay, C.; Brereton, T.; Brotons, L.; Chamberlain, D.; Heliola, J.; Herrando, S.; Julliard, R.; Kuussaari, M.; Lindstrom, A.; et al. Differences in the climatic debts of birds and butterflies at a continental scale. Nat. Clim. Chang. 2012, 2, 121–124. [Google Scholar] [CrossRef]
- Parmesan, C.; Ryrholm, N.; Stefanescu, C.; Hill, J.K.; Thomas, C.D.; Descimon, H.; Huntley, B.; Kaila, L.; Kullberg, J.; Tammaru, T.; et al. Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature 1999, 399, 579–583. [Google Scholar] [CrossRef]
- Gill, J.A.; Alves, J.A.; Sutherland, W.J.; Appleton, G.F.; Potts, P.M.; Gunnarsson, T.G. Why is timing of bird migration advancing when individuals are not? Proc. R. Soc. B 2014, 281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ovaskainen, O.; Skorokhodova, S.; Yakovleva, M.; Sukhov, A.; Kutenkov, A.; Kutenkova, N.; Shcherbakov, A.; Meyke, E.; del Mar Delgado, M. Community-level phenological response to climate change. Proc. Natl. Acad. Sci. USA 2013, 110, 13434–13439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merilä, J.; Hendry, A.P. Climate change, adaptation, and phenotypic plasticity: The problem and the evidence. Evol. Appl. 2014, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, E.D.; Nogues-Bravo, D.; Orlando, L.; Weinstock, J.; Binladen, J.; Marske, K.A.; Ugan, A.; Borregaard, M.K.; Gilbert, M.T.P.; Nielsen, R.; et al. Species-specific responses of late quaternary megafauna to climate and humans. Nature 2011, 479, 359–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.N.; de Siqueira, M.F.; Grainger, A.; Hannah, L.; et al. Extinction risk from climate change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urban, M.C.; de Meester, L.; Vellend, M.; Stoks, R.; Vanoverbeke, J. A crucial step toward realism: Responses to climate change from an evolving metacommunity perspective. Evol. Appl. 2012, 5, 154–167. [Google Scholar] [CrossRef] [PubMed]
- NASA’s Earth Observatory: Global Warming. Available online: http://earthobservatory.nasa.gov/Features/GlobalWarming/page2.php (accessed on 11 November 2016).
- Stewart, R.I.A.; Dossena, M.; Bohan, D.A.; Jeppesen, E.; Kordas, R.L.; Ledger, M.E.; Meerhoff, M.; Moss, B.; Mulder, C.; Shurin, J.B.; et al. Mesocosm experiments as a tool for ecological climate-change research. In Advances in Ecological Research; Guy, W., Eoin, J.O.G., Eds.; Academic Press: Cambridge, MA, USA, 2013; Volume 48, pp. 71–181. [Google Scholar]
- Griffen, B.D.; Drake, J.M. A review of extinction in experimental populations. J. Anim. Ecol. 2008, 77, 1274–1287. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.T.; Dijkstra, P.; Koch, G.W.; Penuelas, J.; Hungate, B.A. Responses of terrestrial ecosystems to temperature and precipitation change: A meta-analysis of experimental manipulation. Glob. Chang. Biol. 2011, 17, 927–942. [Google Scholar] [CrossRef]
- Levin, S.A. The problem of pattern and scale in ecology: The robert h. Macarthur Award Lect. Ecol. 1992, 73, 1943–1967. [Google Scholar]
- Wennersten, L.; Forsman, A. Population-level consequences of polymorphism, plasticity and randomized phenotype switching: A review of predictions. Biol. Rev. 2012, 87, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.R.; Inouye, B.D.; Johnson, M.T.J.; Underwood, N.; Vellend, M. Ecological consequences of genetic diversity. Ecol. Lett. 2008, 11, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Reusch, T.B.H.; Ehlers, A.; Hämmerli, A.; Worm, B. Ecosystem recovery after climatic extremes enhanced by genotypic diversity. Proc. Natl. Acad. Sci. USA 2005, 102, 2826–2831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forsman, A. Effects of genotypic and phenotypic variation on establishment are important for conservation, invasion and infection biology. Proc. Natl. Acad. Sci. USA 2014, 111, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Skelly, D.K. Experimental venue and estimation of interaction strength. Ecology 2002, 83, 2097–2101. [Google Scholar] [CrossRef]
- Carmel, Y.; Kent, R.; Bar-Massada, A.; Blank, L.; Liberzon, J.; Nezer, O.; Sapir, G.; Federman, R. Trends in ecological research during the last three decades—A systematic review. PLoS ONE 2013, 8, e59813. [Google Scholar] [CrossRef] [PubMed]
- Wirta, H.K.; Hebert, P.D.N.; Kaartinen, R.; Prosser, S.W.; Várkonyi, G.; Roslin, T. Complementary molecular information changes our perception of food web structure. Proc. Natl. Acad. Sci. USA 2014, 111, 1885–1890. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.J.; Lewis, O.T.; Godfray, H.C.J. Experimental evidence for apparent competition in a tropical forest food web. Nature 2004, 428, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Levins, R. Evolution in Changing Environments; Princeton University Press: Princeton, NJ, USA, 1968. [Google Scholar]
- Forsman, A.; Betzholtz, P.E.; Franzén, M. Faster poleward range shifts in moths with more variable colour patterns. Sci. Rep. 2016, 6, 36265. [Google Scholar] [CrossRef] [PubMed]
- Rosenblatt, A.E.; Schmitz, O.J. Interactive effects of multiple climate change variables on trophic interactions: A meta-analysis. Clim. Chang. Responses 2014, 1, 1–10. [Google Scholar] [CrossRef]
- Forsman, A.; Wennersten, L. Inter-individual variation promotes ecological success of populations and species: Evidence from experimental and comparative studies. Ecography 2016, 39, 630–648. [Google Scholar] [CrossRef]
- Gruner, D.S.; Bracken, M.E.S.; Berger, S.A.; Eriksson, B.K.; Gamfeldt, L.; Matthiessen, B.; Moorthi, S.; Sommer, U.; Hillebrand, H. Effects of experimental warming on biodiversity depend on ecosystem type and local species composition. Oikos 2016. [Google Scholar] [CrossRef]
- Reichman, O.J.; Jones, M.B.; Schildhauer, M.P. Challenges and opportunities of open data in ecology. Science 2011, 331, 703–705. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Community cleverness required. Nature 2008, 455. [Google Scholar] [CrossRef]
- The Tree of Life Web Project. Available online: http://tolweb.org/tree/ (accessed on 30 September 2015).
- International Union for Conservation of Nature. The IUCN Red List of Threatened Species; Version 2013.2; IUCN: Gland, Switzerland, 2013. [Google Scholar]
- International Nucleotide Sequence Database Collaboration. Available online: http://www.insdc.org/ (accessed on 30 September 2015).
- Perrings, C.; Duraiappah, A.; Larigauderie, A.; Mooney, H.A. The biodiversity and ecosystem services science-policy interface. Science 2011, 331, 1139–1140. [Google Scholar] [CrossRef] [PubMed]
- Global Crop Diversity Trust Website. Available online: https://www.croptrust.org/ (accessed on 30 September 2015).
- Chew, L.L.; Chong, V.C.; Wong, R.C.; Lehette, P.; Ng, C.C.; Loh, K.H. Three decades of sea water abstraction by kapar power plant (Malaysia): What impacts on tropical zooplankton community? Mar. Pollut. Bull. 2015, 101, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Bamber, R.N.; Spencer, J.F. The benthos of a coastal power station thermal discharge canal. J. Mar. Biol. Assoc. UK 1984, 64, 603–623. [Google Scholar] [CrossRef]
- Lardicci, C.; Rossi, F.; Maltagliati, F. Detection of thermal pollution: Variability of benthic communities at two different spatial scales in an area influenced by a coastal power station. Mar. Pollut. Bull. 1999, 38, 296–303. [Google Scholar] [CrossRef]
- Saenger, P.; Stephenson, W.; Moverley, J. Macrobenthos of the cooling watre discharge canal of the Gladstone Power Station, Queensland. Mar. Freshw. Res. 1982, 33, 1083–1095. [Google Scholar] [CrossRef]
- Suresh, K.; Ahamed, M.S.; Durairaj, G.; Nair, K.V.K. Impact of power plant heated effluent on the abundance of sedentary organisms, off Kalpakkam, East coast of India. Hydrobiologia 1993, 268, 109–114. [Google Scholar] [CrossRef]
- Ehlin, U.; Borenäs, K.; Neuman, E.; Sandström, O. Miljöeffekter Av Stora Kylvattenutsläpp I Ett Varmare Klimat; Elforsk: Stockholm, Sweden, 2012. (In Swedish) [Google Scholar]
- Hairston, N.G.J.; van Brunt, R.A.; Kearns, C.M.; Engstrom, D.R. Age and survivorship of diapausing eggs in a sediment egg bank. Ecology 1995, 76, 1706–1711. [Google Scholar] [CrossRef]
- Kerfoot, W.C.; Robbins, J.A.; Weider, L.J. A new approach to historical reconstruction: Combining descriptive and experimental paleolimnology. Limnol. Oceanogr. 1999, 44, 1232–1247. [Google Scholar] [CrossRef]
- Boyd, P.W. Framing biological responses to a changing ocean. Nat. Clim. Chang. 2013, 3, 530–533. [Google Scholar] [CrossRef]
- Merilä, J.; Hoffman, A.A. Evolutionary impacts of climate change. In Oxford Research Encyclopedia of Environmental Science; Oxford University Press: New York, NY, USA, 2016. [Google Scholar]
- Forsman, A. Rethinking phenotypic plasticity and its consequences for individuals, populations and species. Heredity 2015, 115, 276–284. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forsman, A.; Berggren, H.; Åström, M.; Larsson, P. To What Extent Can Existing Research Help Project Climate Change Impacts on Biodiversity in Aquatic Environments? A Review of Methodological Approaches. J. Mar. Sci. Eng. 2016, 4, 75. https://doi.org/10.3390/jmse4040075
Forsman A, Berggren H, Åström M, Larsson P. To What Extent Can Existing Research Help Project Climate Change Impacts on Biodiversity in Aquatic Environments? A Review of Methodological Approaches. Journal of Marine Science and Engineering. 2016; 4(4):75. https://doi.org/10.3390/jmse4040075
Chicago/Turabian StyleForsman, Anders, Hanna Berggren, Mats Åström, and Per Larsson. 2016. "To What Extent Can Existing Research Help Project Climate Change Impacts on Biodiversity in Aquatic Environments? A Review of Methodological Approaches" Journal of Marine Science and Engineering 4, no. 4: 75. https://doi.org/10.3390/jmse4040075





