Quantitative Analysis of 3D Reconstruction Parameters of Multi-Materialsin Soft Clay
Abstract
:1. Introduction
2. Data and Methods
Clay Component Classification Based on the Data Constrained Modelling
3. Resultsand Discussions
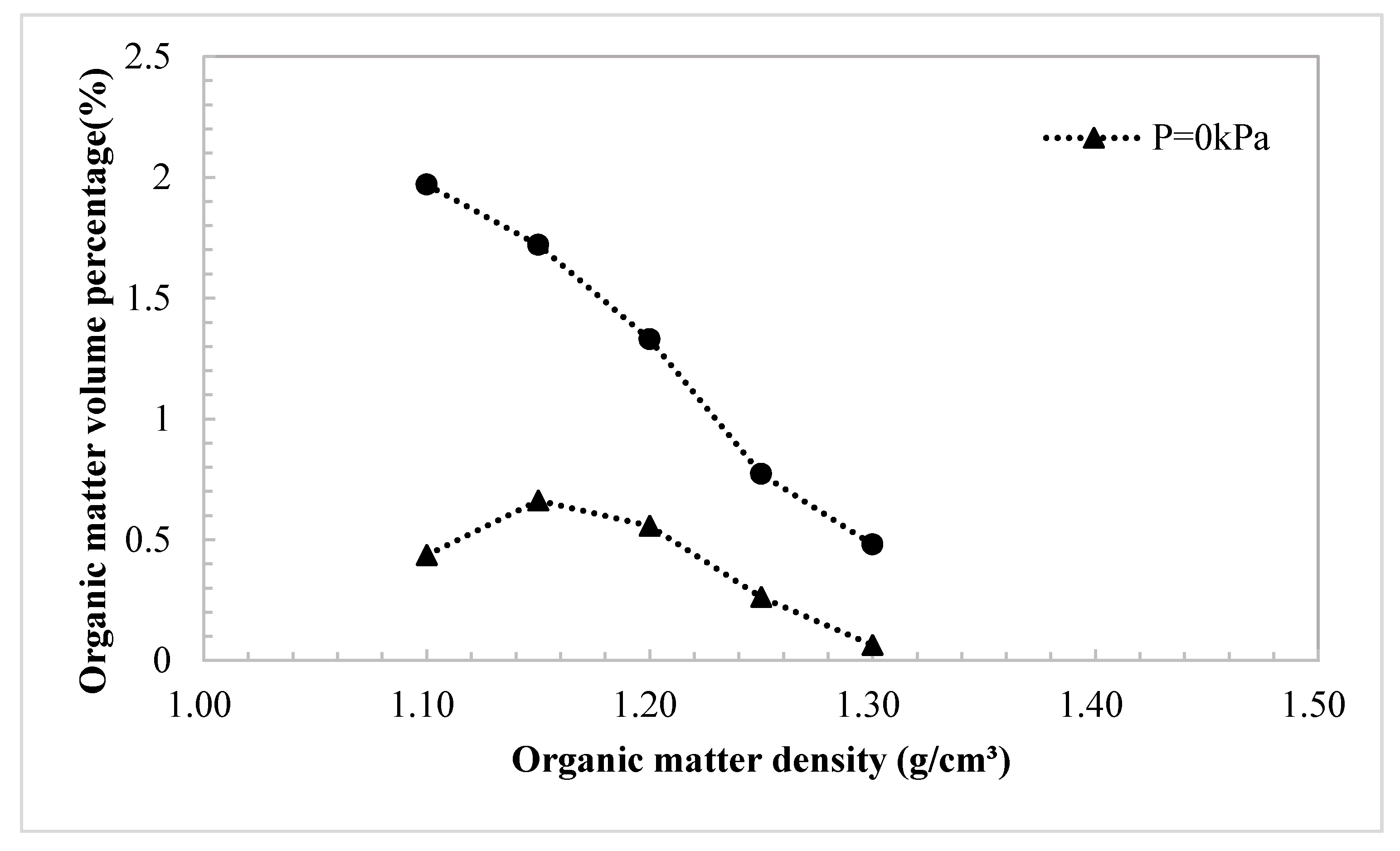
3.1. The Effect of the Density of Organic Matter on the Percentage
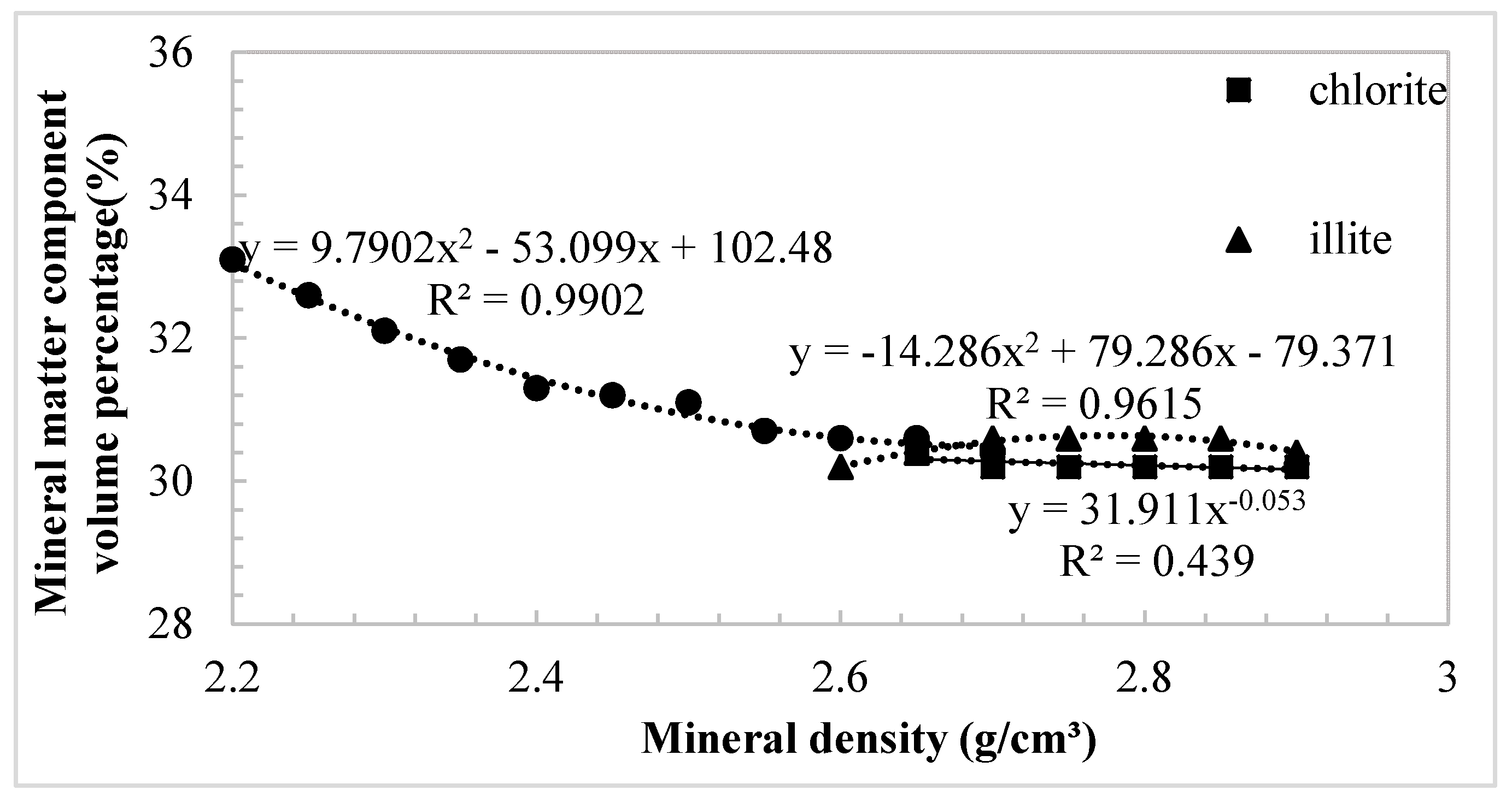
3.2. The Effect of the Density of Clay Minerals on the Percentage
4. Conclusions
- (1)
- Based on the density value of the simple material, combined with the volume and mass percentage of the material, a reasonable multi-component density was calculated. The microstructure of soft clay was not sensitive to the change of illite density.
- (2)
- The density significantly affected the volume percentage data, which directly limited the accuracy of the material distribution analysis. The organic component was the most significant parameter. Montmorillonite, as one of the clay minerals, was the most sensitive material in soil.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Le, T.M.; Fatahi, B.; Khabbaz, H. Numerical optimisation to obtain elastic viscoplastic model parameters for soft clay. Int. J. Plast. 2015, 65, 1–21. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Guo, L.; Wang, J.; Cai, Y.; Fu, H.; Cai, Y. Cyclic behavior of saturated soft clay under stress path with bidirectional shear stresses. Soil Dyn. Earthq. Eng. 2018, 104, 319–328. [Google Scholar] [CrossRef]
- Sun, L.; Gu, C.; Wang, P. Effects of cyclic confining pressure on the deformation characteristics of natural soft clay. Soil Dyn. Earthq. Eng. 2015, 78, 99–109. [Google Scholar] [CrossRef]
- Cui, Z.D.; Tang, Y. Microstructures of different soil layers caused by the high-rise building group in Shanghai. Environ. Earth Sci. 2011, 63, 109–119. [Google Scholar] [CrossRef]
- Buck, B.J.; Brock, A.L.; Johnson, W.H.; Ulery, A.L. Corrosion of depleted uranium in an arid environment: Soil-geomorphology, SEM/EDS, XRD, and electron microprobe analyses. Soil Sediment Contam. 2004, 13, 545–561. [Google Scholar] [CrossRef]
- Seaman, J.C. Thin-Foil SEM Analysis of soil and groundwater colloids: Reducing instrument and operator bias. Environ. Sci. Technol. 2000, 34, 187–191. [Google Scholar] [CrossRef]
- Guyot, J.L.; Jouanneau, J.M.; Soares, L.; Boaventura, G.R.; Maillet, N.; Lagane, C. Clay mineral composition of river sediments in the Amazon Basin. Catena 2007, 71, 340–356. [Google Scholar] [CrossRef]
- Steinke, S.; Hanebuth, T.J.J.; Vogt, C.; Stattegger, K. Sea level induced variations in clay mineral composition in the southwestern South China Sea over the past 17,000 yr. Mar. Geol. 2008, 250, 199–210. [Google Scholar] [CrossRef]
- Kennedy, M.J.; Pevear, D.R.; Hill, R.J. Mineral surface control of organic carbon in black shale. Science 2002, 295, 657–660. [Google Scholar] [CrossRef] [PubMed]
- Blachier, C.; Jacquet, A.; Mosquet, M.; Michot, L.; Baravian, C. Impact of clay mineral particle morphology on the rheological properties of dispersions: A combined X-ray scattering, transmission electronic microscopy and flow rheology study. Appl. Clay Sci. 2014, 87, 87–96. [Google Scholar] [CrossRef]
- Markgraf, W.; Watts, C.W.; Whalley, W.R.; Hrkac, T.; Horn, R. Influence of organic matter on rheological properties of soil. Appl. Clay Sci. 2012, 64, 25–33. [Google Scholar] [CrossRef]
- Schulten, H.R.; Leinweber, P.; Sorge, C. Composition of organic matter in particle-size fractions of an agricultural soil. Eur. J. Soil Sci. 1993, 44, 677–691. [Google Scholar] [CrossRef]
- Shen, Y.H. Sorption of humic acid to soil: The role of soil mineral composition. Chemosphere 1999, 38, 2489–2499. [Google Scholar] [CrossRef]
- Rathossi, C.E.; Lampropoulou, P.G.; Skourlis, K.C.; Katagas, C.G. Mineralogy and microfabrics of claybearing sediments of NE Peloponnese (Greece): Indices for physical behaviour in civil engineering works. Clay Mine. 2012, 47, 259–274. [Google Scholar] [CrossRef]
- Ma, R.; Cai, C.; Li, Z.; Wang, J.; Xiao, T.; Peng, G.; Yang, W. Evaluation of soil aggregate microstructure and stability under wetting and drying cycles in two Ultisols using synchrotron-based X-ray micro-computed tomography. Soil Tillage Res. 2015, 149, 1–11. [Google Scholar] [CrossRef]
- Sleutel, S.; Leinweber, P.; Van Ranst, E.; Kader, M.A.; Jegajeevagan, K. Organic matter in clay density fractions from sandy cropland soils with differing land-use history. Soil Sci. Soc. Am. J. 2011, 75, 521–532. [Google Scholar] [CrossRef]
- Wang, Y.D.; Yang, Y.S.; Cole, I.; Trinchi, A.; Xiao, T.Q. Investigation of the microstructure of an aqueously corroded zinc wire by data-constrained modelling with multi-energy X-ray CT. Mater. Corros. 2013, 64, 180–184. [Google Scholar] [CrossRef]
- Yang, S.; Gao, D.C.; Muster, T.; Tulloh, A.; Furman, S.; Mayo, S.; Trinchi, A. Microstructure of a paint Primer—A data-constrained modeling analysis. Mater. Sci. Forum 2010, 654–656, 1686–1689. [Google Scholar] [CrossRef]
- Yang, Y.S.; Tulloh, A.; Chen, F.; Liu, K.Y.; Clennell, B.; Taylor, J. Data-constrained characterization of sandstone microstructures with multi-energy X-ray CT. J. Phys. 2013, 463, 12048. [Google Scholar] [CrossRef]
- Liu, Z.; Song, J.; Li, X.; Yang, Y.; Ren, Y. Three-dimensional characterization of mineral and organic compositions in process of consolidation of saturated fine-grained soil. J. Eng. Geol. 2017, 8, 273–281. [Google Scholar] [CrossRef]
- Xue, L.I.; Liu, Z.Q.; Song, J.; Yang, Y.S. Micro-Macro characteristics of organic matters in dredger fill consolidation. Period. Ocean Univ. China 2017, 47, 28–35. [Google Scholar] [CrossRef]
- Song, J.; Yang, S.; Ren, Y.Q.; Chu, C.; Maksimenko, A.; Mayo, S. Microstructure Characterizations of Saturated Fine-Grained Soil in Consolidation Process; v3. CSIRO Data Collection; CSIRO: Canberra, Australia, 2017. [Google Scholar] [CrossRef]
- Reijneveld, A.; Wensem, J.V.; Oenema, O. Soil organic carbon contents of agricultural land in the Netherlands between 1984 and 2004. Geoderma 2009, 152, 231–238. [Google Scholar] [CrossRef]
- Golchin, A.; Clarke, P.; Baldock, J.A.; Higashi, T.; Skjemstad, J.O.; Oades, J.M. The effects of vegetation and burning on the chemical composition of soil organic matter in a volcanic ash soil as shown by 13C NMR spectroscopy. I. Whole soil and humic acid fraction. Geoderma 1997, 76, 155–174. [Google Scholar] [CrossRef]
- Fang, X.; Chua, T.; Schmidt-Rohr, K.; Thompson, M.L. Quantitative 13C NMR of whole and fractionated Iowa Mollisols for assessment of organic matter composition. Geochim. Cosmochim. Acta 2010, 74, 584–598. [Google Scholar] [CrossRef]
- Joswig, W. Neutron Diffraction Study of a One-Layer Monoclinic Chlorite. Clays Clay Miner. 1989, 37, 511–514. [Google Scholar] [CrossRef]
- Hong, H.; Fang, Q.; Cheng, L.; Wang, C.; Churchman, G.J. Microorganism-induced weathering of clay minerals in a hydromorphic soil. Geochim. Cosmochim. Acta 2016, 184, 272–288. [Google Scholar] [CrossRef]
- Zanazzi, P.F.; Montagnoli, M.; Nazzareni, S.; Comodi, P. Structural effects of pressure on monoclinic chlorite: A single-crystal study. Am. Mineral. 2006, 91, 1871–1878. [Google Scholar] [CrossRef]
- Vieillard, P. A new method for the prediction of Gibbs free energies of formation of hydrated clay minerals based on the electronegativity scale. Clays Clay Miner. 2000, 48, 459–473. [Google Scholar] [CrossRef]
- Güven, N. Mica Structure and Fibrous Growth of Illite. Clays Clay Miner. 2001, 49, 189–196. [Google Scholar] [CrossRef]
- Ogorodova, L.P.; Kiseleva, I.A.; Melchakova, L.V.; Vigasina, M.F.; Krupskaya, V.V. Thermochemical study of natural montmorillonite. Geochem. Int. 2013, 51, 484–494. [Google Scholar] [CrossRef]
- Harrison, A.D.; Whale, T.F.; Carpenter, M.A.; Holden, M.A.; Neve, L.; O’Sullivan, D.; Vergara Temprado, J.; Murray, B.J. Not all feldspars are equal: a survey of ice nucleating properties across the feldspar group of minerals. Atmos. Chem. Phys. 2016, 16, 10927–10940. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, R.; Martirena, F.; Scrivener, K.L. The origin of the pozzolanic activity of calcined clay minerals: A comparison between kaolinite, illite and montmorillonite. Cem. Concr. Res. 2011, 41, 113–122. [Google Scholar] [CrossRef]
- Wang, H.P.; Yang, Y.S.; Wang, Y.D.; Yang, J.L.; Jia, J.; Nie, Y.H. Data-constrained modelling of an anthracite coal physical structure with multi-spectrum synchrotron X-ray CT. Fuel 2013, 106, 219–225. [Google Scholar] [CrossRef]
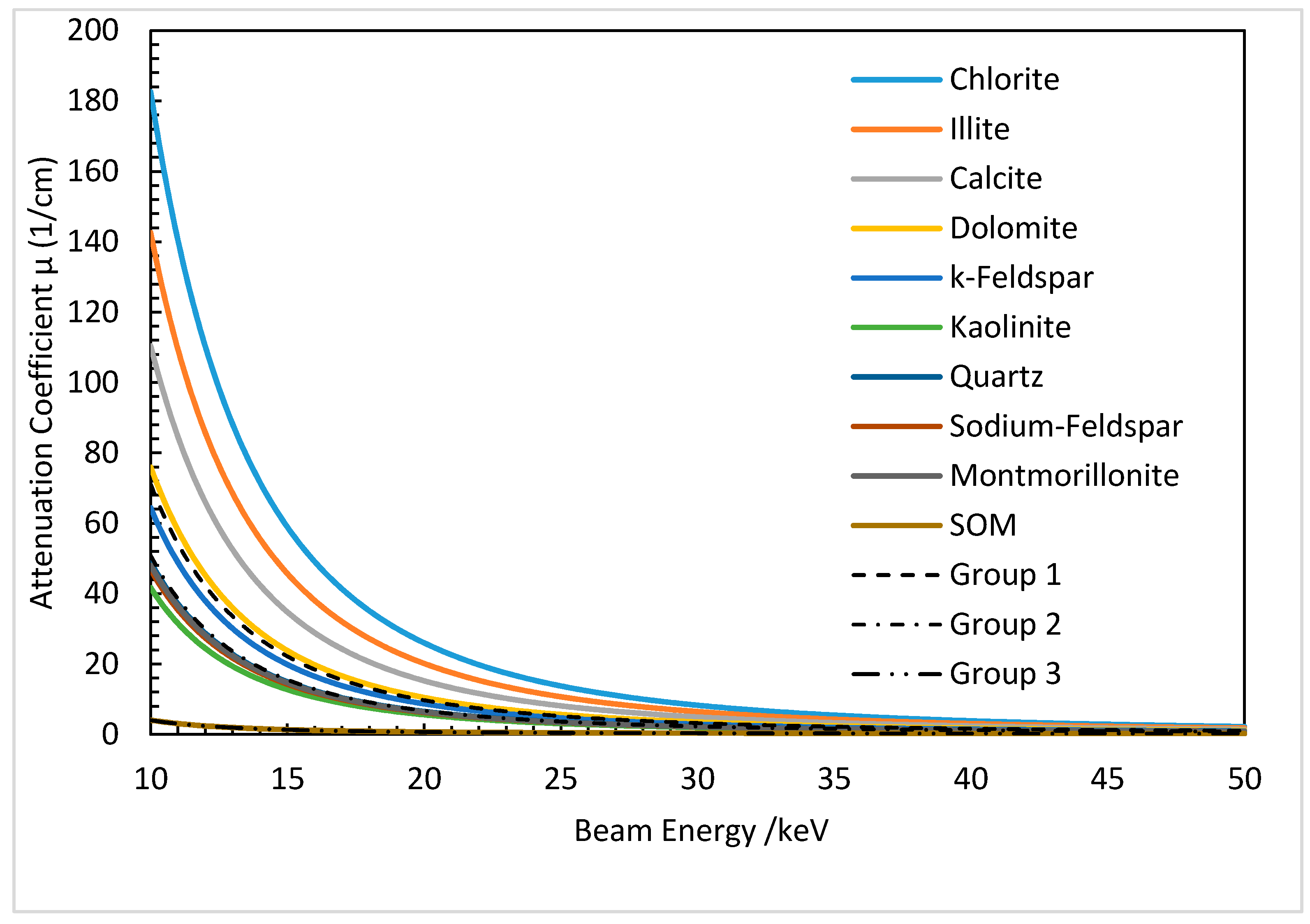
| Material Name | Molecular Formula | Molecular Weight (g/mol) | Reproduced Densities (g/cm3) | Average Density (g/cm3) | Weight Percentage (%) | |
|---|---|---|---|---|---|---|
| Chlorite [26,27,28] | (Mg,Fe,Al)6(Si,Al)4O8(OH)8 | 1126 | 2.65–2.90 | 2.78 | 2.01 | |
| Illite [29,30] | (K,H2O)2Si8(Al,Mg,Fe)4O20(OH)4 | 1154 | 2.60–2.90 | 2.75 | 19.14 | |
| Calcite [31] | CaCO3 | 100 | 2.60–2.90 | 2.75 | 1.75 | |
| Dolomite [31] | CaMg(CO3)2 | 184 | 2.80–2.86 | 2.83 | 3.79 | |
| K-Feldspar [32] | KNaAlSi3O8 | 301 | 2.50–2.60 | 2.55 | 1.96 | |
| Kaolinite [33] | (OH)8Si4Al4O1 | 516 | 2.60–2.63 | 2.62 | 5.31 | |
| Quartz [34] | SiO2 | 60 | 2.65 | 2.65 | 33.20 | |
| Sodium-Feldspar [32] | NaAlSi3O8 | 262 | 2.61–2.76 | 2.68 | 2.00 | |
| Montmorillonite [33] | (OH)4Si8Al4O102H2O | 596 | 2.20–2.80 | 2.50 | 28.46 | |
| Organic matrix [34] | C64H55O26N4 | 1295 | 1.2 | 1.2 | 2.39 |
| Mineral Names | Molecular Formula | Weight Percentage (%) | Molecular Weight (g/mol) | Mole (Mole = Weight Percentage/Molecular Weight) (%g/mol) |
| SOM | C64H55O26N4 | 2.39 | 1295 | 0.001846 |
| O (26 × Mole) | C (64 × Mole) | H (55 × Mole) | N (4 × Mole) | Group Molecular formula |
| 0.04798 | 0.11812 | 0.10151 | 0.00738 | O0.04798C0.11812H0.10151N0.00738 |
| Chlorite density | 2.65 | 2.70 | 2.75 | 2.80 | 2.85 | 2.90 | |||||
| Mineral composition density | 2.72 | 2.73 | 2.73 | 2.73 | 2.73 | 2.73 | |||||
| Illite density | 2.60 | 2.65 | 2.70 | 2.75 | 2.80 | 2.85 | 2.90 | ||||
| Mineral composition density | 2.67 | 2.68 | 2.69 | 2.70 | 2.71 | 2.72 | 2.72 | ||||
| Montmorillonite density | 2.20 | 2.25 | 2.30 | 2.35 | 2.40 | 2.45 | 2.50 | 2.55 | 2.60 | 2.65 | 2.70 |
| Mineral composition density | 2.55 | 2.57 | 2.59 | 2.61 | 2.63 | 2.64 | 2.66 | 2.68 | 2.69 | 2.71 | 2.72 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Song, J.; Yang, Y.; Li, X. Quantitative Analysis of 3D Reconstruction Parameters of Multi-Materialsin Soft Clay. J. Mar. Sci. Eng. 2018, 6, 23. https://doi.org/10.3390/jmse6010023
Liu Z, Song J, Yang Y, Li X. Quantitative Analysis of 3D Reconstruction Parameters of Multi-Materialsin Soft Clay. Journal of Marine Science and Engineering. 2018; 6(1):23. https://doi.org/10.3390/jmse6010023
Chicago/Turabian StyleLiu, Zhiqing, Jing Song, Yushuang Yang, and Xue Li. 2018. "Quantitative Analysis of 3D Reconstruction Parameters of Multi-Materialsin Soft Clay" Journal of Marine Science and Engineering 6, no. 1: 23. https://doi.org/10.3390/jmse6010023





