Antimacrofouling Efficacy of Innovative Inorganic Nanomaterials Loaded with Booster Biocides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Compounds
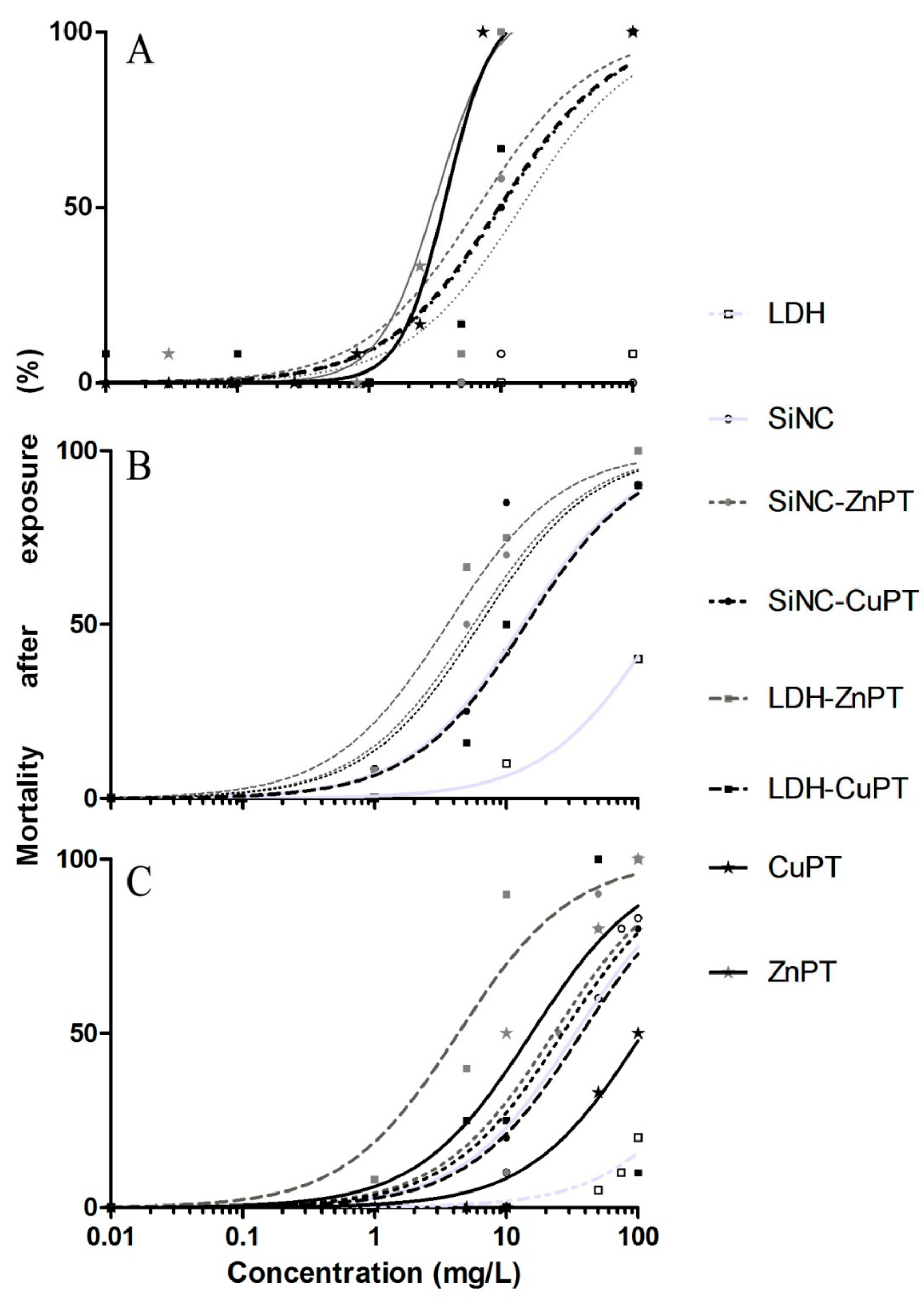
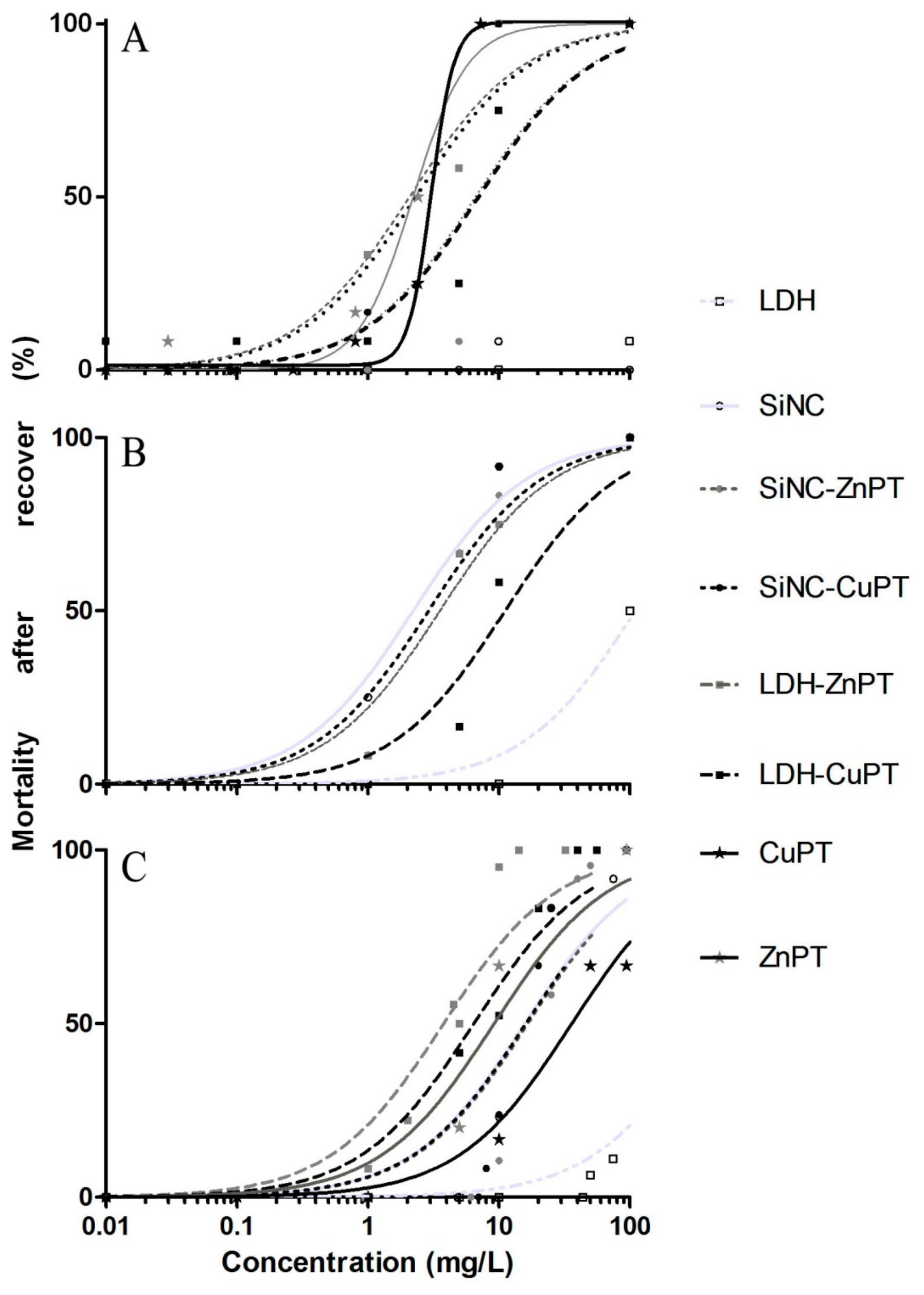
2.2. Antimacrofouling Assays on Mussels
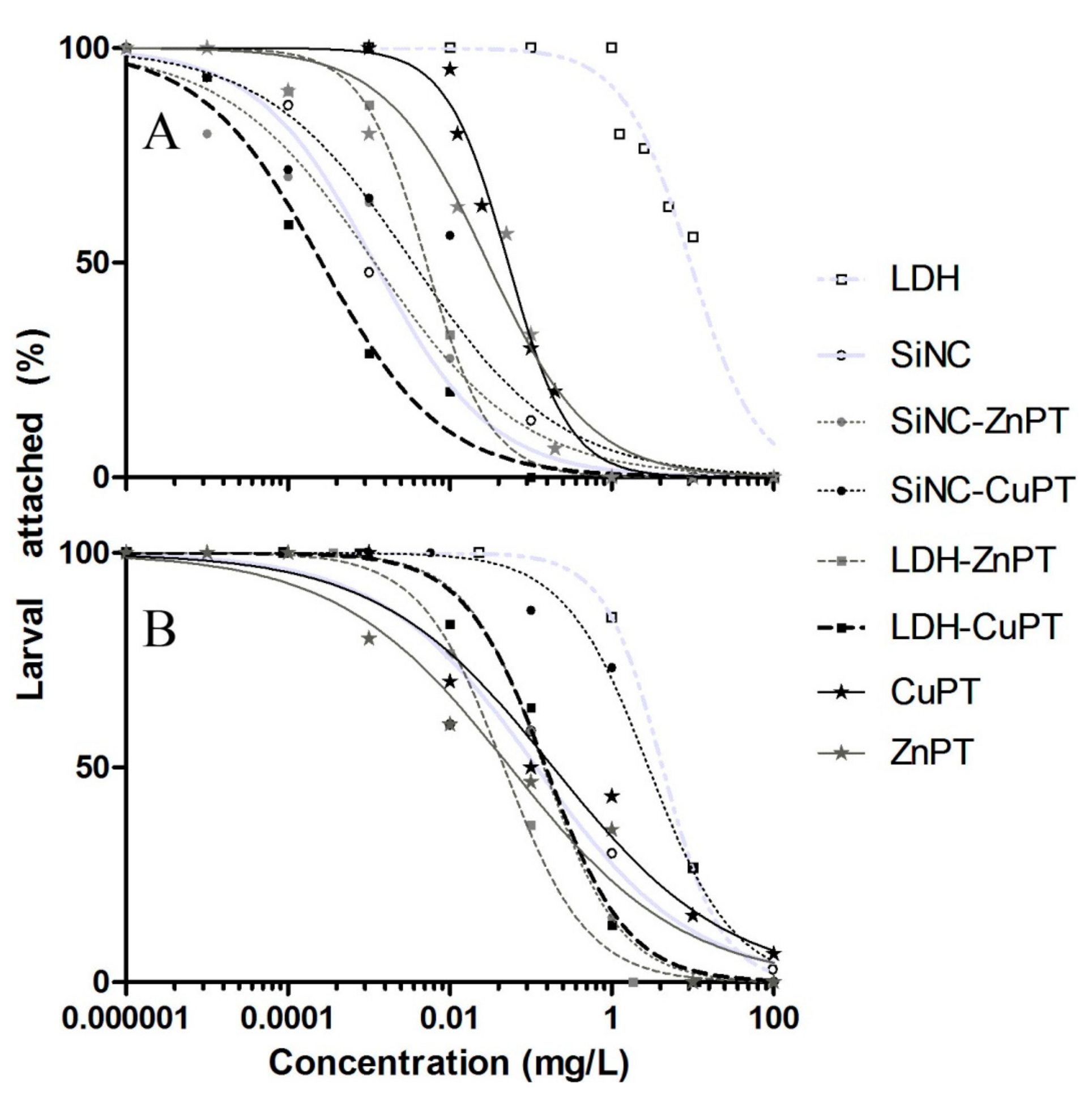
2.3. Antimacrofouling Assays on Bryozoan Larvae
2.4. Statistical Analysis
3. Results and Discussion
3.1. Unloaded Nanocarriers LDH and SiNC
3.2. ZnPT and CuPT Loaded Nanomaterials
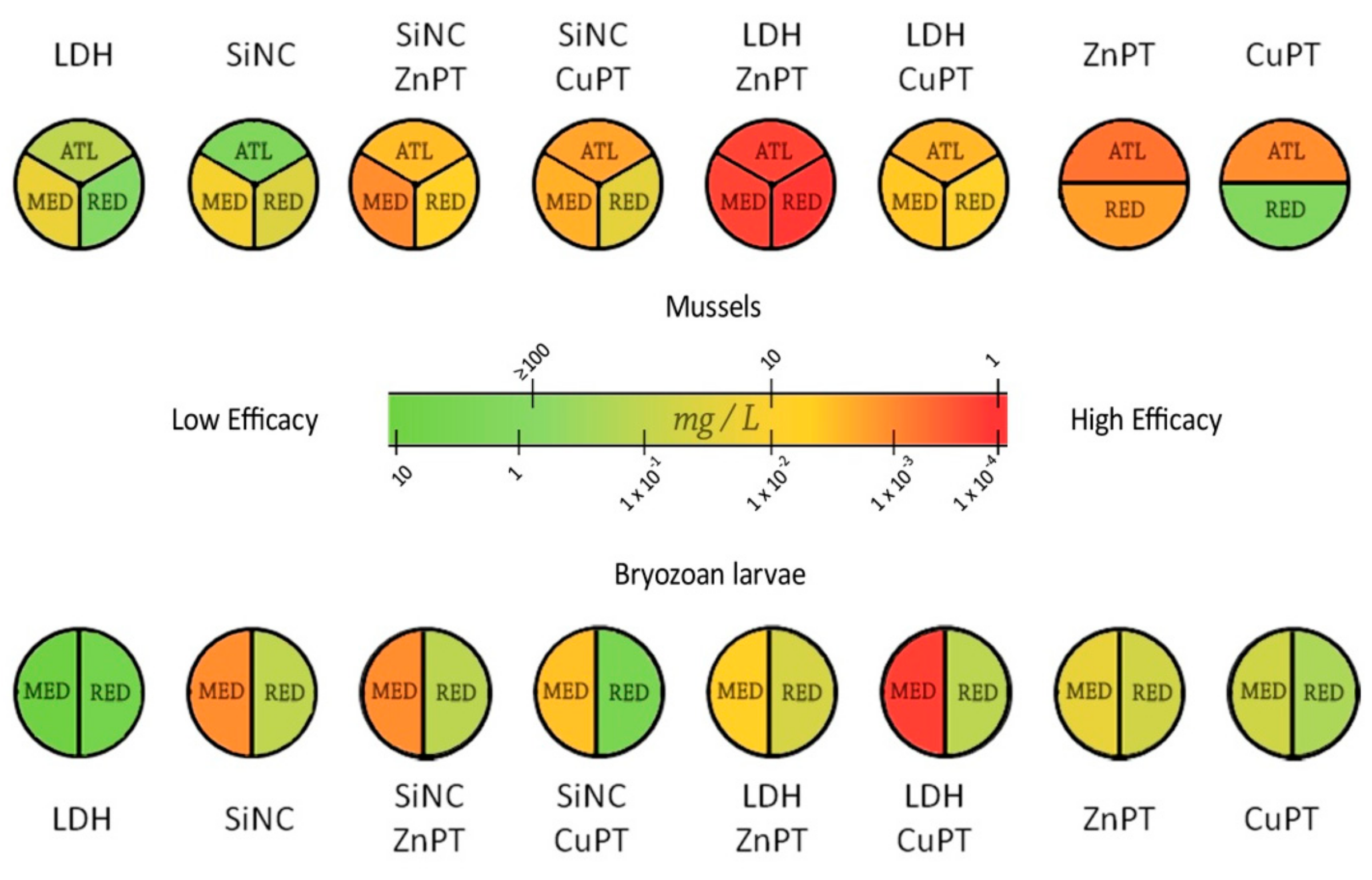
3.3. Model Systems in Geographic Perspective
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wahl, M. Marine epibiosis. I. Fouling and antifouling: Some basic aspects. Mar. Ecol. Prog. Ser. 1989, 58, 175–189. [Google Scholar] [CrossRef] [Green Version]
- Railkin, A.I. Marine Biofouling: Colonization Processes and Defenses; CRC Press: Boca Raton, FL, USA; London, UK, 2003; ISBN 0-8493-1419-4. [Google Scholar]
- Chambers, L.D.; Stroke, K.R.; Walsh, F.C.; Wood, R.J.K. Modern approaches to marine antifouling coatings. Surf. Coat. Technol. 2006, 201, 3642–3652. [Google Scholar] [CrossRef]
- Callow, J.A.; Callow, M.E. Trends in the development of environmentally friendly fouling-resistant marine coatings. Nat. Commun. 2011, 2, 244. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, C.M.; Brennan, A.B. Bio-inspired antifouling strategies. Annu. Rev. Mater. Res. 2012, 42, 211–229. [Google Scholar] [CrossRef]
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling coatings: Recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv. Mater. 2011, 23, 690–718. [Google Scholar] [CrossRef] [PubMed]
- Price, A.R.; Readman, J.W. 12 Booster biocide antifoulants: Is history repeating itself? In Late Lessons from Early Warnings: Science, Precaution, Innovation; European Environment Agency: Copenhagen, Denmark, 2013; ISBN 978-92-9213-349-8. [Google Scholar]
- Huang, X.; Marsh, K.L.; McVerry, B.T.; Hoe, E.M.; Kaner, R.B. Low-Fouling Antibacterial Reverse Osmosis Membranes via Surface Grafting of Graphene Oxide. ACS Appl. Mater. Interfaces 2016, 23, 14334–14338. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Asatekin, A.; Mayers, A.M.; Elimelech, M. Protein antifouling mechanisms of PAN UF membranes incorporating PAN-g-PEO additive. J. Membr. Sci. 2007, 296, 42–50. [Google Scholar] [CrossRef]
- Dobretsov, S.; Teplitski, M.; Bayer, M.; Gunasekera, S.; Proksch, P.; Paul, V.J. Inhibition of marine biofouling by bacterial quorum sensing inhibitors. Biofouling 2011, 27, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Fusetani, N. Antifouling marine natural products. Nat. Prod. Rep. 2011, 28, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Dobretsov, S. Inhibition and Induction of Marine Biofouling by Biofilms; Springer: Berlin/Heidelberg, Germany, 2009; Volume 4, ISBN 978-3-540-69796-1. [Google Scholar]
- Hu, Y.; Zheng, X.T.; Chen, J.S.; Zhou, M.; Li, C.M.; Lou, X.J. Silica-based complex nanorattles as multifunctional carrier for anticancer drug. J. Mater. Chem. 2011, 21, 8052–8056. [Google Scholar] [CrossRef]
- Zeng, G.; Xing, Y.; Gao, J.; Wang, Z.; Zhang, X. Unconventional layer-by-layer assembly of graphene multilayer films for enzyme-based glucose and maltose biosensing. Langmuir 2010, 26, 15022–15026. [Google Scholar] [CrossRef] [PubMed]
- Tedim, J.; Poznyak, S.K.; Kuznetsova, A.; Raps, D.; Hack, T.; Zheludkevich, M.L.; Ferreira, M.G.S. Enhancement of active corrosion protection via combination of inhibitor-loaded nanocontainers. ACS Appl. Mater. Interfaces 2010, 2, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Geiger, T.; Delavy, P.; Hany, R.; Schleuniger, J.; Zinn, M. Encapsulated zosteric acid embedded in poly [3-hydroxyalkanoate] coatings—Protection against biofouling. Polym. Bull. 2004, 52, 65–72. [Google Scholar] [CrossRef]
- Hart, R.L.; Virgallito, D.R.; Work, D.E. Microencapsulation of Biocides and Antifouling Agents. U.S. Patent 7938897, 5 October 2011. [Google Scholar]
- Szabó, T.; Molnár-Nagy, L.; Bognár, J.; Nyikos, L.; Telegdi, J. Self-healing microcapsules and slow release microspheres in paints. Prog. Org. Coat. 2011, 72, 52–57. [Google Scholar] [CrossRef]
- Zheng, Z.; Huang, X.; Schenderlein, M.; Borisova, D.; Cao, R.; Möhwald, H.; Shchukin, D. Self-Healing and Antifouling Multifunctional Coatings Based on pH and Sulfide Ion Sensitive Nanocontainers. Adv. Funct. Mater. 2013, 23, 3307–3314. [Google Scholar] [CrossRef]
- Nooney, R.I.; Thirunavukkarasu, D.; Chen, Y.; Josephs, R.; Ostafin, A.E. Synthesis of nanoscale mesoporous silica spheres with controlled particle size. Chem. Mater. 2002, 14, 4721–4728. [Google Scholar] [CrossRef]
- Maia, F.; Tedim, J.; Lisenkov, A.D.; Salak, A.N.; Zheludkevich, M.L.; Ferreira, M.G. Silica nanocontainers for active corrosion protection. Nanoscale 2012, 4, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Maia, F.; Silva, A.P.; Fernandes, S.; Cunha, A.; Almeida, A.; Tedim, J.; Zheludkevich, M.L.; Ferreira, M.G.S. Incorporation of biocides in nanocapsules for protective coatings used in maritime applications. Chem. Eng. J. 2015, 270, 150–157. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Poznyak, S.K.; Rodrigues, L.M.; Raps, D.; Hack, T.; Dick, L.F.; Nunes, T.; Ferreira, M.G.S. Active protection coatings with layered double hydroxide nanocontainers of corrosion inhibitor. Corros. Sci. 2010, 52, 602–611. [Google Scholar] [CrossRef]
- Newman, S.P.; Jones, W. Synthesis, characterization and applications of layered double hydroxides containing organic guests. New J. Chem. 1998, 22, 105–115. [Google Scholar] [CrossRef]
- Avelelas, F.; Martins, R.; Oliveira, T.; Maia, F.; Malheiro, E.; Soares, A.; Loureiro, S.; Tedim, J. Efficacy and Ecotoxicity of Novel Anti-Fouling Nanomaterials in Target and Non-Target Marine Species. Mar. Biochechnol. 2017, 19, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Ina, K.; Takasawa, R.; Yagi, A.; Yamashita, N.; Ltoh, H.; Sakata, K. An improved assay method for antifouling substances using the blue mussel, Mytilus edulis. Agric. Biol. Chem. 1989, 53, 3319–3321. [Google Scholar]
- Wilsanand, V.; Wagh, A.B.; Bapuji, M. Effect of alcohol extracts of demospongiae on growth of periphytic diatoms. Indian J. Mar. Sci. 1999, 28, 274–279. [Google Scholar]
- Bryan, P.J.; Rittschof, D.; Qian, P.Y. Settlement inhibition of bryozoan larvae by bacterial films and aqueous leachates. Bull. Mar. Sci. 1997, 61, 849–857. [Google Scholar]
- Dahms, H.U.; Dobretsov, S.; Qian, P.Y. The effect of bacterial and diatom biofilms on the settlement of the bryozoan Bugula neritina. J. Exp. Mar. Biol. Ecol. 2004, 313, 191–209. [Google Scholar] [CrossRef]
- Brancato, M.S.; Woollacott, R.M. Effect of microbial films on settlement of bryozoan larvae (Bugula simplex, B. stolonifera and B. turrita). Mar. Biol. 1982, 71, 51–56. [Google Scholar] [CrossRef]
- Qi, S.H.; Xu, Y.; Gao, J.; Qian, P.Y.; Zhang, S. Antibacterial and antilarval compounds from marine bacterium Pseudomonas rhizosphaerae. Ann. Microbiol. 2009, 59, 229. [Google Scholar] [CrossRef]
- Clarke, G.L. Poisoning and recovery in barnacles and mussels. Biol. Bull. 1947, 92, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, M.S.; Murugan, A. Fouling deterrent chemical defense in three muricid gastropod egg masses from the Southeast coast of India. Biofouling 2007, 23, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.; Oliveira, T.; Santos, C.; Kuznetsova, A.; Ferreira, V.; Avelelas, F.; Caetano, A.; Tedim, J.; Ferreira, M.; Freitas, R.; et al. Effects of a novel anticorrosion engineered nanomaterial on the bivalve Ruditapes philippinarum. Environ. Sci. Nano 2017, 4, 1064–1076. [Google Scholar] [CrossRef]
- Zhang, C.; Cui, F.; Zeng, G.M.; Jiang, M.; Yang, Z.Z.; Yu, Z.G.; Zhu, M.Y.; Shen, L.Q. Quaternary ammonium compounds (QACs): A review on occurrence, fate and toxicity in the environment. Sci. Total Environ. 2015, 518, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Connor, P.M. Acute toxicity of heavy metals to some marine larvae. Mar. Pollut. Bull. 1972, 3, 190–192. [Google Scholar] [CrossRef]
- Marcheselli, M.; Cecilia, R.; Mauri, M. Novel antifouling agent zinc pyrithione: Determination, acute toxicity, and bioaccumulation in marine mussels (Mytilus galloprovincialis). Environ. Toxicol. Chem. 2010, 29, 2583–2592. [Google Scholar] [CrossRef] [PubMed]
- Chandler, C.J.; Segel, I.H. Mechanism of the antimicrobial action of pyrithione: Effects on membrane transport, ATP levels, and protein synthesis. Antimicrob. Agents Chemother. 1978, 14, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Bragadin, M.; Manente, S.; Marton, D.; Cima, F.; Rigobello, M.P.; Bindoli, A. The interaction of zinc pyrithione with mitochondria from rat liver and a study of the mechanism of inhibition of ATP synthesis. Appl. Organomet. Chem. 2003, 17, 869–874. [Google Scholar] [CrossRef] [Green Version]
- Yasokawa, D.; Murata, S.; Iwahashi, Y.; Kitagawa, E.; Kishi, K.; Okumura, Y.; Iwahashi, H. DNA microarray analysis suggests that zinc pyrithione causes iron starvation to the yeast Saccharomyces cerevisiae. J. Biosci. Bioeng. 2010, 109, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, R.; Flesch, S.; Mattos, J.J.; Milani, M.R.; Bainy, A.C.D.; Dafre, A.L. Zinc causes acute impairment of glutathione metabolism followed by coordinated antioxidant defenses amplification in gills of brown mussels Perna perna. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2014, 159, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Dinning, A.J.; Al-Adam, I.S.I.; Austin, P.; Charlton, M.; Collier, P.J. Pyrithione biocide interactions with bacterial phospholipid head groups. J. Appl. Microbiol. 1998, 85, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Emolayeva, E.; Sanders, D. Mechanism of pyrithione-induced membrane depolarization in Neurospora crassa. Appl. Environ. Microbiol. 1995, 61, 3385–3390. [Google Scholar]
- Busnaina, A.A.; Mead, J.; Isaacs, J.; Somu, S. Nanomanufacturing and sustainability: Opportunities and challenges. J. Nanopart. Res. 2013, 15, 1984. [Google Scholar] [CrossRef]
- Shutava, T.G.; Fakhrullin, R.F.; Lvov, Y.M. Spherical and tubule nanocarriers for sustained drug release. Curr. Opin. Pharmacol. 2014, 18, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; Ramasubburayan, R.; Iyapparaj, P.; Arthi, A.P.R.; Ahila, N.K.; Ramkumar, V.S.; Immanuel, G.; Palavesam, A. Environmentally benign antifouling potentials of triterpene-glycosides from Streptomyces fradiae: A mangrove isolate. RSC Adv. 2015, 5, 29524–29534. [Google Scholar] [CrossRef]
- Leandro, S.M.; Tiselius, P.; Queiroga, H. Spatial and temporal scales of environmental forcing of Acartia populations (Copepoda: Calanoida) in the Canal de Mira (Ria de Aveiro, Portugal). ICES J. Mar. Sci. 2013, 71, 585–596. [Google Scholar] [CrossRef]
- Raveh, O.; David, N.; Rilov, G.; Rahav, E. The temporal dynamics of coastal phytoplankton and bacterioplankton in the Eastern Mediterranean Sea. PLoS ONE 2015, 10, e0140690. [Google Scholar] [CrossRef] [PubMed]
- Carlson, D.F.; Fredj, E.; Gildor, H. The annual cycle of vertical mixing and restratification in the Northern Gulf of Eilat/Aqaba (Red Sea) based on high temporal and vertical resolution observations. Deep Sea Res. Part 1 Oceanogr. Res. Pap. 2014, 84, 1–17. [Google Scholar] [CrossRef]
- Sogorb, A.; Andreu-Moliner, E.S.; Almar, M.M.; del Rame, J.; Núñez, A. Temperature-toxicity relationships of fluvalinate (Synthetic pyrethroid) on Procambarus clarkii (Girard) under laboratory conditions. Bull. Environ. Contam. Toxicol. 1988, 40, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Hall, L.W.; Ziegenfuss, M.C.; Anderson, R.D.; Lewis, B.L. The effect of salinity on the acute toxicity of total and free cadmium to a Chesapeake Bay copepod and fish. Mar. Pollut. Bull. 1995, 30, 376–384. [Google Scholar] [CrossRef]
- Kwok, K.W.H.; Leung, K.M. Toxicity of antifouling biocides to the intertidal harpacticoid copepod Tigriopus japonicus (Crustacea, Copepoda): Effects of temperature and salinity. Mar. Pollut. Bull. 2005, 51, 830–837. [Google Scholar] [CrossRef] [PubMed]

| Compound Abbreviation | Chemical Specification of Compounds |
|---|---|
| ZnPT | Zinc pyrithione (Zinc Omadine™) |
| CuPT | Copper pyrithione (Copper Omadine™) |
| LDHs | Zn-Al layered double hydroxide (without biocide) |
| SiNCs | Hollow silica nanocapules (without biocide) |
| SiNC-ZnPT | Zinc pyrithione encapsulated into silica nano-capsules |
| SiNC-CuPT | Copper pyrithione encapsulated into silica nano-capsules |
| LDH-ZnPT | Zinc pyrithione immobilized in layered double hydroxide |
| LDH-CuPT | Copper pyrithione immobilized in layered double hydroxide |
| ATL | Exposure (72 h) | Recover (72 h + 72 h) | ||||
|---|---|---|---|---|---|---|
| Efficacy (Inhibition of Settlement) | Mortality | Mortality | ||||
| EC50 | 95% CI-EC50 | LC50 | 95% CI-LC50 | LC50 | 95% CI-LC50 | |
| LDH | 35.7 | 17.54–72.70 | >100 | >100 | ||
| SiNC | >100 | >100 | >100 | |||
| SiNC-ZnPT | 5.5 | 3.47–8.86 | 14.1 | 9.25–21.64 | 6.66 | 4.48–9.91 |
| SiNC-CuPT | 4.3 | 2.88–6.36 | 10.0 | 6.43–15.59 | 2.34 | 1.57–3.47 |
| LDH-ZnPT | 1.6 | 1.07–2.57 | 6.6 | 4.48–9.91 | 2.09 | 1.27–3.46 |
| LDH-CuPT | 5.0 | 3.81–6.67 | 9.6 | 6.00–15.51 | 7.04 | 4.30–11.51 |
| ZnPT | 2.4 | 1.85–3.03 | 3.2 | 2.57–4.13 | 2.23 | 1.77–2.82 |
| CuPT | 3.2 | 1.60–6.56 | 3.8 | 3.18–4.67 | 3.07 | 1.90–4.94 |
| MED | Exposure (72 h) | Recover (72 h + 72 h) | ||||
| Efficacy (Inhibition of Settlement) | Mortality | Mortality | ||||
| EC50 | 95% CI-EC50 | LC50 | 95% CI-LC50 | LC50 | 95% CI-LC50 | |
| LDH | 15.6 | 4.9–49.6 | >100 | >100 | ||
| SiNC | 11.8 | 6.6–21.0 | 13.2 | 11.6–15.0 | 2.2 | 1.1–4.3 |
| SiNC-ZnPT | 3.4 | 1.7–6.7 | 5.6 | 3.3–9.7 | 3.5 | 1.5–8.1 |
| SiNC-CuPT | 4.9 | 1.3–19.0 | 6.3 | 2.4–16.9 | 2.9 | 1.4–6.2 |
| LDH-ZnPT | 1.5 | 0.6–3.6 | 3.6 | 2.0–6.2 | 3.6 | 2.1–6.1 |
| LDH-CuPT | 6.3 | 1.7–23.3 | 14.1 | 8.4–23.8 | 11.2 | 5.4–23.3 |
| ZnPT | ||||||
| CuPT | ||||||
| RED | Exposure (72 h) | Recover (72 h + 72 h) | ||||
| Efficacy (Inhibition of Settlement) | Mortality | Mortality | ||||
| EC50 | 95% CI-EC50 | LC50 | 95% CI-LC50 | LC50 | 95% CI-LC50 | |
| LDH | >100 | >100 | >>100 | |||
| SiNC | 20.9 | 12.7–34.5 | 33.5 | 19.6–57.2 | 15.7 | 6.2–40.0 |
| SiNC-ZnPT | 9.3 | 4.2–20.8 | 23.1 | 9.5–55.9 | 16.6 | 6.5–42.4 |
| SiNC-CuPT | 17.3 | 8.1–36.7 | 26.9 | 13.1–55.1 | 16.1 | 6.3–41.3 |
| LDH-ZnPT | 1.3 | 0.7–2.5 | 4.3 | 1.8–10.4 | 3.8 | 2.1–6.8 |
| LDH-CuPT | 9.6 | 3.9–23.6 | 37.4 | 3.4–407.6 | 6.4 | 3.6–11.4 |
| ZnPT | 4.2 | 1.4–12.2 | 15.5 | 6.0–39.9 | 9.2 | 4.5–18.4 |
| CuPT | >100 | >100 | 36.1 | 25.9–50.1 | ||
| Efficacy of B. neritina (Inhibition of Settlement) | ||||
| Compound | MED | RED | ||
| EC50 (mg/L) | 95% CI-EC50 | EC50 (mg/L) | 95% CI-LC50 | |
| LDH | 9.4 | 6.0–14.5 | 4.3 | 4.2–4.4 |
| SiNC | 0.001 | 0.0006–0.002 | 0.1 | 0.04–0.3 |
| SiNC-ZnPT | 0.001 | 0.0004–0.003 | 0.1 | 0.14–0.15 |
| SiNC-CuPT | 0.003 | 0.0008–0.01 | 2.9 | 1.9–4.2 |
| LDH-ZnPT | 0.005 | 0.003–0.007 | 0.046 | 0.03–0.06 |
| LDH-CuPT | 0.0002 | 0.0001–0.0004 | 0.1 | 0.1–0.2 |
| ZnPT | 0.02 | 0.01–0.05 | 0.05 | 0.02–0.1 |
| CuPT | 0.05 | 0.04–0.06 | 0.19 | 0.07–0.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutner-Hoch, E.; Martins, R.; Oliveira, T.; Maia, F.; Soares, A.M.V.M.; Loureiro, S.; Piller, C.; Preiss, I.; Weis, M.; Larroze, S.B.; et al. Antimacrofouling Efficacy of Innovative Inorganic Nanomaterials Loaded with Booster Biocides. J. Mar. Sci. Eng. 2018, 6, 6. https://doi.org/10.3390/jmse6010006
Gutner-Hoch E, Martins R, Oliveira T, Maia F, Soares AMVM, Loureiro S, Piller C, Preiss I, Weis M, Larroze SB, et al. Antimacrofouling Efficacy of Innovative Inorganic Nanomaterials Loaded with Booster Biocides. Journal of Marine Science and Engineering. 2018; 6(1):6. https://doi.org/10.3390/jmse6010006
Chicago/Turabian StyleGutner-Hoch, Eldad, Roberto Martins, Tania Oliveira, Frederico Maia, Amadeu M. V. M. Soares, Susana Loureiro, Chen Piller, Iris Preiss, Michal Weis, Severine B. Larroze, and et al. 2018. "Antimacrofouling Efficacy of Innovative Inorganic Nanomaterials Loaded with Booster Biocides" Journal of Marine Science and Engineering 6, no. 1: 6. https://doi.org/10.3390/jmse6010006





