Ocean Thermal Energy Conversion Using Double-Stage Rankine Cycle
Abstract
:1. Introduction
2. Analysis Method
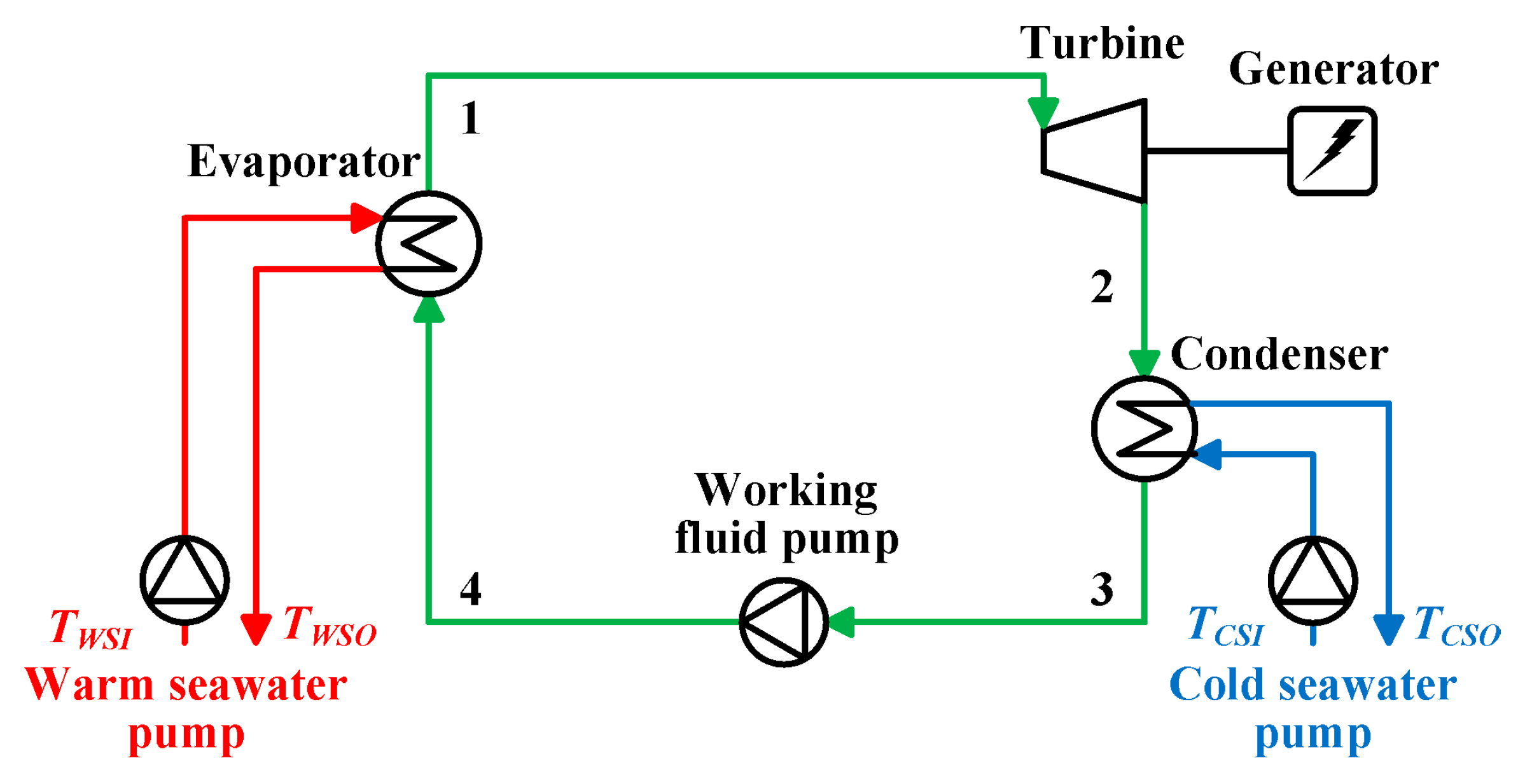
2.1. Rankine Cycle
- (1)
- The working fluid is saturated vapor at the evaporator outlet.
- (2)
- The working fluid is saturated liquid at the condenser outlet.
- (3)
- The heat transfer part is negligible without the evaporator and in the condenser.
- (4)
- The pressure loss of the working fluid is negligible in the laying pipes, the evaporator, and the condenser.
- (5)
- The potential energy of the working fluid is negligible.
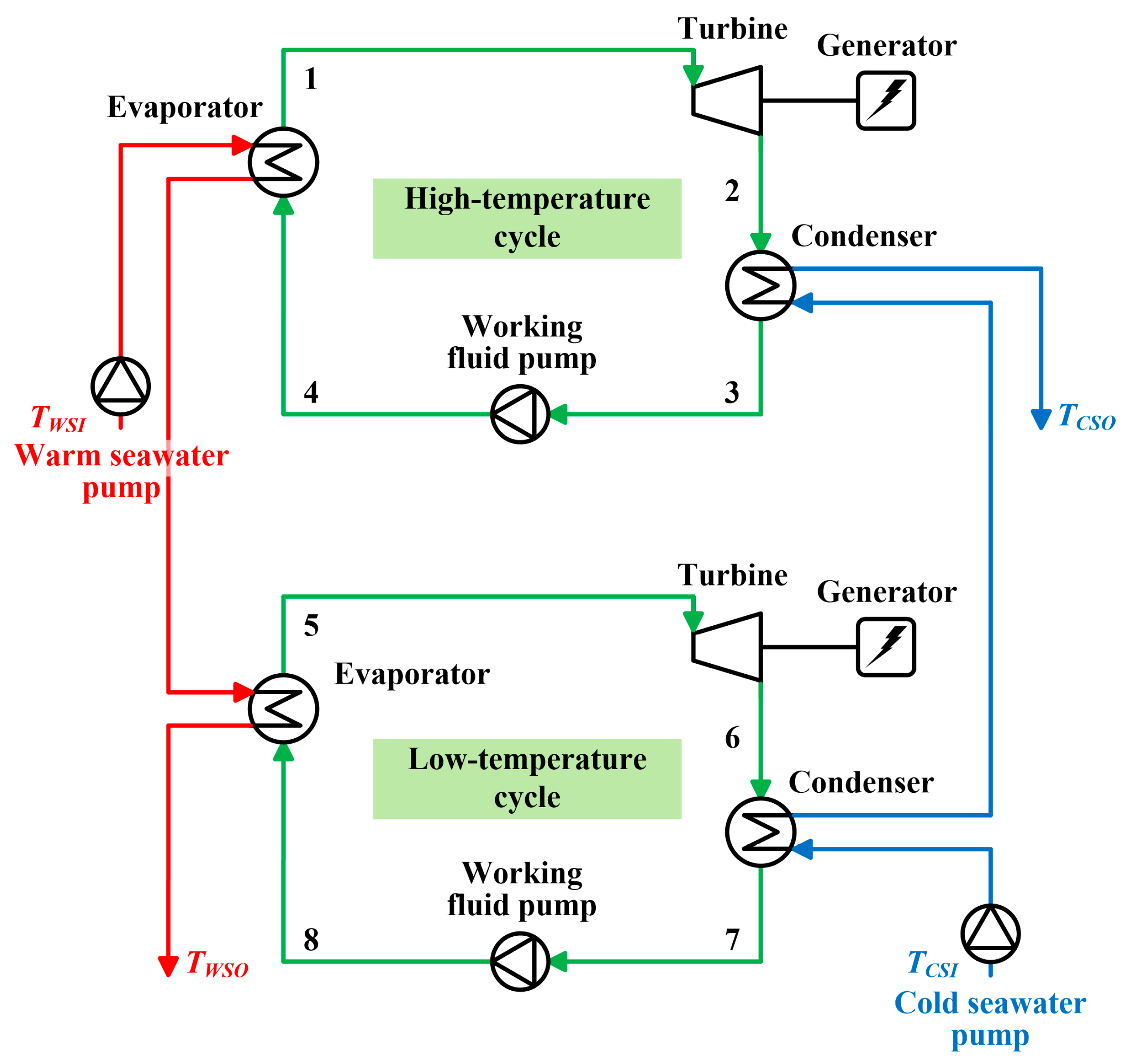
2.2. Double-Stage Rankine Cycle
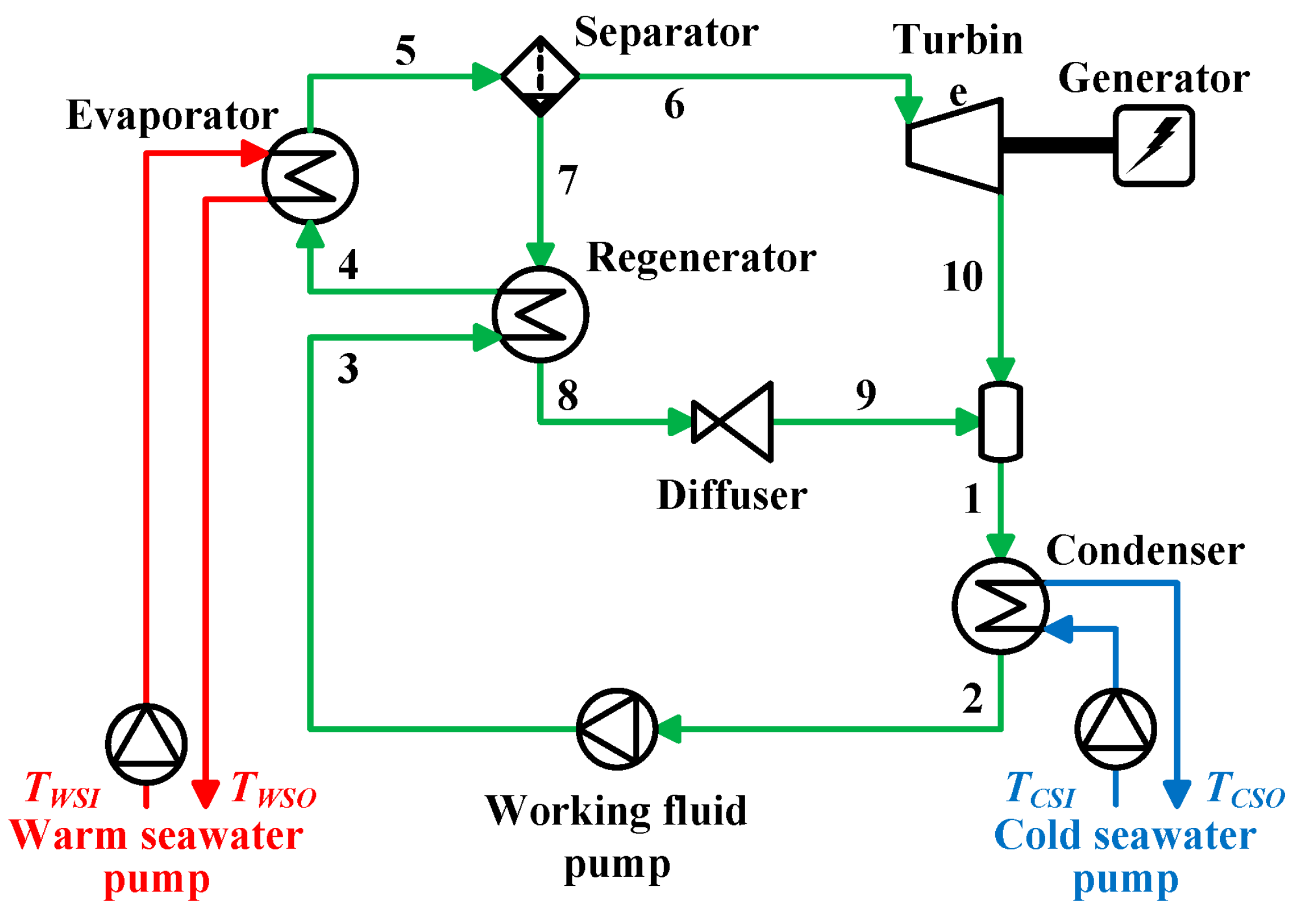
2.3. Kalina Cycle
- (1)
- The working fluid is saturated liquid at condenser outlet and diffuser outlet.
- (2)
- The working fluid is separated into a saturated vapor and a saturated liquid in isothermal and isobaric process by the separator.
- (3)
- The heat transfer part is negligible without the evaporator and in the condenser.
- (4)
- The process in the diffuser is isenthalpic.
- (5)
- The pressure loss of the working fluid is negligible in the laying pipes, the evaporator, and the condenser.
- (6)
- The potential energy of the working fluid is negligible.
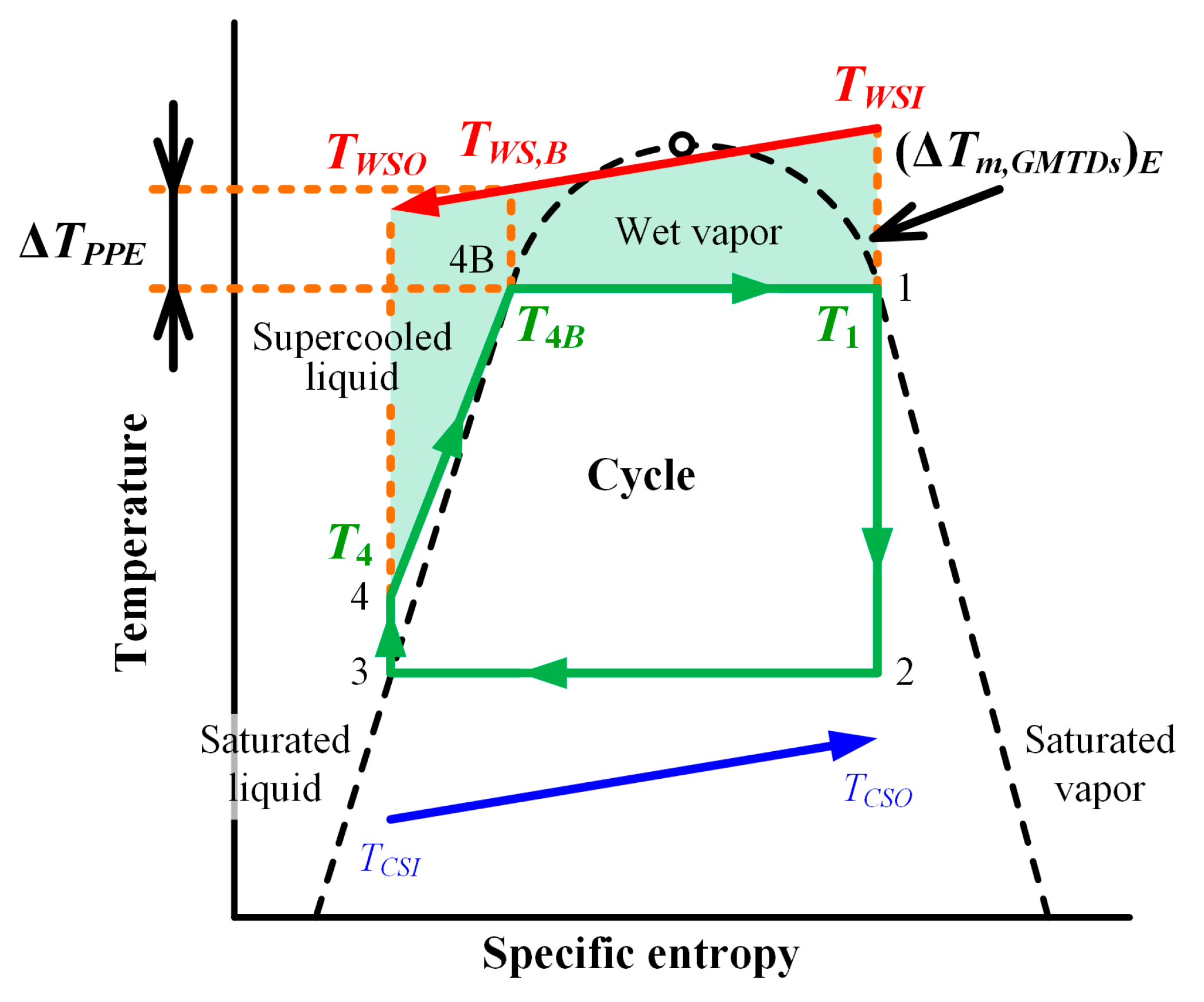
2.4. Evaluation of Heat Transfer Process
2.5. Maximum Power Evaluation
2.6. Calculation Condition
3. Results and Discussion
3.1. Comparison of Single Rankine, Double-Stage Rankine and Kalina Cycles
3.2. The System Characteristics of the Double-Stage Rankine Cycle
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ikegami, Y.; Yasunaga, T.; Urata, K.; Nishimura, R.; Kamano, T.; Nishida, T. Oceanic Observation and Investigation for compound use of OTEC in Kumejima. OTEC 2016, 21, 25–34. (In Japanese) [Google Scholar]
- Ikegami, Y.; Bejan, A. On the Thermodynamic Optimization of Power Plants with Heat Transfer and Fluid Flow Irreversibilities. Trans. ASME J. Sol. Energy Eng. 1998, 120, 139–144. [Google Scholar] [CrossRef]
- Morisaki, T.; Ikegami, Y. Maximum power of a multistage Rankine cycle in low-grade thermal energy conversion. Appl. Therm. Eng. 2014, 69, 78–85. [Google Scholar] [CrossRef]
- Owens, W.L. Optimization of closed-cycle OTEC plants. Proc. ASME-JSME Therm. Eng. Jt. Conf. 1983, 2, 227–239. [Google Scholar]
- Uehara, H.; Nakaoka, T. OTEC Plant Consisting of Plate Type Heat Exchangers. Heat Transf. Jpn. Res. 1985, 14, 19–35. [Google Scholar]
- Uehara, H.; Nakaoka, T. OTEC for Small Island. Sol. Eng. 1987, 2, 1029–1034. [Google Scholar]
- Wu, C. Specific power optimization of closed-cycle OTEC plants. Ocean Eng. 1990, 17, 307–314. [Google Scholar] [CrossRef]
- Sun, F.; Ikegami, Y.; Jia, B.; Arima, H. Optimization design and exergy analysis of organic rankine cycle in ocean thermal energy conversion. Appl. Ocean Res. 2012, 35, 38–46. [Google Scholar] [CrossRef]
- Uehara, H.; Ikegami, Y. Parametric Performance Analysis of OTEC Using Kalina Cycle. ASME Jt. Sol. Eng. Conf. 1993, 60, 3519–3525. [Google Scholar]
- Uehara, H.; Ikegami, Y.; Nishida, T. OTEC System using a New Cycle with Absorption and Extraction Processes. In Proceedings of the 12th International Conference Properties of Water and Steam, Orlando, FL, USA, 11–16 September 1994; pp. 862–869. [Google Scholar]
- Panchal, C.B.; Hillis, D.L.; Lorenz, J.J.; Yung, D.T. OTEC Performance Tests of the Trane Plate-Fin Heat Exchanger; US DOE Report 1981, ANL/OTEC-PS-7; Argonne National Lab.: Lemont, IL, USA, 1981. [Google Scholar]
- Starling, K.E.; Fish, L.W.; Christensen, J.H.; Iqbal, K.; Lawson, C.; Yieh, D. Use of Mixtures as Working Fluids in Ocean Thermal Energy Conversion Cycles: Phase I; US DOE Report 1974, ORO-4918-9; Argonne National Lab.: Lemont, IL, USA, 1974. [Google Scholar]
- Starling, K.E.; Johnson, D.W.; Hafezzadeh, H.; Fish, L.W.; West, H.H.; Iqbal, K.Z. Use of Mixtures as Working Fluids in Ocean Thermal Energy Conversion Cycles: Phase II; US DOE Report 1978, ORO-4918-11; Argonne National Lab.: Lemont, IL, USA, 1978. [Google Scholar]
- Morisaki, T.; Ikegami, Y. Influence of temperature difference calculation method on the evaluation of Rankine cycle performance. J. Therm. Sci. 2014, 23, 68–76. [Google Scholar] [CrossRef]
- Anderson, J.H.; Anderson, J.H.J. Thermal power from seawater. Mech. Eng. 1986, 88, 41–46. [Google Scholar]
- Ibrahim, O.M.; Klein, S.A. High-Power Multi-Stage Rankine Cycles. J. Energy Resour. Technol. 1995, 117, 192–196. [Google Scholar] [CrossRef]
- Johnson, D. The exergy of the ocean thermal resource and analysis of second-law efficiencies of idealized ocean thermal energy conversion power cycles. Energy 1983, 8, 927–946. [Google Scholar] [CrossRef]
- Kanoglu, M. Exergy analysis of a dual-level binary geothermal power plant. Geothermics 2002, 31, 709–724. [Google Scholar] [CrossRef]
- Utamura, M.; Nikitin, K.; Kato, Y. Generalization of Logarithmic Mean Temperature Difference Method for Heat Exchanger Performance Analysis. Therm. Sci. Eng. 2007, 15, 163–173. (In Japanese) [Google Scholar]
- National Institute of Standards and Technolog (NIST). NIST Reference Fluid Thermodynamic and Transport Properties—REFPROP, Version9.0. 2009. Available online: http://www.nknotes.com/html/nist/pdf/indexed/UsersGuideREFPROP9.1.pdf (accessed on 27 February 2018).
- Program Package for Thermo Physical Properties of Fluids (PROPATH) Group. A Program Package of Thermo Physical Properties of Fluids, Version 13.1. 2008. Available online: http://www.mech.kyushu-u.ac.jp/~heat/propath/ (accessed on 28 February 2009).




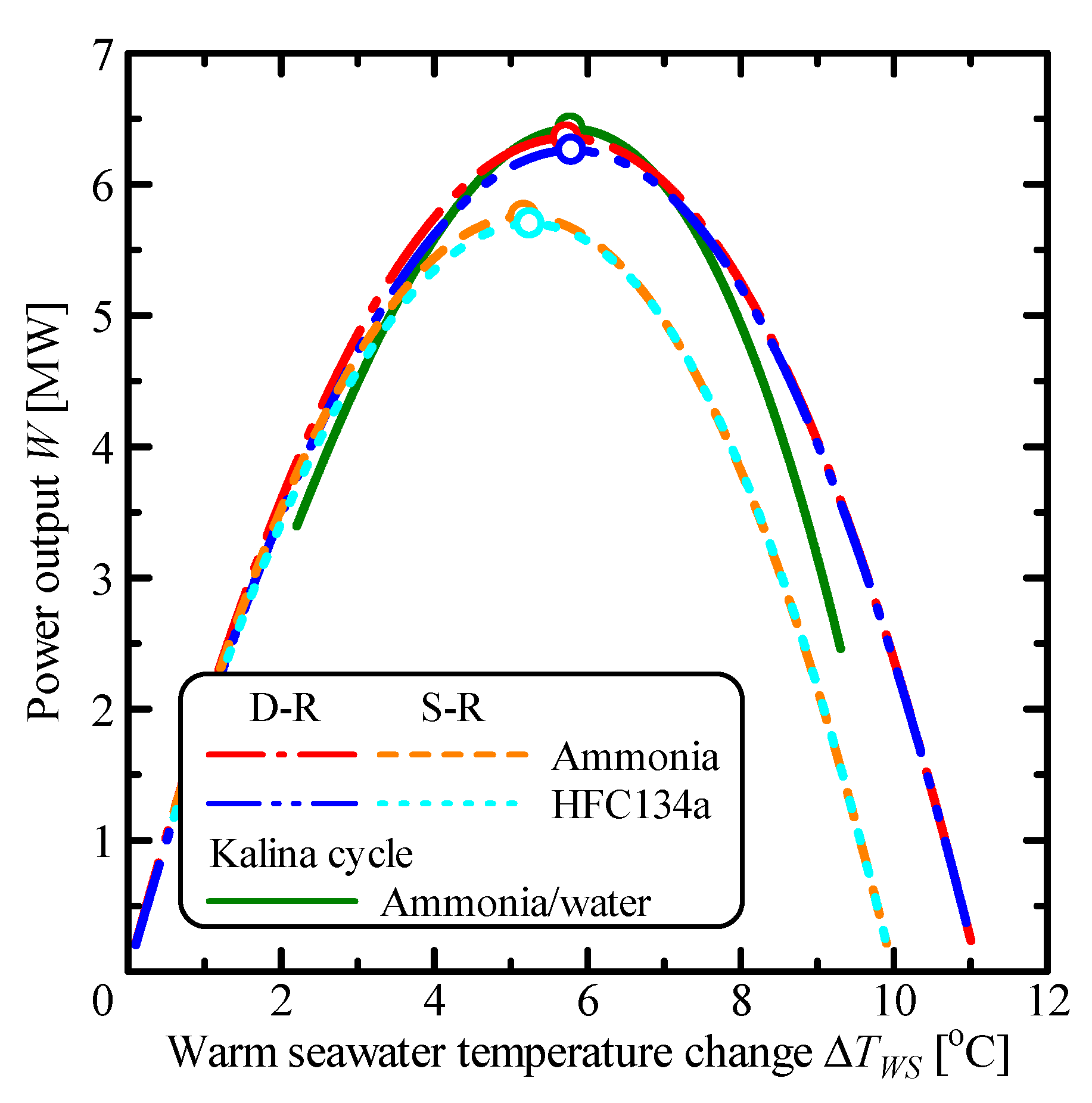
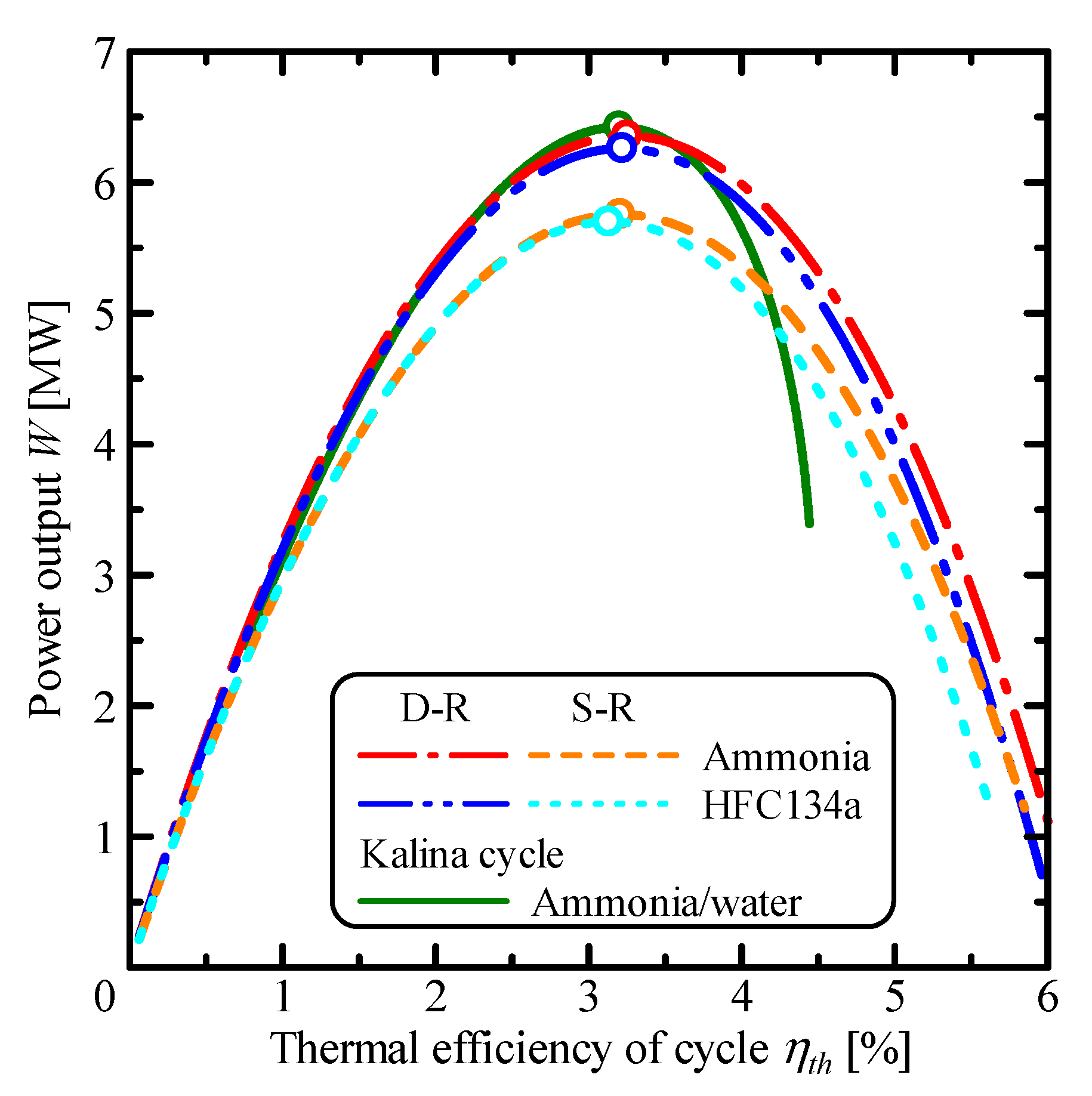
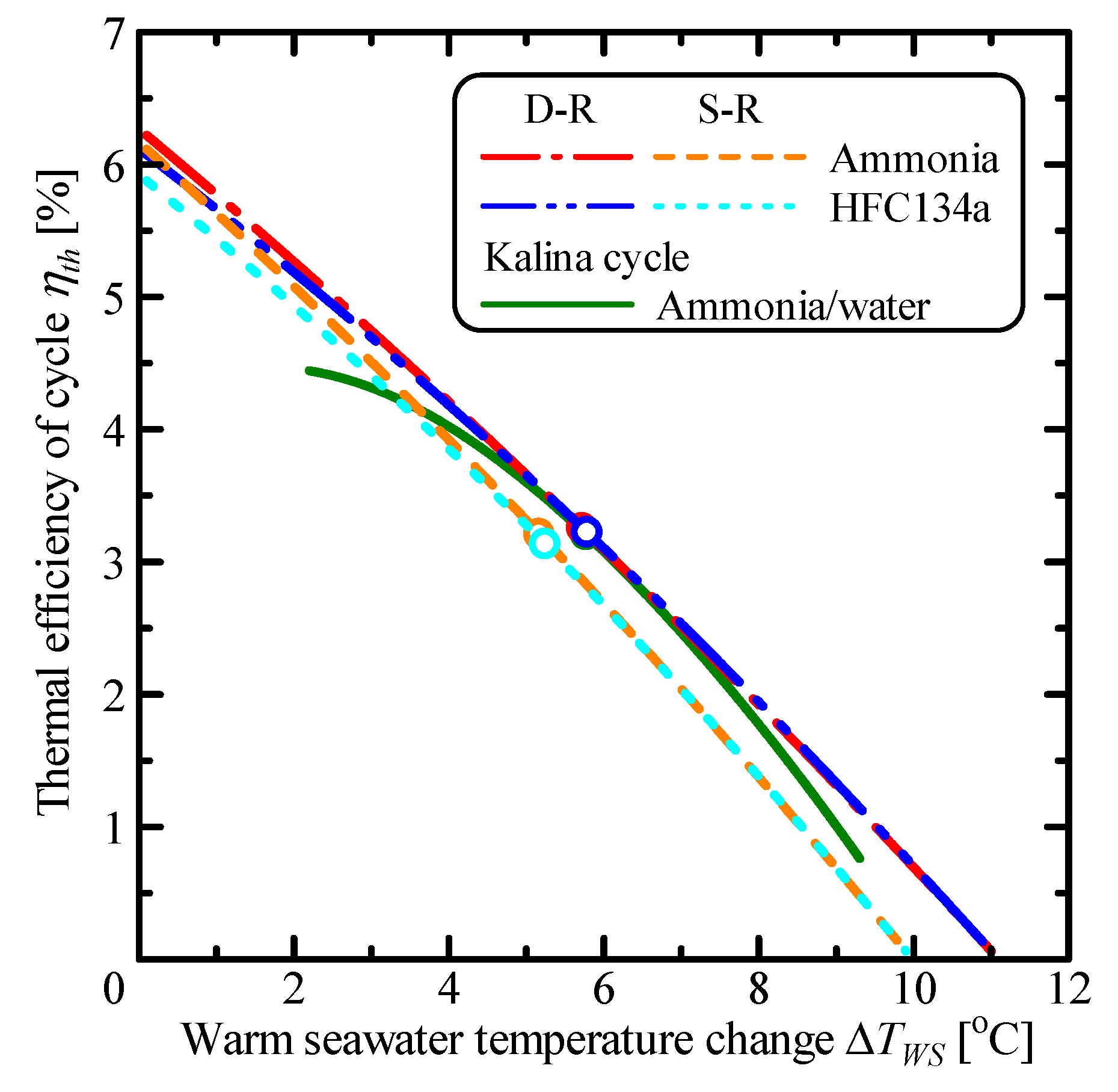
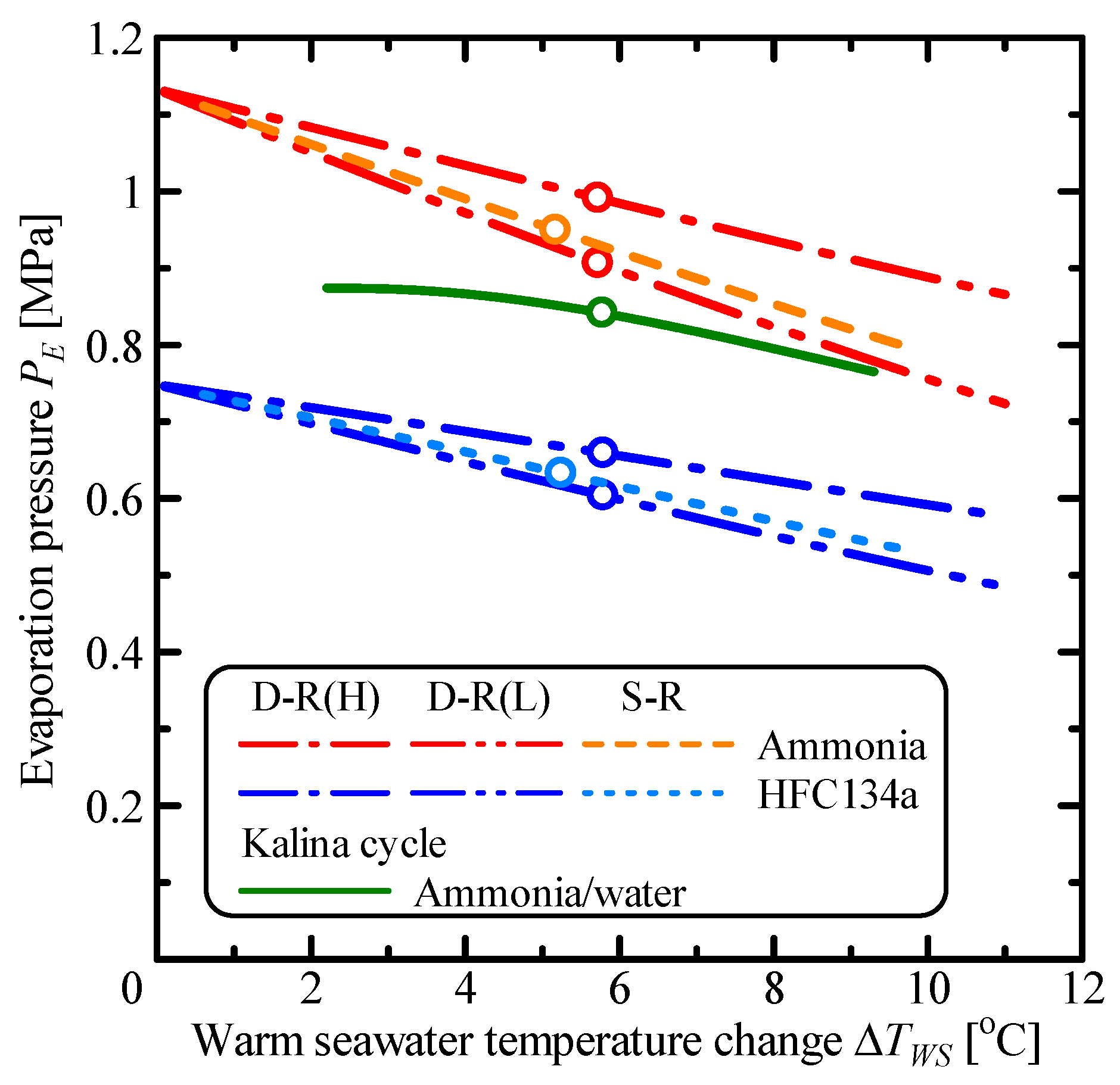

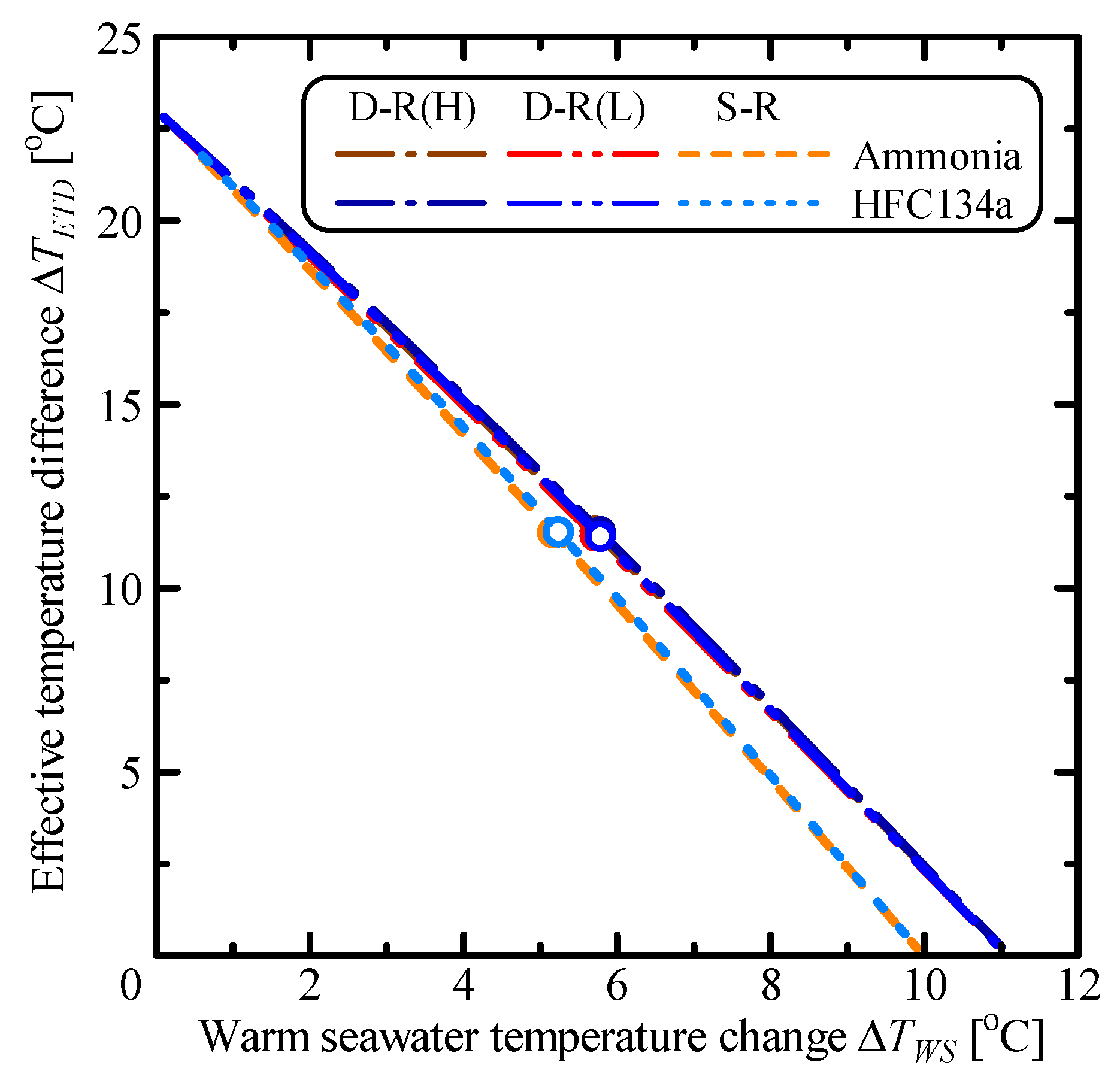
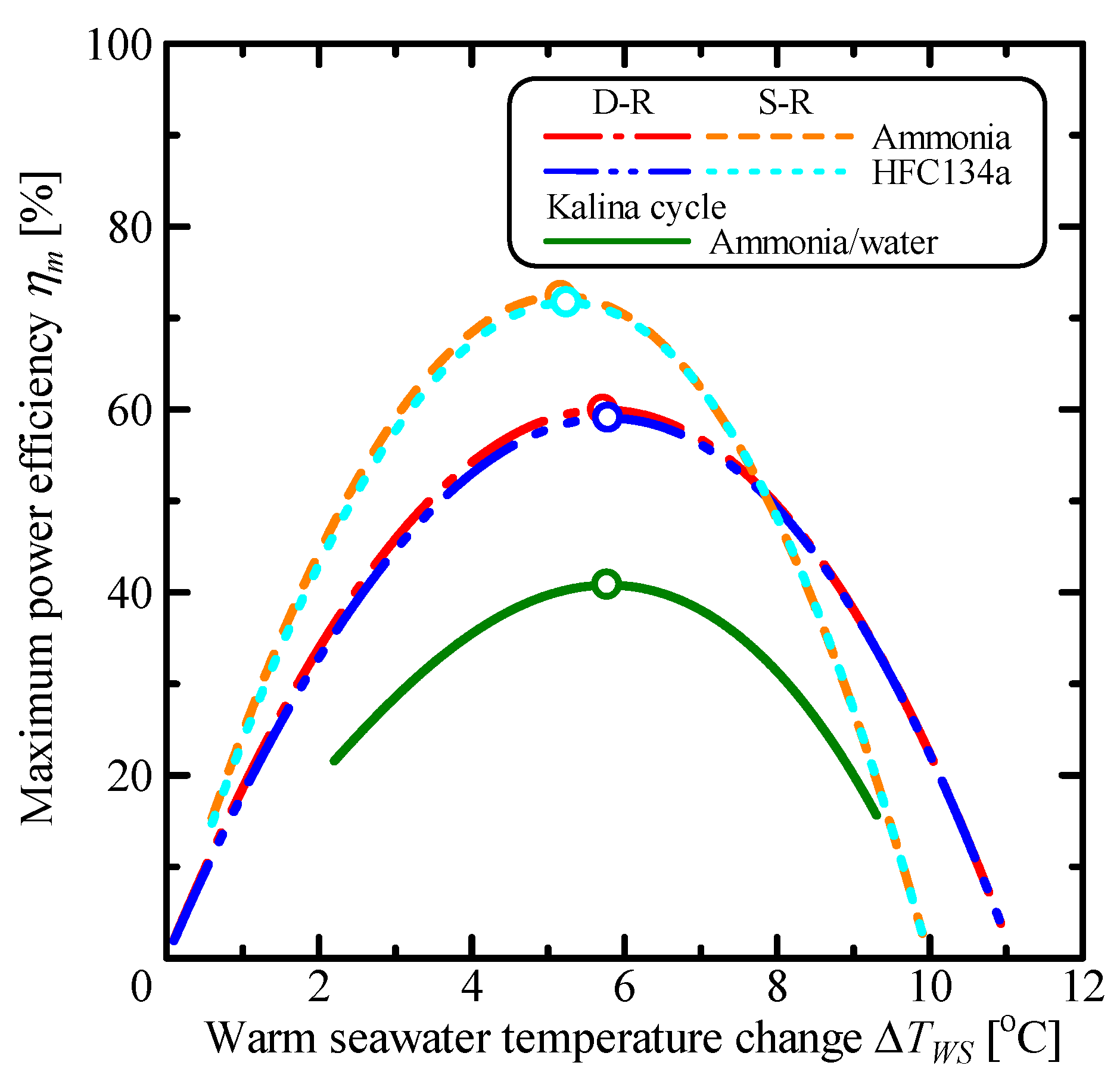


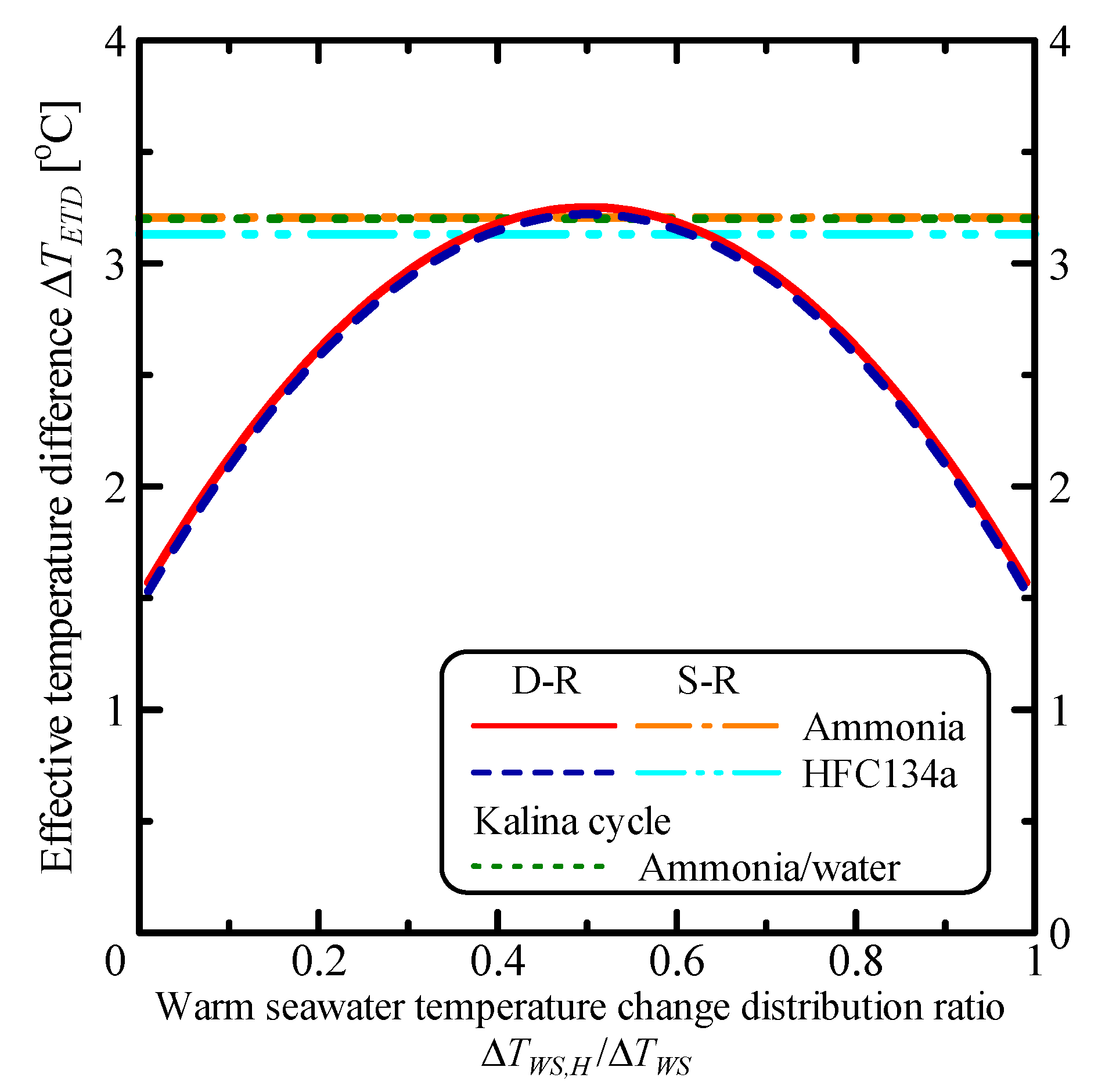
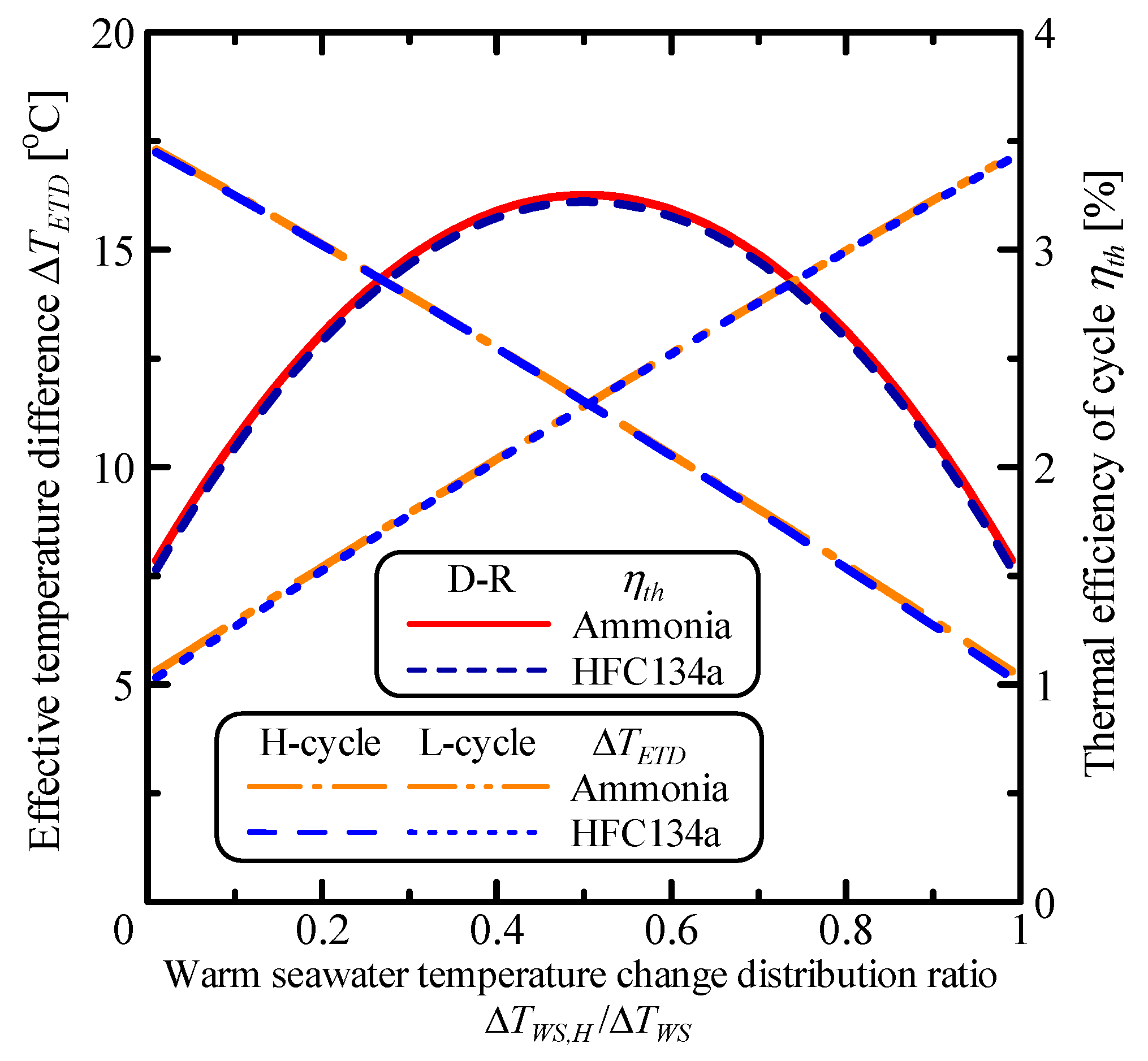
| Parameter | Unit | S-R Ammonia | S-R HFC134a | D-R Ammonia | D-R HFC134a | Kalina |
|---|---|---|---|---|---|---|
| Wm | MW | 5.75 | 5.70 | 6.35 | 6.26 | 6.42 |
| ΔTWS,m | °C | 5.17 | 5.24 | 5.72 | 5.79 | 5.78 |
| ηth,m | % | 3.20 | 3.13 | 3.25 | 3.22 | 3.20 |
| Wm,utilizable | MW | 7.95 | 7.95 | 10.6 | 10.6 | 15.7 |
| ηm | % | 72.4 | 71.7 | 59.9 | 59.0 | 40.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikegami, Y.; Yasunaga, T.; Morisaki, T. Ocean Thermal Energy Conversion Using Double-Stage Rankine Cycle. J. Mar. Sci. Eng. 2018, 6, 21. https://doi.org/10.3390/jmse6010021
Ikegami Y, Yasunaga T, Morisaki T. Ocean Thermal Energy Conversion Using Double-Stage Rankine Cycle. Journal of Marine Science and Engineering. 2018; 6(1):21. https://doi.org/10.3390/jmse6010021
Chicago/Turabian StyleIkegami, Yasuyuki, Takeshi Yasunaga, and Takafumi Morisaki. 2018. "Ocean Thermal Energy Conversion Using Double-Stage Rankine Cycle" Journal of Marine Science and Engineering 6, no. 1: 21. https://doi.org/10.3390/jmse6010021





