Short-Term Nitrogen and Phosphorus Release during the Disturbance of Surface Sediments: A Case Study in an Urbanised Estuarine System (Gold Coast Broadwater, Australia)
Abstract
:1. Introduction
2. Materials and Methods
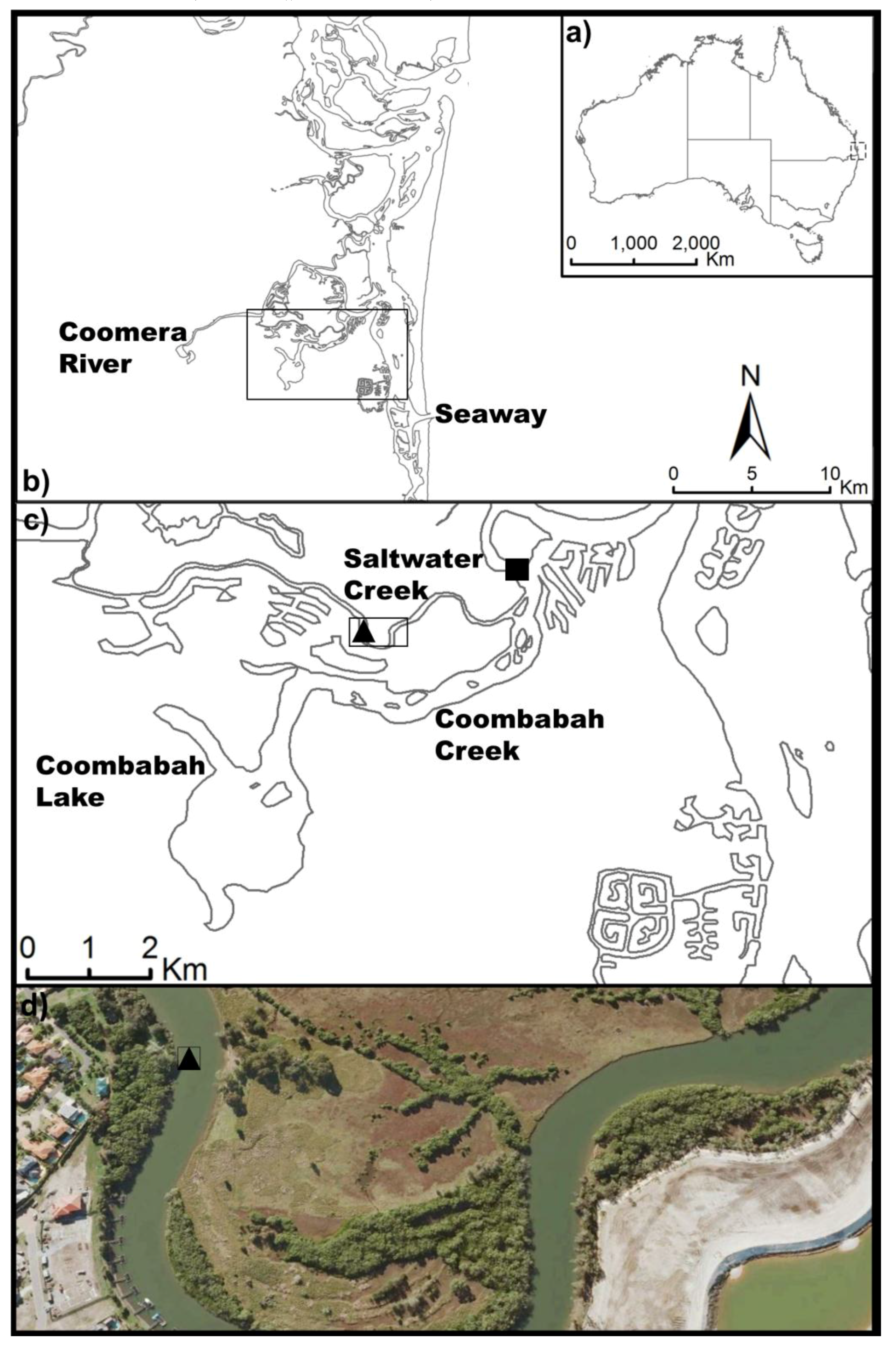
2.1. Study Location
2.2. Sample Collection
2.3. Sediment Resuspension Flux Measurements
2.4. Sample Handling and Analytical Techniques
2.5. Data Presentation and Statistical Analysis
3. Results and Discussion
3.1. Background Sediment and Water Column Conditions
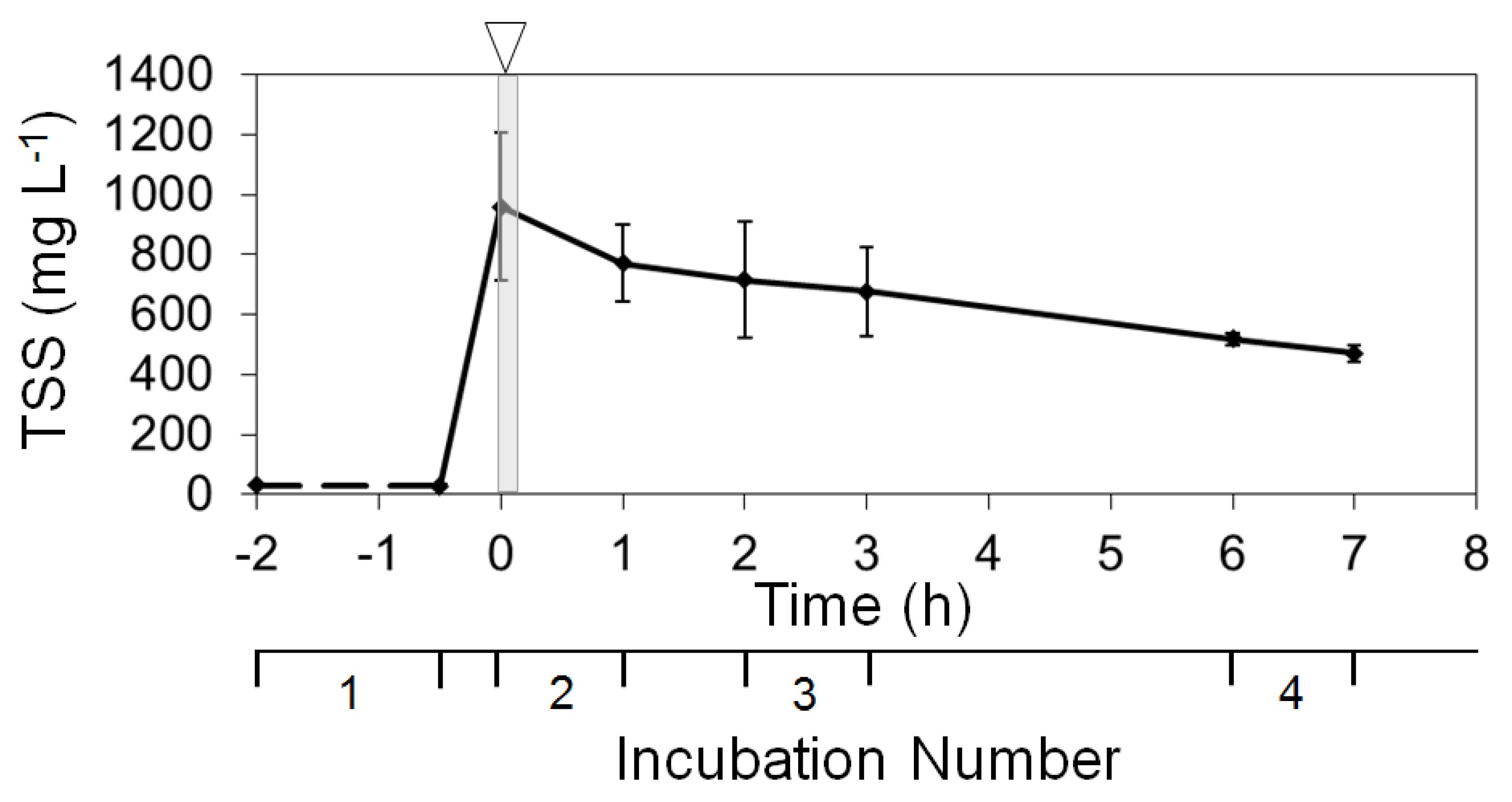
3.2. Sediment Disturbance Simulation and Sediment Incubations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nixon, S.W. Remineralization and nutrient cycling in coastal marine ecosystems. In Estuaries and Nutrients; Neilson, B.J., Cronin, L.E., Eds.; Humana Press Inc.: Clifton, NJ, USA, 1981; pp. 111–138. [Google Scholar]
- Dunn, R.J.K.; Welsh, D.T.; Teasdale, P.R.; Lee, J.; Lemckert, C.J.; Meziane, T. Investigating the distribution and sources of organic matter in surface sediment of Coombabah Lake (Australia) using elemental, isotopic and fatty acid biomarkers. Cont. Shelf. Res. 2008, 28, 2525–2549. [Google Scholar] [CrossRef]
- Lijklema, L.; Koelmans, A.A.; Portielje, R. Water quality impacts of sediment pollution and the role of early diagenesis. Water Sci. Technol. 1993, 28, 1–12. [Google Scholar]
- Eyre, B.D.; Ferguson, A.J.P.; Webb, A.; Maher, D.; Oakes, J.M. Denitrification, N-fixation and nitrogen and phosphorus fluxes in different benthic habitats and their contribution to the nitrogen and phosphorus budgets of a shallow oligotrophic sub-tropical coastal system (southern Moreton Bay, Australia). Biogeochemistry 2010, 102, 111–133. [Google Scholar] [CrossRef]
- Dunn, R.J.K.; Welsh, D.T.; Jordan, M.A.; Waltham, N.J.; Lemckert, C.J.; Teasdale, P.R. Benthic metabolism and nitrogen dynamics in a sub-tropical coastal lagoon: Microphytobenthos stimulate nitrification and nitrate reduction through photosynthetic oxygen evolution. Estuar. Coast. Shelf Sci. 2012, 113, 272–282. [Google Scholar] [CrossRef]
- Graf, G.; Rosenberg, R. Biodeposition and bioresuspension: A review. J. Mar. Syst. 1997, 11, 269–278. [Google Scholar] [CrossRef]
- Couceiro, F.; Fones, G.R.; Thompson, C.E.L.; Statham, P.J.; Sivyer, D.B.; Parker, R.; Kelly-Gerreyn, B.A.; Amos, C.L. Impact of resuspension of cohesive sediments at the Oyster Grounds (North Sea) on nutrient exchange across the sediment–water interface. Biogeochemistry 2013, 1331, 37–52. [Google Scholar] [CrossRef]
- Eggleton, J.; Thomas, K.V. A review of factors affecting the release and bioavailability of contaminants during sediment disturbance events. Environ. Int. 2004, 30, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, R.W.; Hoyer, M.V.; Canfield, D.E., Jr. The potential for wave disturbance in shallow Florida Lakes. Lake Reserv. Manag. 2000, 16, 281–291. [Google Scholar] [CrossRef]
- Beachler, M.M.; Hill, D.F. Stirring up trouble? Resuspension of bottom sediments by recreational watercraft. Lake Reserv. Manag. 2003, 19, 15–25. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, Q.; Xu, D.; Lin, J.; Cheng, S.; Wu, Z. Effects of sediment dredging on water quality and zooplankton community structure in a shallow of eutrophic lake. J. Environ. Sci. 2010, 22, 218–224. [Google Scholar] [CrossRef]
- Lohrer, A.M.; Wetz, J.J. Dredging-induced nutrient release from sediments to the water column in a southeastern saltmarsh tidal creek. Mar. Pollut. Bull. 2003, 46, 1156–1163. [Google Scholar] [CrossRef]
- Nayar, S.; Miller, D.J.; Hunt, A.; Goh, B.P.L.; Chou, L.M. Environmental effects of dredging on sediment nutrients, carbon and granulometry in a tropical estuary. Environ. Monit. Assess. 2007, 127, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.D.; Wu, C.X.; Liu, J.T.; Wang, H.G.; Ao, H.Y. The effects of dredging on nitrogen balance in sediment-water microcosms and implications to dredging projects. Ecol. Eng. 2013, 52, 167–174. [Google Scholar] [CrossRef]
- Wulff, A.; Sundbaeck, K.; Nilsson, C.; Carlson, L.; Jönsson, B. Effect of sediment load on the microbenthic community of a shallow-water sandy sediment. Estuaries 1997, 20, 547–558. [Google Scholar] [CrossRef]
- Cabrita, M.T. Phytoplankton community indicators of changes associated with dredging in the Tagus estuary (Portugal). Environ. Pollut. 2014, 191, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Tengberg, A.; Almroth, E.; Hall, P. Resuspension and its effects on organic carbon recycling and nutrient exchange in coastal sediments: In situ measurements using new experimental technology. J. Exp. Mar. Biol. Ecol. 2003, 285–286, 119–142. [Google Scholar] [CrossRef]
- Dyer, K.R. Sediment transport processes in estuaries. In Geomorphology and Sedimentology of Estuaries; Perillo, G.M.E., Ed.; Elsevier Science B.V.: Amsterdam, The Netherlands, 1995; pp. 423–450. [Google Scholar]
- Sloth, N.P.; Riemann, B.; Nielsen, L.P.; Blackburn, T. Resilience of pelagic and benthic microbial communities to sediment resuspension in a coastal ecosystem, Knebel Vig, Denmark. Estuar. Coast. Shelf Sci. 1996, 42, 405–415. [Google Scholar] [CrossRef]
- Reddy, K.; Fisher, M.; Wang, Y.; White, J.R.; James, R.T. Potential effects of sediment dredging on internal phosphorus loading in a shallow, subtropical lake. Lakes Reserv. Manag. 2007, 23, 27–38. [Google Scholar] [CrossRef]
- Wu, M.; Sun, X.-M.; Huang, S.-L.; Tang, X.-Q.; Scholz, M. Laboratory analyses of nutrient release processes from Haihe River sediment. Int. J. Sediment Res. 2012, 27, 61–72. [Google Scholar] [CrossRef]
- Ailstock, M.S.; Hornor, S.G.; Norman, C.M.; Davids, E.M. Resuspension of sediments by watercraft operated in shallow water habitats of Anne Arundel County, Maryland. J. Coast. Res. 2002, 37, 18–32. [Google Scholar]
- Waltham, N.J.; Barry, M.; McAlister, T.; Weber, T.; Groth, D. Protecting the green behind the gold: Catchment-wide restoration efforts necessary to achieve nutrient and sediment load reduction targets in Gold Coast City, Australia. Environ. Manag. 2014, 54, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Dunn, R.J.K.; Waltham, N.J.; Benfer, N.P.; King, B.A.; Lemckert, C.J.; Zigic, S. Gold Coast Broadwater: Southern Moreton Bay, Southeast Queensland (Australia). In Estuaries of Australia in 2050 and beyond; Wolanski, E., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 93–109. [Google Scholar]
- Benfer, N.P.; King, B.A.; Lemckert, C.J. Salinity observations in a subtropical estuarine system on the Gold Coast, Australia. J. Coast. Res. 2007, 50, 646–651. [Google Scholar]
- Webster, T.; Lemckert, C. Sediment resuspension within a microtidal estuary/embayment and the implication to channel management. J. Coast. Res. 2002, 36, 753–759. [Google Scholar]
- Dunn, R.J.K.; Robertson, D.; Teasdale, P.R.; Waltham, N.J.; Welsh, D.T. Benthic metabolism and nitrogen dynamics in an urbanised tidal creek: Domination of DNRA over denitrification as a nitrate reduction pathway. Estuar. Coast. Shelf Sci. 2013, 131, 271–281. [Google Scholar] [CrossRef]
- Tomlinson, R.; Teasdale, P.; Connolly, R.; Lemckert, C.; Robertson, A.; Preston, K.; O′Halloran, K.; Webster, T.; Burns, P.; Cheung, L.; et al. Saltwater Creek Environmental Inventory: Scoping Study Report and Recommendations; Research Report No. 6; Griffith University Centre for Coastal Management: Gold Coast, Australia, 2006. [Google Scholar]
- Lee, S.Y.; Connolly, R.M.; Dale, P.E.R.; Dunn, R.J.K.; Knight, J.M.; Lemckert, C.J.; McKinnon, S.; Powell, B.; Teasdale, P.R.; Welsh, D.T.; Young, R. The Impact of Urbanisation on Coastal Wetlands: A Case Study of Coombabah Lake, Southeast Queensland; Technical Report No. 54; Cooperative Research Centre for Coastal Zone, Estuary and Waterway Management: Gold Coast, Australia, 2006. [Google Scholar]
- Dunn, R.J.K.; Ali, A.; Lemckert, C.J.; Teasdale, P.R.; Welsh, D.T. Short-term variability of physico-chemical parameters and the estimated transport of filterable nutrients and chlorophyll-a in the urbanised Coombabah Lake and Coombabah Creek system, southern Moreton Bay, Australia. J. Coast. Res. 2007, 50, 1062–1068. [Google Scholar]
- Dunn, R.J.K.; Lemckert, C.J.; Teasdale, P.R.; Welsh, D.T. Distribution of nutrients in surface and sub-surface sediments of Coombabah Lake, southern Moreton Bay (Australia). Mar. Pollut. Bull. 2007, 54, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Nizzoli, D.; Welsh, D.T.; Viaroli, P. Seasonal nitrogen and phosphorus dynamics during benthic clam and suspended mussel cultivation. Mar. Pollut. Bull. 2011, 62, 1276–1287. [Google Scholar] [CrossRef] [PubMed]
- Azzoni, R.; Giordani, G.; Bartoli, M.; Welsh, D.T.; Viaroli, P. Iron, sulphur and phosphorus cycling in the rhizosphere sediments of a eutrophic Ruppia cirrhosa meadow (Valle Smarlacca, Italy). J. Sea Res. 2001, 45, 15–26. [Google Scholar] [CrossRef]
- Burdige, D.J. Geochemistry of Marine Sediments; Princeton University Press: Princeton, NJ, USA, 2006. [Google Scholar]
- Corbett, D.R. Resuspension and estuarine nutrient cycling: Insights from the Neuse River Estuary. Biogeosciences 2010, 7, 3289–3300. [Google Scholar] [CrossRef]
- Fanning, K.A.; Carder, K.L.; Betzer, P.R. Sediment resuspension by coastal waters: A potential mechanism for nutrient re-cycling on the ocean’s margins. Deep Sea Res. 1982, 29, 953–965. [Google Scholar] [CrossRef]
- Kristensen, P.; Sondergaard, M.; Jeppesen, E. Resuspension in a shallow eutrophic lake. Hydrobiologia 1992, 228, 101–109. [Google Scholar] [CrossRef]
- Sondergaard, M.; Kristensen, P.; Jeppesen, E. Phosphorus release from resuspended sediment in the shallow and wind-exposed Lake Arreso, Denmark. Hydrobiologia 1992, 228, 91–99. [Google Scholar] [CrossRef]
- De Jong, V.N.; van Beusekom, J.E.E. Wind- and tide-induced resuspension of sediment and microphytobenthos from tidal flats in the Ems estuary. Limnol. Oceanog. 1995, 40, 766–778. [Google Scholar]
- Zhong, J.; Liu, G.; Fan, C.; Li, B.; Ding, S. Environmental effect of sediment dredging in lake. II. The role of sediment dredging in reducing internal nitrogen release. J. Lake Sci. 2009, 21, 335–344. [Google Scholar]
- Cornwell, J.C.; Owens, M.S. Quantifying sediment nitrogen release associated with estuarine dredging. Aquat. Geochem. 2011, 17, 499–517. [Google Scholar] [CrossRef]
- Berner, R.A. Early Diagenesis: A Theoretical Approach; Princeton University Press: Princeton, NJ, USA, 1980. [Google Scholar]
- Aller, R.C. Diagenetic processes near the sediment-water interface of Long Island Sound. Adv. Geophys. 1980, 22, 237–350. [Google Scholar]
- Krom, M.D.; Berner, R.A. The diagenesis of phosphorus in a nearshore marine sediment. Geochim. Cosmochim. Acta 1981, 45, 207–216. [Google Scholar] [CrossRef]
- Gomez, E.; Durillon, C.; Rofes, G.; Picot, B. Phosphate adsorption and release from sediments of brackish lagoons: pH, O2 and loading influence. Water Res. 1999, 33, 2437–2447. [Google Scholar] [CrossRef]

| Depth (cm) | Wet-Bulk Density (g cm−3) | Porosity (%) | LOI550 (%) | <63 µm (%) | 63–180 µm (%) | >180 µm (%) | NH4+bio | PO43−bio |
|---|---|---|---|---|---|---|---|---|
| (nmol g−1 dry wt) | ||||||||
| 0–1 | 1.37 ± 0.05 | 76.1 ± 6.91 | 9.23 ± 3.56 | 24.1 ± 4.08 | 60.8 ± 2.39 | 15.1 ± 5.43 | 478 ± 189 | 8.56 ± 1.03 |
| 1–2 | 1.38 ± 0.07 | 70.1 ± 5.22 | 9.16 ± 2.88 | 36.7 ± 22.17 | 63.2 ± 8.04 | 13.1 ± 7.64 | 208 ± 85.1 | 7.72 ± 1.95 |
| 2–4 | 1.51 ± 0.16 | 68.4 ± 1.47 | 7.68 ± 2.01 | 26.5 ± 3.33 | 61.3 ± 6.40 | 12.2 ± 3.24 | 181 ± 103 | 5.57 ± 1.01 |
| 4–6 | 1.53 ± 0.15 | 66.3 ± 2.76 | 7.77 ± 2.50 | 27.9 ± 2.70 | 60.6 ± 4.77 | 11.4 ± 6.48 | 212 ± 90.7 | 4.27 ± 1.67 |
| 6–10 | 1.66 ± 0.07 | 66.1 ± 3.78 | 6.72 ± 1.12 | 29.7 ± 5.13 | 59.7 ± 3.71 | 10.5 ± 7.97 | 186 ± 93.1 | 1.50 ± 0.56 |
| 10–20 | 1.68 ± 0.51 | 66.9 ± 5.93 | 7.66 ± 0.81 | 34.9 ± 4.60 | 55.4 ± 4.63 | 9.32 ± 2.88 | 161 ± 51.9 | 2.53 ± 0.32 |
| 20–40 | 1.73 ± 0.16 | 65.1 ± 4.95 | 6.51 ± 1.25 | 30.7 ± 3.86 | 51.3 ± 5.15 | 16.5 ± 5.72 | 150 ± 87.6 | 2.11 ± 0.71 |
| >40 | 1.78 ± 0.32 | 64.2 ± 5.87 | 4.12 ± 2.11 | 34.8 ± 4.47 | 57.7 ± 4.86 | 6.94 ± 6.80 | 155 ± 60.4 | 2.96 ± 1.13 |
| Parameter | Porosity (%) | LOI550 (%) | <63 µm (%) | 63–180 µm (%) | >180 µm (%) | NH4+bio | PO43−bio | |
|---|---|---|---|---|---|---|---|---|
| (nmol g−1 dry wt) | ||||||||
| Wet-bulk density (g cm−3) | r | −0.850 ** | −0.891 ** | 0.365 | 0.755 * | −0.470 | −0.684 | 0.922 ** |
| p | 0.008 | 0.003 | 0.374 | 0.030 | 0.240 | 0.61 | 0.001 | |
| Porosity (%) | r | 0.784 * | 0.473 | 0.498 | 0.495 | 0.919 ** | 0.868 ** | |
| p | 0.021 | 0.236 | 0.209 | 0.213 | 0.001 | 0.005 | ||
| LOI550 (%) | r | −0.301 | 0.488 | 0.576 | 0.581 | 0.726 * | ||
| p | 0.468 | 0.220 | 0.135 | 0.131 | 0.041 | |||
| <63 µm (%) | r | −0.182 | −0.455 | −0.611 | −0.278 | |||
| p | 0.666 | 0.258 | 0.108 | 0.505 | ||||
| 63-180 µm (%) | r | −0.126 | 0.381 | 0.672 | ||||
| p | 0.766 | 0.352 | 0.068 | |||||
| >180 µm (%) | r | 0.432 | 0.407 | |||||
| p | 0.285 | 0.316 | ||||||
| NH4+bio (nmol g−1 dry wt) | r | 0.725 * | ||||||
| p | 0.042 | |||||||
| Time (h) | Initial Concentration | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TSS (mg L−1) | NO3− (µmol L−1) | NOx (µmol L−1) | NH4+ (µmol L−1) | PO43− (µmol L−1) | ||||||
| -2 | 29 ± 1 | 0.50 ± 0.08 | 0.90 ± 0.16 | 0.21 ± 0.05 | 0.22 ± 0.05 | |||||
| Time (h) | Δ TSS | Δ NO3− | Δ NOx | Δ NH4+ | Δ PO43− | |||||
| (mg L−1) | % | (µmol L−1) | % | (µmol L−1) | % | (µmol L−1) | % | (µmol L−1) | % | |
| -0.5 | −1 | −3 | 0.12 | 24.7 | 0.14 | 15.8 | 0.27 | 125.2 | −0.01 | −3.5 |
| 0 | 932 | >1000 | 0.28 | 44.8 | 0.30 | 28.6 | 6.26 | >1000 | 0.03 | 13.5 |
| +1 | −189 | −20 | 0.38 | 42.5 | 0.49 | 36.5 | 5.67 | 84.1 | 0.08 | 33.8 |
| +2 | −56 | −7 | −0.29 | −22.4 | −0.39 | −21.6 | −11.10 | −89.5 | 0.05 | 14.8 |
| +3 | −39 | −5 | 0.21 | 21.3 | 0.19 | 13.5 | 0.29 | 22.2 | −0.01 | −3.7 |
| +6 | −159 | −23 | −0.85 | −70.1 | −0.84 | −51.5 | −0.27 | −17.3 | −0.13 | −34.9 |
| +7 | −46 | −9 | 0.03 | 7.1 | 0.07 | 8.4 | 0.20 | 15.0 | 0.02 | 9.6 |
| Nutrient | Time (hours) | |||||||
|---|---|---|---|---|---|---|---|---|
| -2 | -0.5 | 0 | +1 | +2 | +3 | +6 | +7 | |
| NH4+ | 0.09 | 0.21 | 2.99 | 5.50 | 0.58 | 0.71 | 0.58 | 0.67 |
| PO43− | 4.89 | 4.72 | 5.36 | 7.16 | 8.22 | 7.92 | 5.15 | 5.65 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dunn, R.J.K.; Waltham, N.J.; Teasdale, P.R.; Robertson, D.; Welsh, D.T. Short-Term Nitrogen and Phosphorus Release during the Disturbance of Surface Sediments: A Case Study in an Urbanised Estuarine System (Gold Coast Broadwater, Australia). J. Mar. Sci. Eng. 2017, 5, 16. https://doi.org/10.3390/jmse5020016
Dunn RJK, Waltham NJ, Teasdale PR, Robertson D, Welsh DT. Short-Term Nitrogen and Phosphorus Release during the Disturbance of Surface Sediments: A Case Study in an Urbanised Estuarine System (Gold Coast Broadwater, Australia). Journal of Marine Science and Engineering. 2017; 5(2):16. https://doi.org/10.3390/jmse5020016
Chicago/Turabian StyleDunn, Ryan J.K., Nathan J. Waltham, Peter R. Teasdale, David Robertson, and David T. Welsh. 2017. "Short-Term Nitrogen and Phosphorus Release during the Disturbance of Surface Sediments: A Case Study in an Urbanised Estuarine System (Gold Coast Broadwater, Australia)" Journal of Marine Science and Engineering 5, no. 2: 16. https://doi.org/10.3390/jmse5020016





