Enhancement of Protein and Pigment Content in Two Chlorella Species Cultivated on Industrial Process Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Growth Media
2.3. Microalgal Growth Experiments
2.4. Analytical Methods
2.4.1. Growth Curve
2.4.2. Proteins and Amino Acids
2.4.3. Lipids, Fatty Acid, and Tocopherols
2.4.4. Pigments
2.4.5. Statistical Analysis
3. Results and Discussion
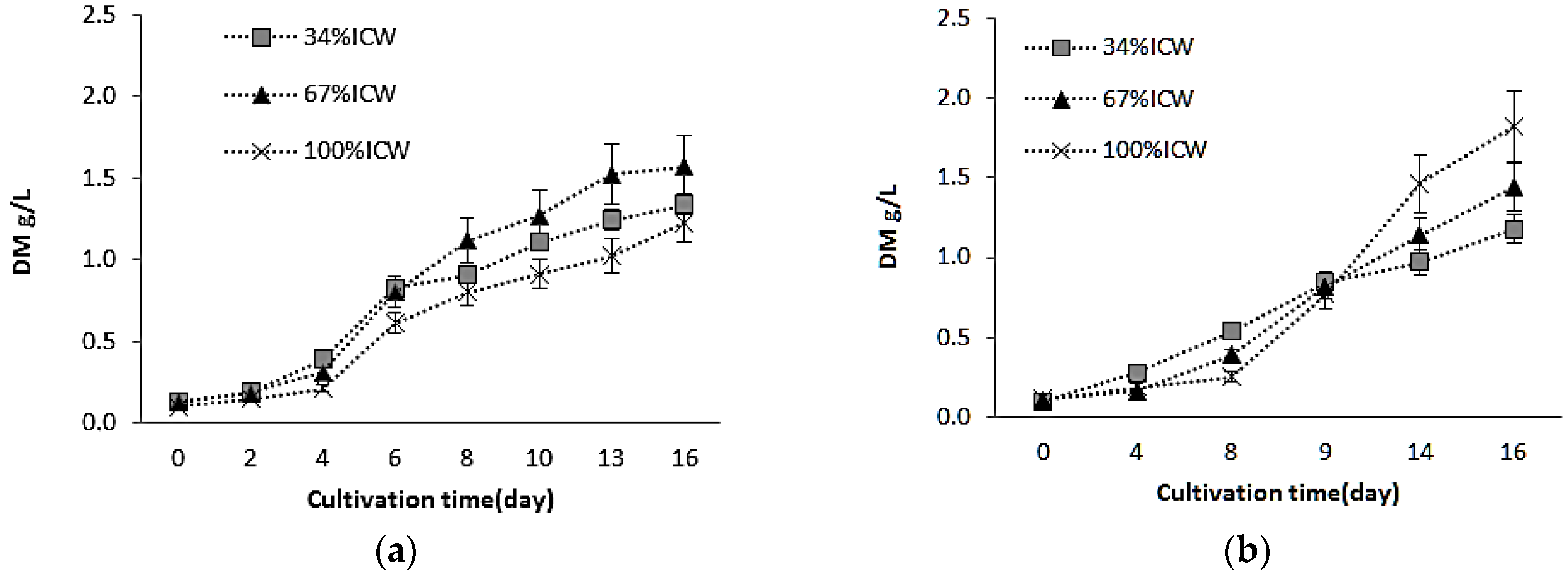
3.1. Growth and Biomass Production
3.2. Proteins and Lipids
3.2.1. Proteins
3.2.2. Lipids
3.3. Fatty Acid Composition
3.4. Tocopherols
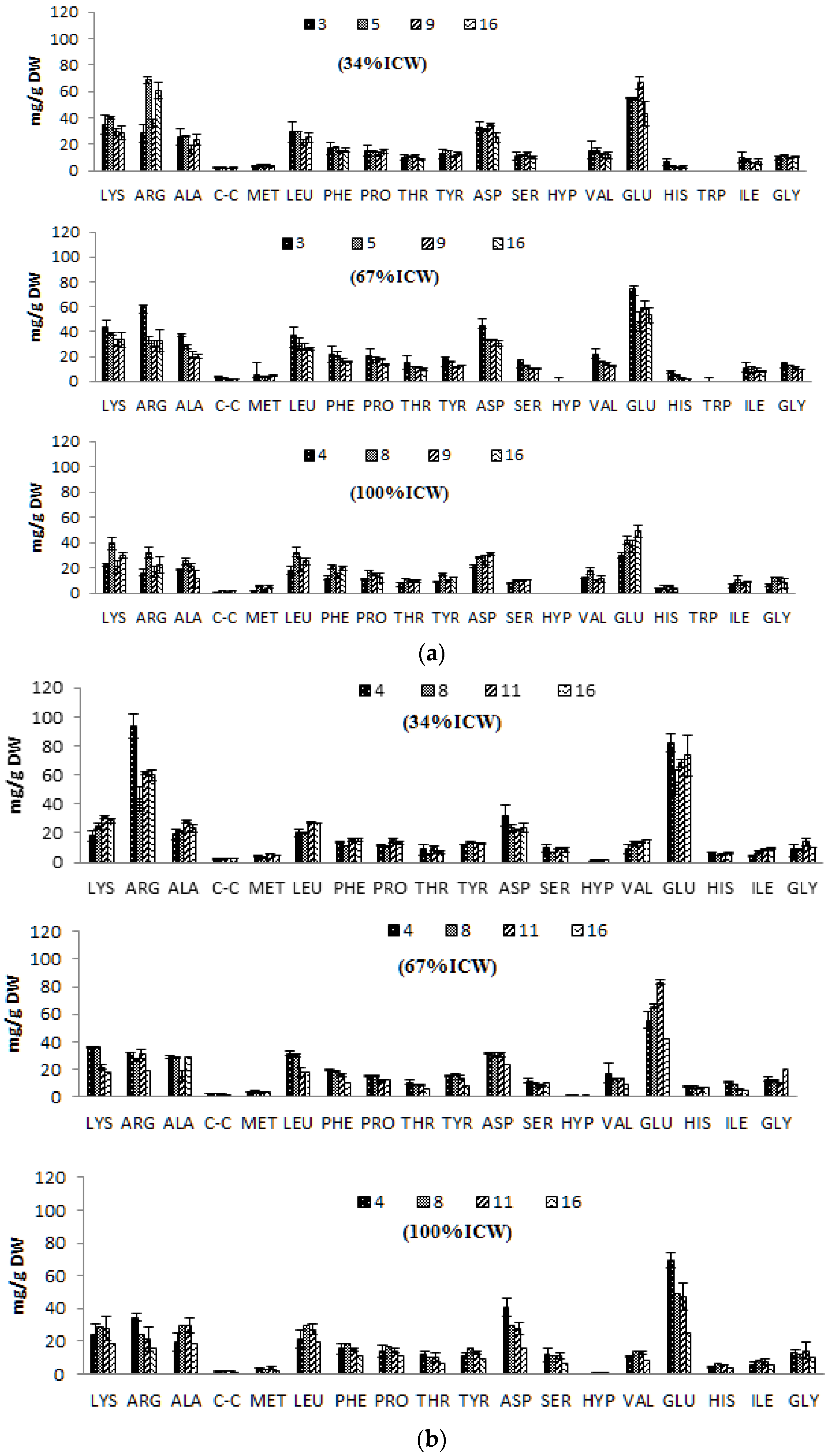
3.5. Amino Acid Composition
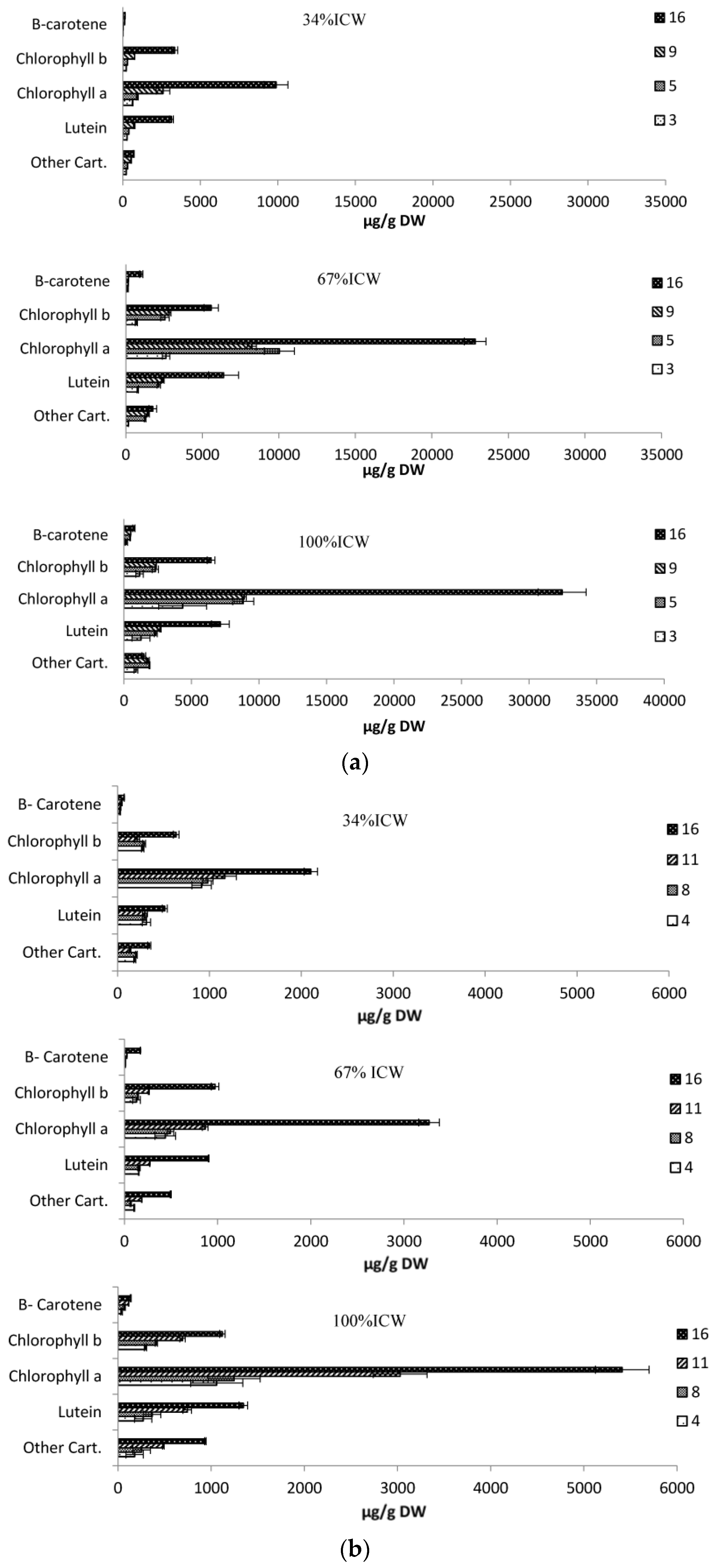
3.6. Pigments
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bishop, W.M.; Zubeck, H. Evaluation of Microalgae for use as Nutraceuticals and Nutritional Supplements. J. Nutr. Food Sci. 2012. [Google Scholar] [CrossRef]
- Griffiths, M.J.; van Hille, R.P.; Harrison, S.T. The effect of nitrogen limitation on lipid productivity and cell composition in Chlorella vulgaris. Appl. Microbiol. Biotechnol. 2014, 98, 2345–2356. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.V.; Hachemi, I.; Mäki-Arvela, P. Algal products beyond lipids: Comprehensive characterization of different products in direct saponification of green alga Chlorella sp. Algal Res. 2015, 11, 156–164. [Google Scholar] [CrossRef]
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, L.; Choubert, G.; Rema, P. Use of Chlorella vulgaris as a carotenoid source for rainbow trout: Effect of dietary lipid content on pigmentation, digestibility and retention in the muscle tissue. Aquac. Int. 1998, 279, 269–279. [Google Scholar] [CrossRef]
- Samek, D.; Mišurcová, L.; Machů, L. Whole-Cell Protein Profiles of Disintegrated Freshwater Green Algae and Cyanobacterium. J. Aquat. Food Prod. Technol. 2016, 25, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Morris, H.J.; Almarales, A.; Carrillo, O. Utilisation of Chlorella vulgaris cell biomass for the production of enzymatic protein hydrolysates. Bioresour. Technol. 2008, 99, 7723–7729. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.M.; Chen, F.; Yuan, J.P.; Chen, H. Heterotrophic production of lutein by selected Chlorella strains. J. Appl. Phycol. 1997, 9, 445–450. [Google Scholar] [CrossRef]
- Sun, Z.; Li, T.; Zhou, Z.; Jiang, Y. Microalgae as a source of lutein: Chemistry, biosynthesis, and carotenogenesis. Adv. Biochem. Eng. Biotechnol. 2016, 153, 37–58. [Google Scholar] [PubMed]
- He, P.J.; Mao, B.; Shen, C.M. Cultivation of Chlorella vulgaris on wastewater containing high levels of ammonia for biodiesel production. Bioresour. Technol. 2013, 129, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Gismondi, A.; di Pippo, F.; Bruno, L.; Antonaroli, S.; Congestri, R. Phosphorus removal coupled to bioenergy production by three cyanobacterial isolates in a biofilm dynamic growth system. Int. J. Phytoremediat. 2016, 18, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Congestri, R.; di Pippo, F.; de Philippis, R.; Buttino, I.; Paradossi, G.; Albertano, P. Seasonal succession of phototrophic biofilms in an Italian wastewater treatment plant: Biovolume, spatial structure and exopolysaccharides. Aquat. Microb. Ecol. 2006, 45, 301–312. [Google Scholar] [CrossRef]
- McHugh, S.; O’Reilly, C.; Mahony, T. Anaerobic granular sludge bioreactor technology. Rev. Environ. Sci. Biotechnol. 2003, 2, 225–245. [Google Scholar] [CrossRef]
- Safafar, H.; Hass, M.Z.; Møller, P.; Holdt, S.L.; Jacobsen, C. High-EPA Biomass from Nannochloropsis salina Cultivated in a Flat-Panel Photo-Bioreactor on a Process Water-Enriched Growth Medium. Mar. Drugs 2016, 14, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tam, N.F.Y.; Lau, P.S.; Wong, Y.S. Wastewater inorganic N and P removal by immobilised Chlorella vulgaris. Water Sci. Technol. 1994, 30, 369–374. [Google Scholar]
- Tam, N.F.Y.; Wong, Y.S. Wastewater nutrient removal by Chlorella pyrenoidosa and Scenedesmus sp. Environ. Pollut. 1989, 58, 19–34. [Google Scholar] [CrossRef]
- Liu, J.; Hu, Q. Chlorella: Industrial Production of Cell Mass and Chemicals. In Handbook of Microalgal Culture; John Wiley & Sons, Ltd.: Oxford, UK, 2013; pp. 327–338. [Google Scholar]
- Chung, K.; Ferris, D.H. Martinus Willem Beijerinck (1851–1931). J. Am. Med. Assoc. 1963, 185, 40–41. [Google Scholar]
- Griffiths, M.J.; Garcin, C.; van Hille, R.P.; Harrison, S.T.L. Interference by pigment in the estimation of microalgal biomass concentration by optical density. J. Microbiol. Methods 2011, 85, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Firestone, D.A.O.A.C. Official Methods and Recommended Practices of the AOCS, 5th ed.; American Oil Chemist’s Society: Urbana, IL, USA, 2009. [Google Scholar]
- Safafar, H.; van Wagenen, J.; Møller, P.; Jacobsen, C. Carotenoids, Phenolic Compounds and Tocopherols Contribute to the Antioxidative Properties of Some Microalgae Species Grown on Industrial Wastewater. Mar. Drugs 2015, 13, 7339–7356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, S.Y.; Kao, C.Y.; Chen, T.Y. Cultivation of microalgal Chlorella for biomass and lipid production using wastewater as nutrient resource. Bioresour. Technol. 2015, 184, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Lee, N.; Park, S. Microalgae cultivation for bioenergy production using wastewaters from a municipal waste water treatment plant, as nutritional sources. Bioresour. Technol. 2013, 131, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.M.; Cheng, L.H.; Xu, X.H. Enhanced lipid production of Chlorella vulgaris by adjustment of cultivation conditions. Bioresour. Technol. 2010, 101, 6797–6804. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q. Environmental effects on cell composition. In Handbook of Microalgal Culture; John Wiley & Sons, Ltd.: Oxford, UK, 2003; pp. 83–94. [Google Scholar]
- Leonardos, N.; Geider, R.J. Responses of elemental and biochemical composition of Chaetoceros muelleri to growth under varying light and nitrate:phosphate supply ratios and their influence on critical N:P. Limnol. Oceanogr. 2004, 49, 2105–2114. [Google Scholar] [CrossRef]
- Guccione, A.; Biondi, N.; Sampietro, G. Chlorella for protein and biofuels: From strain selection to outdoor cultivation in a Green Wall Panel photo bioreactor. Biotechnol. Biofuels 2014, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Waghmare, A.G.; Salve, M.K.; LeBlanc, J.G.; Arya, S.S. Concentration and characterization of microalgae proteins from Chlorella pyrenoidosa. Bioresour. Bioprocess. 2016, 3, 16. [Google Scholar] [CrossRef]
- Alonso, D.L.; Belarbi, E.H.; Fernandez-Sevilla, J.M. Acyl lipid composition variation related to culture age and nitrogen concentration in continuous culture of the microalga Phaeodactylum tricornutum. Phytochemistry 2000, 54, 461–471. [Google Scholar] [CrossRef]
- Guedes, A.C.; Malcata, F.X. Nutritional Value and Uses of Microalgae in Aquaculture; Muchlisin, Z., Ed.; INTECH Open Access Publisher: Rijeka, Croatia, 2012. [Google Scholar]
- Zhang, W.; Zhang, Z.; Yan, S. Effects of various amino acids as organic nitrogen sources on the growth and biochemical composition of Chlorella pyrenoidosa. Bioresour. Technol. 2015, 197, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.S.; Pal, R. Microalgae in Aquaculture: A Review with Special References to Nutritional Value and Fish Dietetics. Proc. Zool. Soc. 2015, 68, 1–8. [Google Scholar] [CrossRef]
- Kim, S.S.; Rahimnejad, S.; Kim, K.W.; Lee, K.J. Feeding ecology of the topmouth gudgeon Pseudorasbora parva (Temminck and Schlegel, 1846) in the Gelingüllü Reservoir, Turkey. Turk. J. Fish. Aquat. Sci. 2013, 13, 87–94. [Google Scholar]
- National Research Council (NRC). Nutrient Requirements of Fish; National Academy Press: Washington, DC, USA, 1993; p. 114. [Google Scholar]
- Del Campo, J.A.; Moreno, J.; Rodriguez, H. Carotenoid content of Chlorophycean microalgae: Factors determining lutein accumulation in Muriellopsis sp. (Chlorophyta). J. Biotechnol. 2000, 76, 51–59. [Google Scholar] [CrossRef]
- Cordero, B.F.; Obraztsova, I.; Couso, I. Enhancement of lutein production in Chlorella sorokiniana (chorophyta) by improvement of culture conditions and random mutagenesis. Mar. Drugs 2011, 9, 1607–1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| (a) Chemical Composition of Industrial Process Water | ||
| Item | Unit | Amount |
| pH | - | 8.1 |
| Suspended solids | mg/L | 20 |
| Total N | mg/L | 190 |
| Ammonia + ammonium-N | mg/L | 150 |
| Nitrite + nitrate | mg/L | <0.1 |
| Total P | mg/L | 11 |
| Sulphate | mg/L | 3.6 |
| Total cyanide | µg/L | 2.5 |
| Total Alkalinity | mmol/L | 62.5 |
| EDTA (Ethylenediaminetetraacetic acid) | mg/L | <0.5 |
| Sodium (Na) | mg/L | 1500 |
| Cadmium (Cd) | µg/L | <0.05 |
| Copper (Cu) | µg/L | 3.4 |
| Iron (Fe) | mg/l | 0.23 |
| Cobolt (Co) | µg/L | <0.5 |
| (b) Type and Amounts of Nitrogen and Phosphorus in Each Growth Medium | ||
| Growth Media * | -N | Total P |
| 34% ICW | 50 | 3.5 |
| 67% ICW | 100 | 6.8 |
| 100% ICW | 150 | 11 |
| (a) Chlorella vulgaris | |||
| Lipids % DW | |||
| Cultivation Time (days) | ICW% | ||
| 34 | 67 | 100 | |
| 3 | 3.51 ± 0.2 a, x | 4.63 ± 0.0 a, x | 4.60 ± 0.2 a, y |
| 5 | 5.61 ± 0.1 b, x | 5.80 ± 0. 3 b, x | 7.20 ± 0. 1 b, y |
| 9 | 9.70 ± 0.3 c, y | 9.00 ± 0.1 c, x | 9.87 ± 0.1 c, y |
| 16 | 17.0 ± 0.1 d, z | 11.4 ± 0. 4 d, x | 13.0 ± 0. 4 d, y |
| Proteins % DW | |||
| Cultivation Time (days) | ICW% | ||
| 34 | 67 | 100 | |
| 3 | 42.3 ± 2.8 a, x | 44.8 ± 4.1 a, y | 42.4 ± 1.1 a, x |
| 5 | 55.1 ± 1.4 c, x | 54.4 ± 3.3 c, x | 55.2 ± 1.8 d, x |
| 9 | 49.9 ± 1.0 b, xy | 47.7 ± 2. 3 b, x | 51.1 ± 0.9 c, x |
| 16 | 42.0 ± 0.9 a, x | 44.4 ± 1.3 a, y | 47.4 ± 2.1 d, z |
| (b) Chlorella pyrenoidosa | |||
| Lipids % DW | |||
| Cultivation Time (days) | ICW% | ||
| 34 | 67 | 100 | |
| 4 | 2.51 ± 0.2 a, x | 5.91 ± 0.0 a, y | 5.75 ± 0.1 a, y |
| 8 | 3.52 ± 0.1 b, x | 8.80 ± 0.1 b. y | 8.20 ± 0.2 b, y |
| 11 | 9.30 ± 0.1 c, y | 10.0 ± 0.3 c, z | 7.87 ± 0. 3 b, x |
| 16 | 17.6 ± .0.1 d, z | 13.1 ± 0.5 d, y | 10.0 ± 0.3 c, x |
| Proteins % DW | |||
| Cultivation Time (days) | ICW% | ||
| 34 | 67 | 100 | |
| 4 | 52.3 ± 2.2 c, x | 60.3 ± 3.7 c, y | 53.1 ± 1.2 a, x |
| 8 | 55.8 ± 1.0 d, x | 65.2 ± 1.3 d, z | 58.2 ± 3.5 b, y |
| 11 | 47.8 ± 1.9 ab, x | 57.4 ± 2.0 b, y | 59.1 ± 3.1 bc, z |
| 16 | 46.3 ± 2.8 a, x | 54.6 ± 2.7 a, y | 57.8 ± 3.2 b, z |
| (a) Chlorella vulgaris | ||||||||||||
| ICW % | 34 | 67 | 100 | |||||||||
| Fatty Acid | 3 | 5 | 9 | 16 | 3 | 5 | 9 | 16 | 3 | 5 | 9 | 16 |
| 14:00 | 1.03 ± 0.34 | 0.57 ± 0.03 | 0.61 ± 0.07 | 0.47 ± 0.01 | 0.81 ± 0.22 | 1.49 ± 0.62 | 1.51 ± 0.06 | 0.29 ± 0.04 | 0.92 ± 0.04 | 1.97 ± 0.01 | 1.60 ± 0.05 | 0.40 ± 0.01 |
| 14:01 | 0.95 ± 0.00 | 0.32 ± 0.02 | 0.53 ± 0.03 | 0.36 ± 0.01 | 0.37 ± 0.01 | 0.27 ± 0.17 | 0.36 ± 0.04 | 0.39 ± 0.01 | 0.53 ± 0.01 | 0.28 ± 0.00 | 0.25 ± 0.04 | 0.38 ± 0.00 |
| 15:00 | 0.20 ± 0.00 | 0.17 ± 0.01 | 0.17 ± 0.00 | 0.11 ± 0.01 | 0.18 ± 0.01 | 0.16 ± 0.01 | 0.14 ± 0.01 | 0.14 ± 0.00 | 0.23 ± 0.00 | 0.25 ± 0.02 | 0.18 ± 0.01 | 0.15 ± 0.01 |
| 16:00 | 18.1 ± 2.76 | 18.4 ± 0.11 | 20.7 ± 0.04 | 23.3 ± 0.07 | 19.1 ± 0.06 | 19. 0 ± 0.48 | 17.1 ± 0.01 | 17.7 ± 0.04 | 24.0 ± 0.17 | 23.1 ± 0.05 | 21.2 ± 0.01 | 23.1 ± 0.11 |
| 16:1 (n-7) | 1.88 ± 0.56 | 1.34 ± 0.04 | 1.87 ± 0.13 | 7.15 ± 0.01 | 2.56 ± 0.20 | 0.94 ± 0.01 | 1.62 ± 0.10 | 17.6 ± 0.01 | 5.28 ± 0.01 | 3.58 ± 0.01 | 1.43 ± 0.01 | 16.4 ± 0.08 |
| 16:2 (n-4) | 3.46 ± 0.74 | 4.56 ± 0.01 | 4.76 ± 0.08 | 2.51 ± 0.05 | 4.66 ± 0.04 | 5.24 ± 0.12 | 6.18 ± 0.01 | 1.96 ± 0.01 | 3.00 ± 0.04 | 5.90 ± 0.04 | 6.72 ± 0.04 | 2.14 ± 0.01 |
| 16:4(n-1) | 16.2 ± 2.39 | 15.2 ± 0.01 | 10.9 ± 0.23 | 10.9 ± 0.06 | 16.4 ± 0.05 | 16.1 ± 0.35 | 12.4 ± 0.04 | 10.7 ± 0.11 | 12.4 ± 0.11 | 12.7 ± 0.11 | 9.15 ± 0.11 | 8.2 ± 0.19 |
| 18:00 | 1.20 ± 0.10 | 1.03 ± 0.04 | 1.01 ± 0.01 | 2.04 ± 0.01 | 1.14 ± 0.01 | 0.45 ± 0.02 | 0.46 ± 0.01 | 1.50 ± 0.05 | 1.58 ± 0.00 | 0.63 ± 0.01 | 0.85 ± 0.00 | 3.14 ± 0.02 |
| 18:1 (n-9) | 5.91 ± 0.88 | 8.33 ± 0.11 | 11.4 ± 0.30 | 13.2 ± 0.08 | 4.52 ± 0.06 | 5.53 ± 0.13 | 7.1 ± 0.08 | 11.9 ± 0.06 | 5.82 ± 0.02 | 4.51 ± 0.01 | 14.7 ± 0.01 | 13.2 ± 0.04 |
| 18:1 (n-7) | 4.01 ± 0.76 | 3.58 ± 0.01 | 8.26 ± 0.25 | 9.37 ± 0.06 | 3.25 ± 0.01 | 1.35 ± 0.01 | 1.89 ± 0.01 | 7.39 ± 0.01 | 3.99 ± 0.03 | 3.16 ± 0.01 | 2.44 ± 0.00 | 7.23 ± 0.02 |
| 18:2 (n-6) | 7.59 ± 1.07 | 9.73 ± 0.01 | 10.9 ± 0.17 | 7.37 ± 0.01 | 9.68 ± 0.03 | 12.7 ± 0.31 | 17.8 ± 0.06 | 4.72 ± 0.03 | 9.17 ± 0.02 | 12.5 ± 0.01 | 16.7 ± 0.03 | 6.42 ± 0.03 |
| 18:3 (n-3) | 35. 9 ± 4.88 | 35.5 ± 0.13 | 27.6 ± 0.52 | 22.1 ± 0.10 | 35.9 ± 0.25 | 36.2 ± 0.88 | 33.0 ± 0.08 | 24.3 ± 0.19 | 31.7 ± 0.10 | 31.5 ± 0.11 | 24.3 ± 0.15 | 16.7 ± 0.18 |
| 20:4 (n-6) | 1.07 ± 0.13 | 0.28 ± 0.06 | 0.37 ± 0.02 | 0.20 ± 0.01 | 0.49 ± 0.02 | 0.11 ± 0.10 | 0.05 ± 0.00 | 0.46 ± 0.01 | 0.44 ± 0.03 | 0.13 ± 0.02 | 0.06 ± 0.00 | 0.97 ± 0.01 |
| 20:5 (n-3) | 0.81 ± 0.02 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 22:5 (n-3) | 1.72 ± 0.25 | 0.79 ± 0.05 | 0.77 ± 0.02 | 0.79 ± 0.01 | 0.81 ± 0.03 | 0.21 ± 0.10 | 0.12 ± 0.01 | 0.76 ± 0.01 | 0.83 ± 0.01 | 0.34 ± 0.01 | 0.18 ± 0.02 | 1.44 ± 0.03 |
| ∑Sat | 20.3 ± 3.20 | 20.2 ± 0.20 | 22.5 ± 0.12 | 25.3 ± 0.10 | 21.3 ± 0.30 | 21.1 ± 1.12 | 19.2 ± 0.08 | 19.6 ± 0.13 | 26.7 ± 0.21 | 25.2 ± 0.07 | 23.8 ± 0.07 | 26.8 ± 0.20 |
| ∑n-3 | 38.4 ± 5.15 | 36.3 ± 0.18 | 28.4 ± 0.54 | 22.3 ± 0.11 | 36.7 ± 0.28 | 36.5 ± 0.98 | 33.1 ± 0.08 | 25.0 ± 0.19 | 32.6 ± 0.11 | 31.9 ± 0.12 | 24.5 ± 0.15 | 18.1 ± 0.21 |
| ∑n-6 | 8.60 ± 1.20 | 10.0 ± 0.08 | 11.2 ± 0.19 | 7.50 ± 0.01 | 10.1 ± 0.05 | 12.8 ± 0.41 | 17.9 ± 0.06 | 5.20 ± 0.01 | 9.60 ± 0.05 | 12.6 ± 0.04 | 16.8 ± 0.03 | 7.30 ± 0.04 |
| (b) Chlorella pyrenoidosa | ||||||||||||
| ICW % | 34 | 67 | 100 | |||||||||
| Fatty Acid | 4 | 8 | 11 | 16 | 4 | 8 | 11 | 16 | 4 | 8 | 11 | 16 |
| 14:00 | 0.37 ± 0.08 | 0.33 ± 0.10 | 0.49 ± 0.02 | 0.68 ± 0.10 | 0.71 ± 0.26 | 1.06 ± 0.62 | 0.30 ± 0.02 | 0.85 ± 0.03 | 1.50 ± 0.01 | 0.83 ± 0.25 | 2.21 ± 0.08 | 1.79 ± 0.15 |
| 14:01 | 0.10 ± 0.01 | 0.16 ± 0.01 | 0.36 ± 0.02 | 0.52 ± 0.01 | 0.21 ± 0.01 | 0.39 ± 0.01 | 0.79 ± 0.63 | 0.37 ± 0.05 | 0.24 ± 0.02 | 0.52 ± 0.02 | 0.53 ± 0.02 | 0.67 ± 0.01 |
| 15:00 | 0.24 ± 0.02 | 0.08 ± 0.01 | 0.13 ± 0.06 | 0.27 ± 0.01 | 0.25 ± 0.01 | 0.21 ± 0.00 | 0.45 ± 0.37 | 0.16 ± 0.00 | 0.24 ± 0.03 | 0.16 ± 0.03 | 0.30 ± 0.09 | 0.15 ± 0.02 |
| 16:00 | 13.9 ± 0.09 | 17.3 ± 0.23 | 17.8 ± 1.86 | 16.8 ± 0.23 | 20.6 ± 0.21 | 20.7 ± 0.04 | 17.4 ± 1.22 | 19.3 ± 0.08 | 26.1 ± 0.05 | 21.7 ± 0.26 | 19.3 ± 2.43 | 20.3 ± 0.35 |
| 16:1 (n-7) | 1.54 ± 0.02 | 0.86 ± 0.28 | 1.00 ± 0.11 | 1.22 ± 0.28 | 0.98 ± 0.29 | 1.20 ± 0.14 | 1.13 ± 0.03 | 1.71 ± 1.07 | 1.30 ± 0.01 | 0.97 ± 0.04 | 1.56 ± 0.01 | 1.13 ± 0.04 |
| 16:2 (n-4) | 3.24 ± 0.03 | 2.83 ± 0.04 | 2.60 ± 0.30 | 2.65 ± 0.04 | 5.23 ± 0.07 | 4.49 ± 0.14 | 5.75 ± 0.33 | 5.82 ± 0.05 | 2.85 ± 0.02 | 7.04 ± 0.08 | 4.52 ± 0.49 | 3.52 ± 0.08 |
| 16:3 (n-4) | 0.11 ± 0.01 | 0.13 ± 0.02 | 0.35 ± 0.24 | 0.00 ± 0.01 | 0.10 ± 0.01 | 0.27 ± 0.06 | 0.26 ± 0.08 | 0.09 ± 0.00 | ND | 0.18 ± 0.04 | 0.58 ± 0.28 | 0.30 ± 0.13 |
| 16:4(n-1) | 11.0 ± 0.37 | 11.0 ± 0.10 | 10.5 ± 1.35 | 11.6 ± 0.10 | 12.2 ± 0.50 | 12.7 ± 0.31 | 13.7 ± 0.94 | 13.9 ± 0.28 | 10.6 ± 0.09 | 10.1 ± 0.11 | 12.7 ± 1.39 | 12.0 ± 0.24 |
| 17:00 | ND | 0.10 ± 0.01 | 0.17 ± 0.01 | 0.10 ± 0.06 | 0.10 ± 0.14 | ND | ND | ND | ND | 0.11 ± 0.01 | ND | ND |
| 18:00 | 3.07 ± 0.01 | 0.85 ± 0.03 | 1.17 ± 0.17 | 0.80 ± 0.03 | 0.95 ± 0.05 | 1.08 ± 0.01 | 0.56 ± 0.05 | 0.44 ± 0.01 | 1.81 ± 0.01 | 0.62 ± 0.04 | 1.37 ± 0.18 | 0.98 ± 0.06 |
| 18:1 (n-9) | 15.0 ± 0.18 | 28.2 ± 0.84 | 19.3 ± 1.22 | 18.3 ± 0.84 | 7.4 ± 0.11 | 10.6 ± 0.03 | 10.7 ± 0.91 | 5.26 ± 0.04 | 6.51 ± 0.02 | 7.67 ± 0.01 | 8.48 ± 0.40 | 8.03 ± 0.16 |
| 18:1 (n-7) | 7.98 ± 0.07 | 2.70 ± 0.03 | 3.20 ± 0.30 | 4.52 ± 0.03 | 3.36 ± 0.22 | 2.50 ± 0.02 | 2.36 ± 0.21 | 1.80 ± 0.07 | 6.89 ± 0.00 | 2.02 ± 0.01 | 3.14 ± 0.04 | 3.07 ± 0.03 |
| 18:2 (n-6) | 12.3 ± 0.03 | 10.4 ± 0.19 | 11.9 ± 1.29 | 11.7 ± 0.19 | 14.9 ± 0.06 | 11.6 ± 0.38 | 13.3 ± 1.06 | 15.4 ± 0.18 | 9.8 ± 0.01 | 16.8 ± 0.20 | 11.0 ± 1.17 | 11.8 ± 0.28 |
| 18:3 (n-3) | 30.5 ± 0.27 | 24.2 ± 0.08 | 27.7 ± 3.08 | 29 ± 0.08 | 32.0 ± 0.33 | 29.1 ± 0.84 | 32.0 ± 2.47 | 33.3 ± 0.10 | 31.3 ± 0.11 | 30.1 ± 0.28 | 30.8 ± 3.36 | 33.9 ± 0.86 |
| 20:1 (n-9) | ND | ND | ND | ND | ND | 0.16 ± 0.01 | ND | ND | ND | ND | 0.17 ± 0.01 | 0.09 ± 0.01 |
| 20:4 (n-6) | 0.25 ± 0.03 | 0.14 ± 0.01 | 0.96 ± 0.14 | 0.39 ± 0.01 | 0.19 ± 0.02 | 0.87 ± 0.08 | 0.24 ± 0.01 | 0.26 ± 0.04 | 0.25 ± 0.01 | 0.24 ± 0.02 | 0.79 ± 0.09 | 0.47 ± 0.01 |
| 20:5 (n-3) | ND | 0.23 ± 0.02 | 0.60 ± 0.30 | 0.65 ± 0.02 | 0.33 ± 0.01 | 1.32 ± 0.04 | 0.33 ± 0.03 | 0.45 ± 0.16 | ND | 0.33 ± 0.01 | 1.11 ± 0.06 | 0.71 ± 0.01 |
| 22:5 (n-3) | 0.43 ± 0.02 | 0.32 ± 0.05 | 1.67 ± 0.22 | 0.83 ± 0.05 | 0.33 ± 0.02 | 1.62 ± 0.07 | 0.44 ± 0.04 | 0.76 ± 0.12 | 0.53 ± 0.04 | 0.45 ± 0.03 | 1.32 ± 0.12 | 0.94 ± 0.10 |
| ∑Sat | 17.6 ± 0.18 | 18.7 ± 0.43 | 19.7 ± 2.09 | 18.5 ± 0.43 | 22.6 ± 0.66 | 23.1 ± 0.67 | 18.7 ± 1.66 | 20.8 ± 0.09 | 29.6 ± 0.10 | 21.4 ± 0.59 | 23.2 ± 2.78 | 24.2 ± 0.57 |
| ∑n-3 | 31.0 ± 0.29 | 24.7 ± 0.16 | 29.9 ± 3.59 | 30.4 ± 0.16 | 32.7 ± 0.33 | 32.0 ± 0.95 | 32.8 ± 2.53 | 34.5 ± 0.69 | 31.8 ± 0.15 | 30.92 ± 0.29 | 33.2 ± 3.54 | 35.5 ± 0.98 |
| ∑n-6 | 12.5 ± 0.06 | 10.5 ± 0.19 | 12.9 ± 1.44 | 12.1 ± 0.19 | 15.1 ± 0.06 | 12.4 ± 0.46 | 13.6 ± 1.07 | 15.6 ± 0.22 | 10.1 ± 0.02 | 17.0 ± 0.22 | 11.8 ± 1.27 | 12.3 ± 0.28 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safafar, H.; Uldall Nørregaard, P.; Ljubic, A.; Møller, P.; Løvstad Holdt, S.; Jacobsen, C. Enhancement of Protein and Pigment Content in Two Chlorella Species Cultivated on Industrial Process Water. J. Mar. Sci. Eng. 2016, 4, 84. https://doi.org/10.3390/jmse4040084
Safafar H, Uldall Nørregaard P, Ljubic A, Møller P, Løvstad Holdt S, Jacobsen C. Enhancement of Protein and Pigment Content in Two Chlorella Species Cultivated on Industrial Process Water. Journal of Marine Science and Engineering. 2016; 4(4):84. https://doi.org/10.3390/jmse4040084
Chicago/Turabian StyleSafafar, Hamed, Patrick Uldall Nørregaard, Anita Ljubic, Per Møller, Susan Løvstad Holdt, and Charlotte Jacobsen. 2016. "Enhancement of Protein and Pigment Content in Two Chlorella Species Cultivated on Industrial Process Water" Journal of Marine Science and Engineering 4, no. 4: 84. https://doi.org/10.3390/jmse4040084