Optimization of Plating Conditions for the Determination of Polonium Using Copper Foils
Abstract
:1. Introduction
2. Materials and Methodology
2.1. Reagents and Experiment
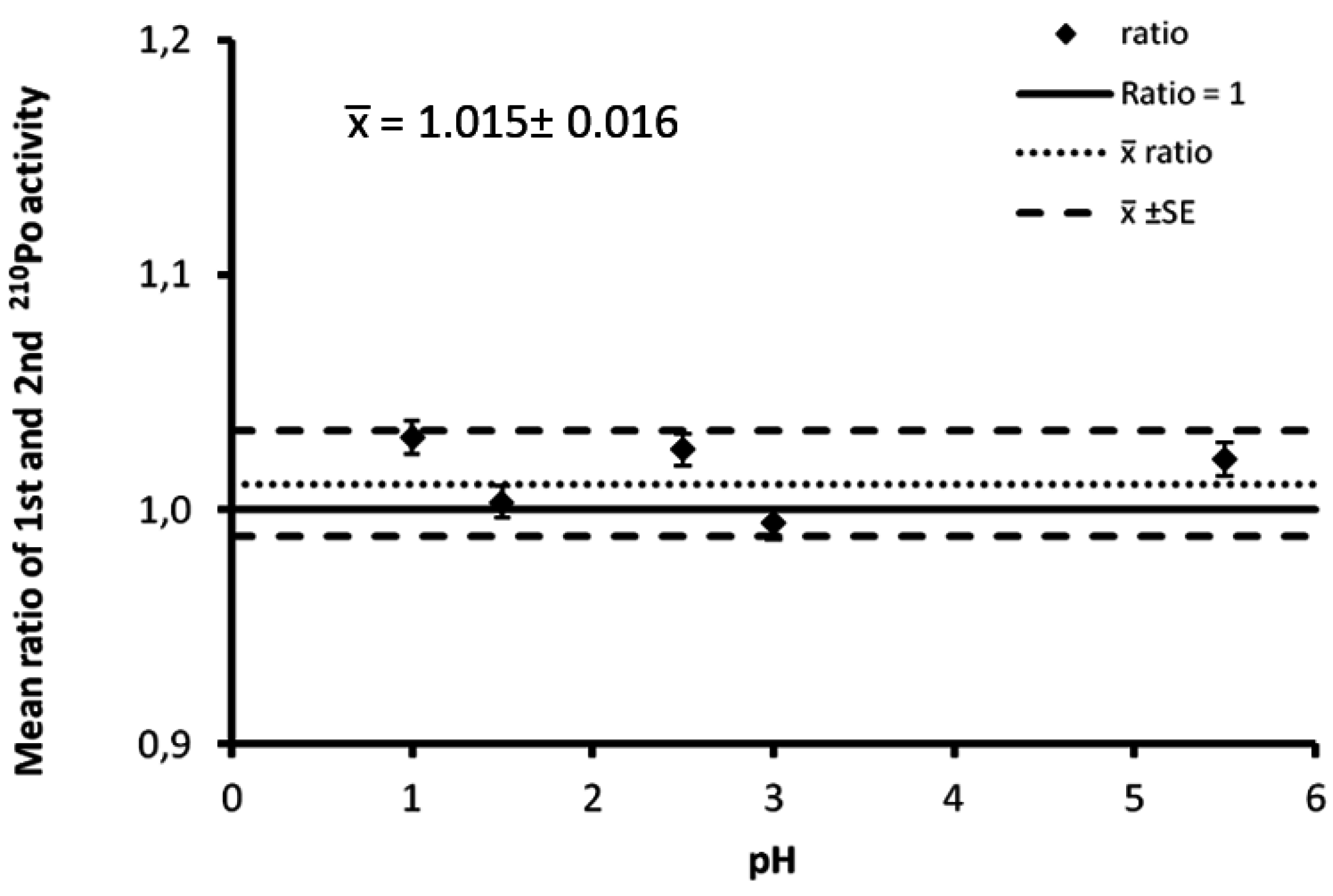
2.2. Measurements of Isotopes
2.3. Verification of Methods Using Certified Reference Material from the International Atomic Energy Agency (IAEA) and Sediment Samples from Nigeria
3. Results and Discussions
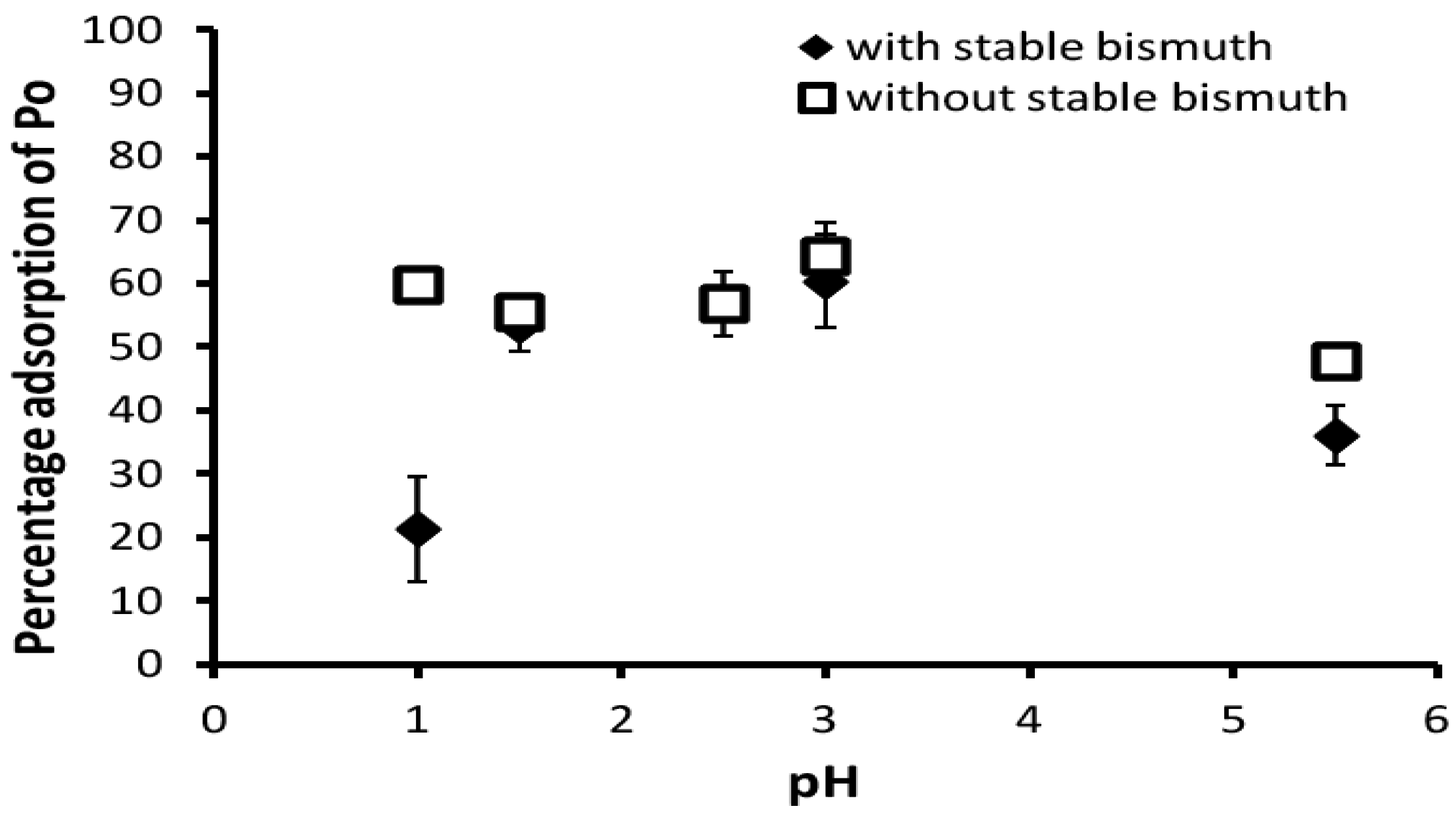
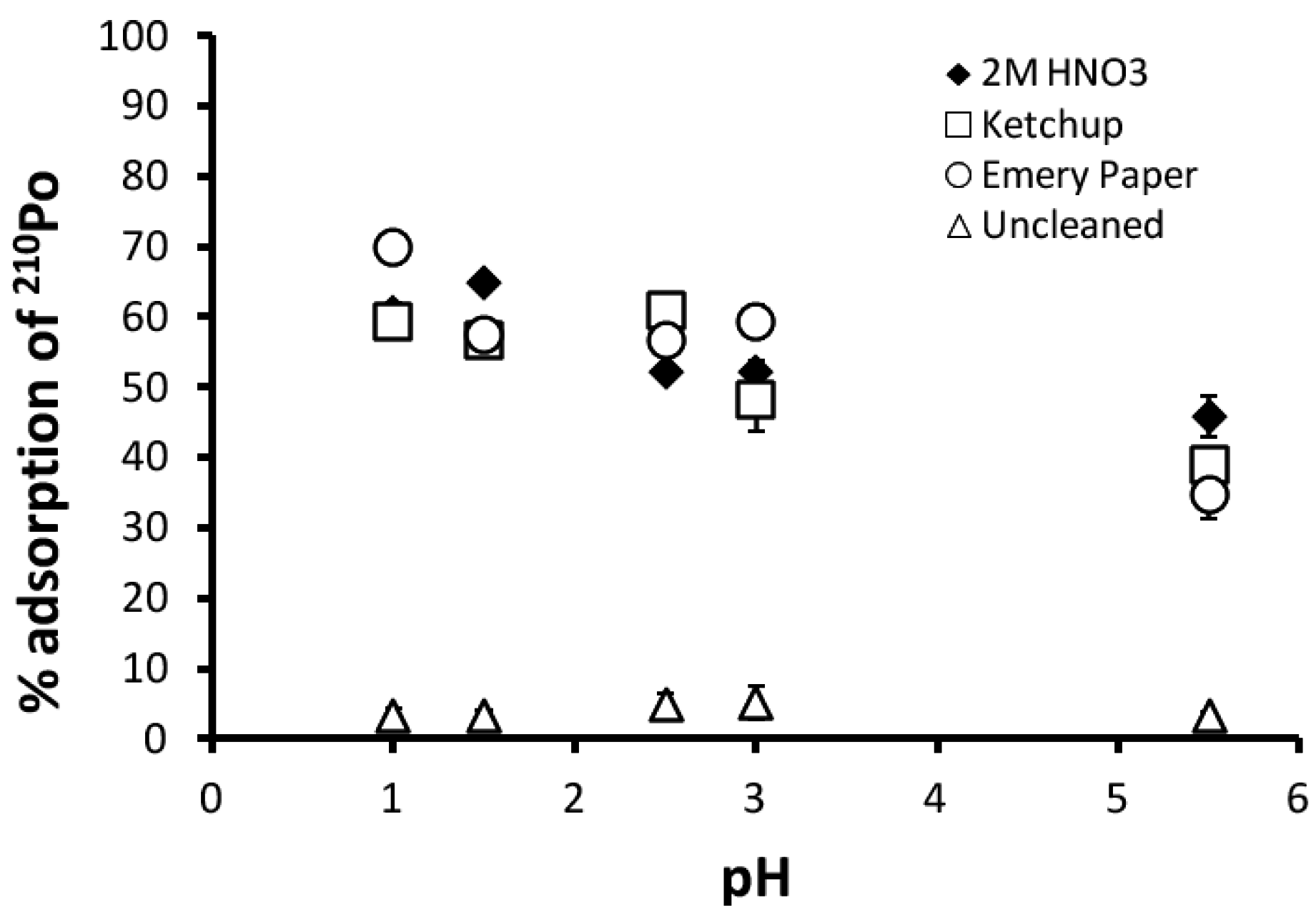
3.1. Adsorption
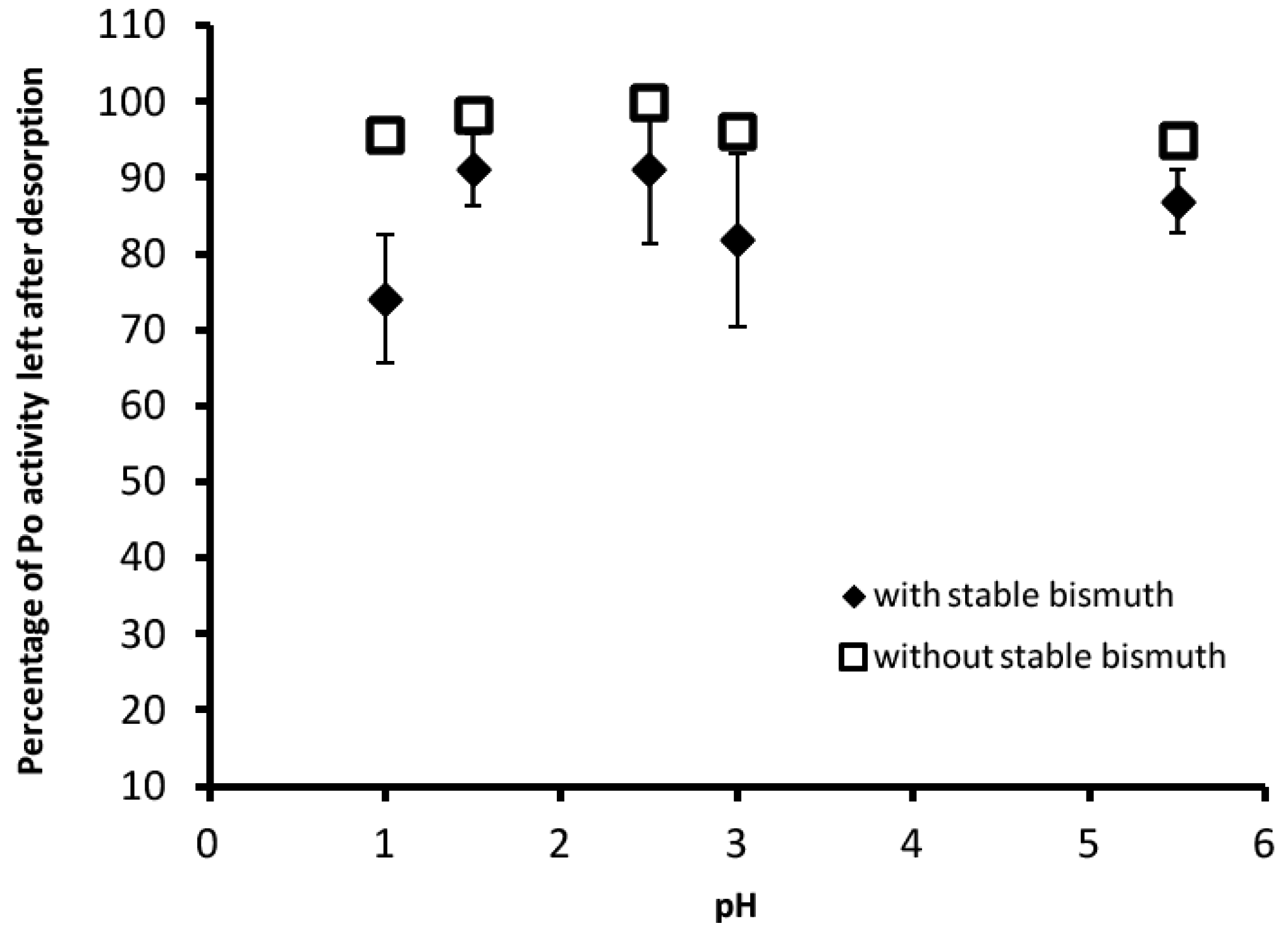
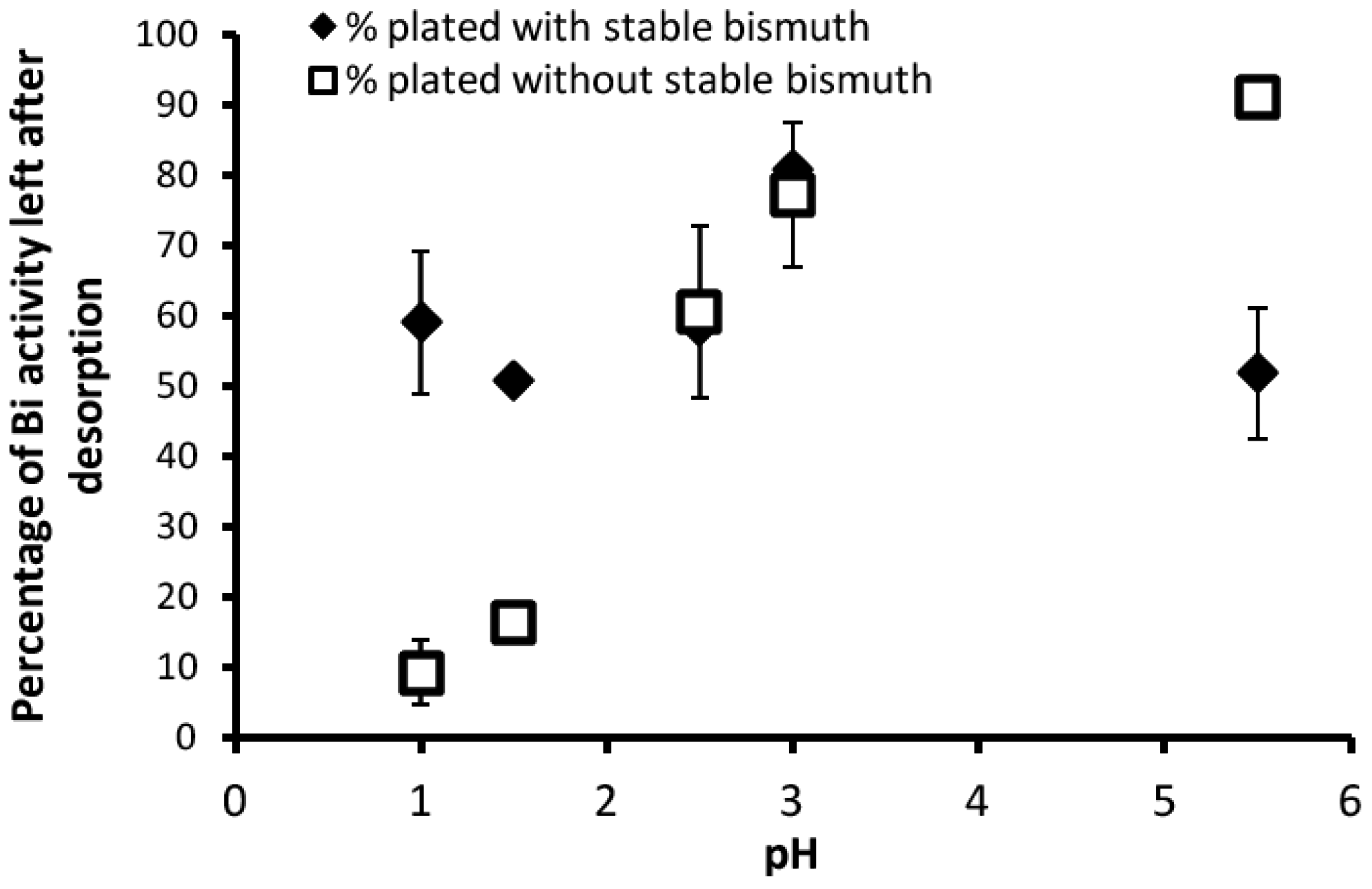
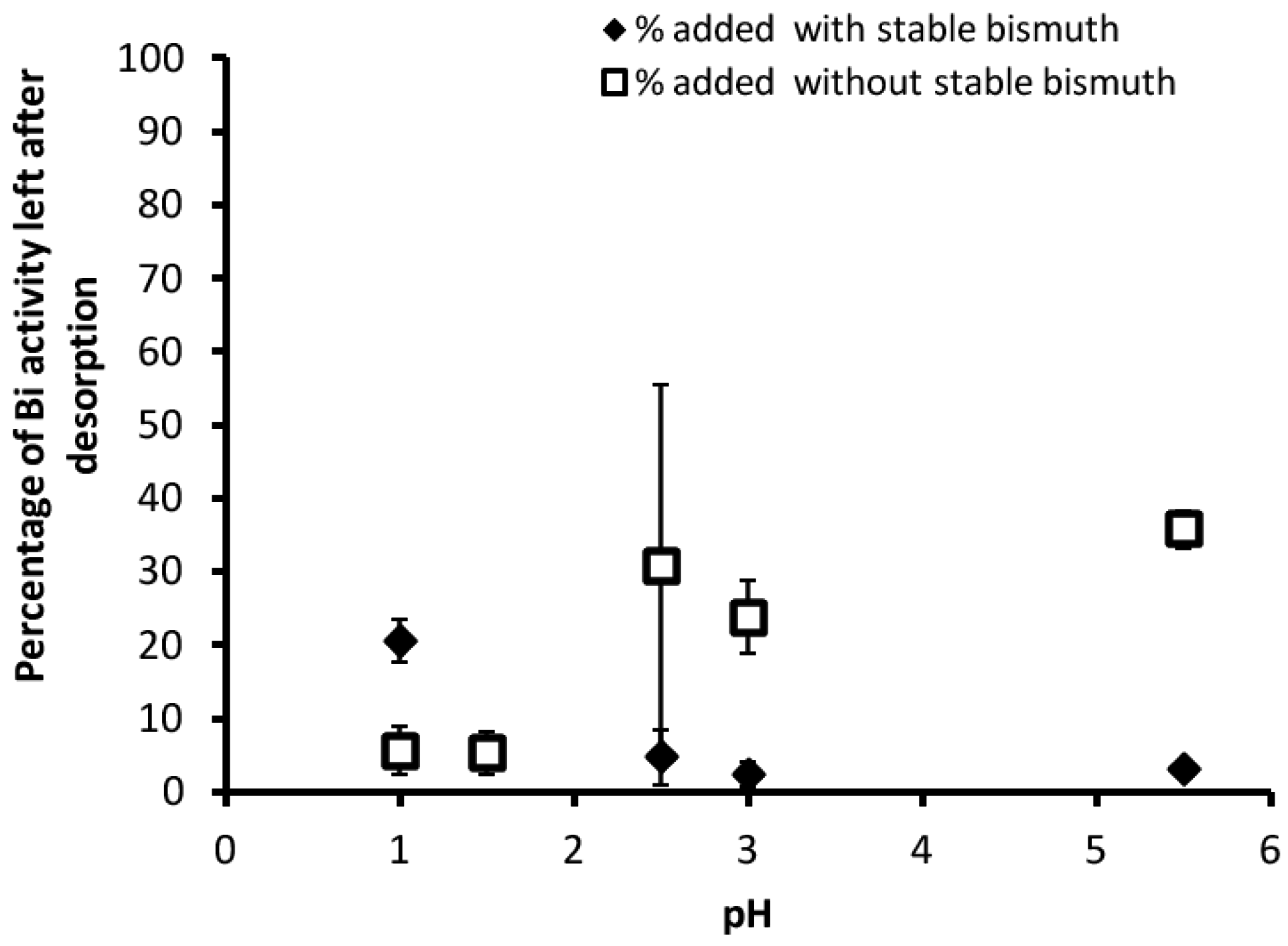
3.2. Desorption
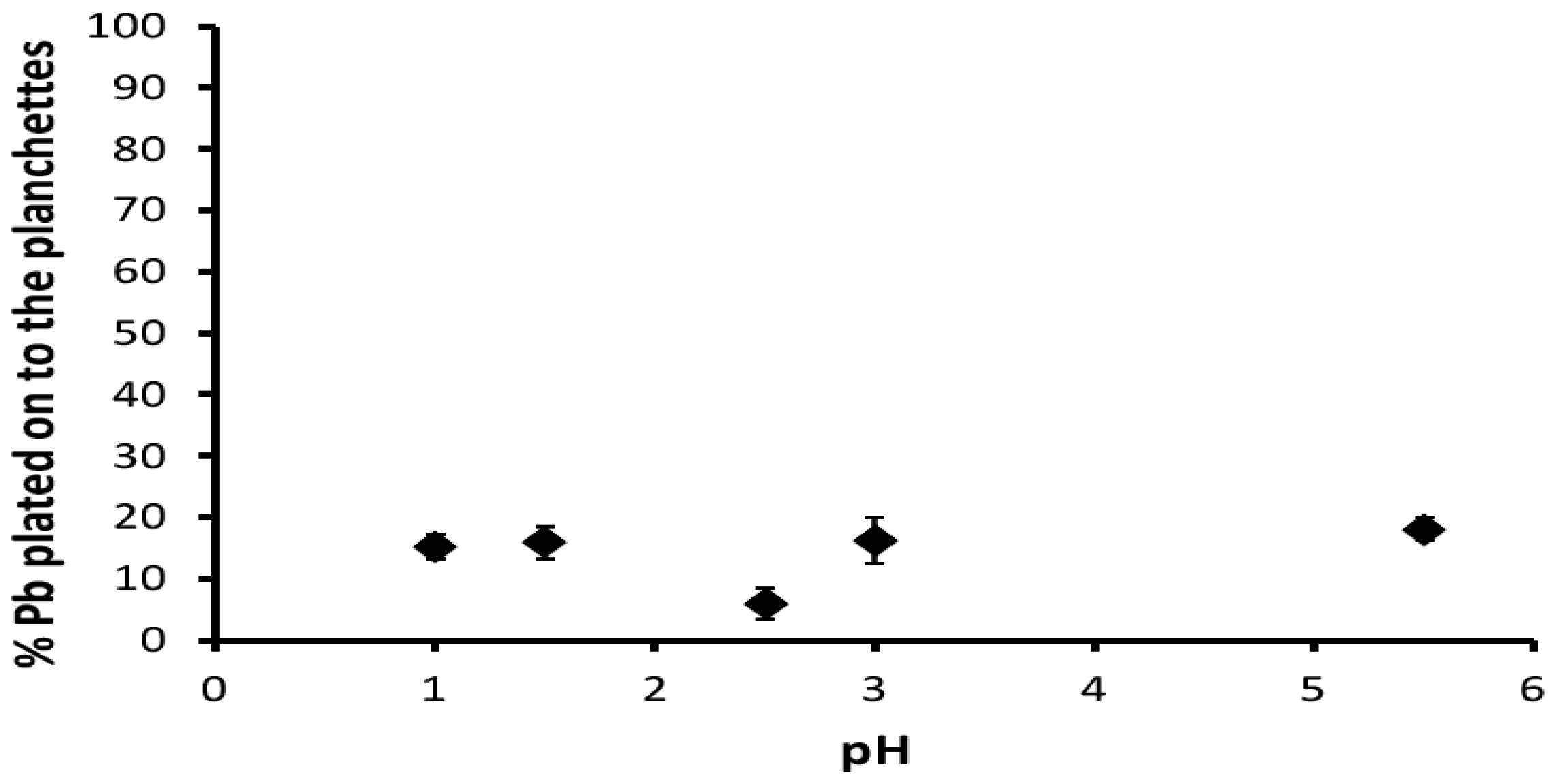
3.3. Coating and Cleaning Methods for the Copper Foils
3.4. Verification of Method Using Certified Reference Material from the International Atomic Energy Agency (IAEA) and Sediment Samples from Nigeria
4. Conclusions
Acknowledgment
Conflicts of Interest
References
- Jia, G.G.; Belli, M.; Blasi, M.; Marchetti, A.; Rosamilia, S.; Sansone, U. Determination of Pb-210 and Po-210 in mineral and biological environmental samples. J. Radioanal. Nucl. Chem. 2001, 247, 491–499. [Google Scholar] [CrossRef]
- Al-Masri, M.S.; Nashawati, A.; Amin, Y.; Al-akel, B. Determination of Po-210 in tea, mate and their infusions and its annual intake by Syrians. J. Radioanal. Nucl. Chem. 2004, 260, 27–34. [Google Scholar] [CrossRef]
- Al-Masri, M.S.; Mukallati, H.; Al-Hamwi, A.; Khalili, H.; Hassan, M.; Assaf, H. Natural radionuclides in Syrian diet and their daily intake. J. Radioanal. Nucl. Chem. 2004, 260, 405–412. [Google Scholar] [CrossRef]
- Henricsson, F.; Ranebo, Y.; Holm, E.; Roos, P. Aspects on the analysis of 210Po. J. Environ. Radioact. 2011, 102, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Mathew, K.M.; Kim, C.K.; Martin, P. Determination of 210Po in environmental materials: A review of analytical methodology. Appl. Radiat. Isotopes 2007, 65, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Cabeza, J.A.; Masque, P.; Ani-Ragolta, I.; Merino, J.; Frignani, M.; Alvisi, F.; Palanques, A.; Puig, P. Sediment accumulation rates in the southern Barcelona continental margin (NW Mediterranean Sea) derived from Pb-210 and Cs-137 chronology. Prog. Oceanogr. 1999, 44, 313–332. [Google Scholar] [CrossRef]
- Stewart, G.M.; Fisher, N.S. Experimental studies on the accumulation of polonium-210 by marine phytoplankton. Limnol. Oceanogr. 2003, 48, 1193–1201. [Google Scholar] [CrossRef]
- Garcia-Orellana, I.; Garcia-Leon, M. An easy method to determine Po-210 and Pb-210 by alpha spectrometry in marine environmental samples. Appl. Radiat. Isotopes 2002, 56, 633–636. [Google Scholar] [CrossRef]
- Su, C.-C.; Huh, C.-A. Atmospheric 210Po anomaly as a precursor of volcano eruptions. Geophys. Res. Lett. 2002, 29, 14. [Google Scholar] [CrossRef]
- Fowler, S.W. 210Po in the marine environment with emphasis on its behaviour within the biosphere. J. Environ. Radioact. 2011, 102, 448–461. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.J.; Hou, X.L.; Dahlgaard, H.; Nielsen, S.P.; Aarkrog, A. A rapid method for the separation of Po-210 from Pb-210 by TIOA extraction. J. Radioanal. Nucl. Chem. 2001, 249, 587–593. [Google Scholar]
- Bustamante, P.; Germain, P.; Leclerc, G.; Miramand, P. Concentration and distribution of Po-210 in the tissues of the scallop Chlamys varia and the mussel Mytilus edulis from the Coasts of Charente-Maritime (France). Mar. Pollut. Bull. 2002, 44, 997–1002. [Google Scholar] [CrossRef]
- Faanhof, A.; Louw, I. The measurement of natural radioactivity in fish and the impact on humans. J. Radioanal. Nucl. Chem. 2001, 249, 227–232. [Google Scholar]
- Babatunde, B.B.; Sikoki, F.D.; Hart, I. Human health impact of natural and artificial radioactivity levels in the sediments and fish of Bonny Estuary, Niger Delta, Nigeria. Challenges 2015, 6, 244–257. [Google Scholar] [CrossRef]
- Din, K.S. Determination of Po-210 in various foodstuffs and its annual effective dose to inhabitants of Qena City, Egypt. Sci. Total Environ. 2011, 409, 5301–5304. [Google Scholar] [CrossRef] [PubMed]
- McDonald, P.; Jackson, D.; Leonard, D.R.P.; Mckay, K. An assessment of 210Pb and 210Po in terrestrial foodstuffs from regions of England and Wales. J. Environ. Radioact. 1999, 43, 15–29. [Google Scholar] [CrossRef]
- Martin, P.; Hancook, G.J. Routine Analysis of Naturally Occurring Radionuclides in Environmental Samples by Alpha-Particle Spectrometry; Supervising Scientist Report 180; AGPS: Canberra, Australia, 2004. [Google Scholar]
- Persson, B.R.R.; Holm, E. Polonium-210 and lead-210 in the terrestrial environment: A historical review. J. Environ. Radioact. 2011, 102, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Flynn, W.W. Determination of low levels of polonium-210 in environmental materials. Anal. Chim. Acta 1968, 43, 221–234. [Google Scholar] [CrossRef]
- Mackenzie, A.B.; Scott, R.D. Separation of Bi-210 and Po-210 from aqueous-solutions by spontaneous adsorption on copper foils. Analyst 1979, 104, 1151–1158. [Google Scholar] [CrossRef]
- Karali, T.; Olmez, S.; Yener, G. Study of spontaneous deposition of Po-210 on various metals and application for activity assessment in cigarette smoke. Appl. Radiat. Isotopes 1996, 47, 409–411. [Google Scholar] [CrossRef]
- Clayton, R.F.; Bradley, E.J. A cost effective method for the determination of Po-210 and Pb-210 in environmental materials. Sci. Total Environ. 1995, 173, 23–28. [Google Scholar] [CrossRef]
- Holtzman, R.B. The determination of Pb-210 and Po-210 in biological and environmental materials. J. Radioanal. Nucl. Chem. 1987, 115, 59–70. [Google Scholar] [CrossRef]
- Jia, G.G.; Belli, M.; Blasi, M.; Marchetti, A.; Rosamilia, S.; Sansone, U. Pb-210 and Po-210 determination in environmental samples. Appl. Radiat. Isotopes 2000, 53, 115–120. [Google Scholar] [CrossRef]
- Vajda, N.; Larosa, J.; Zeisler, R.; Danesi, P.; Kisbenedek, G. A novel technique for the simultaneous determination of Pb-210 and Po-210 using a crown ether. J. Environm. Radioact. 1997, 37, 355–372. [Google Scholar] [CrossRef]
- Benedik, L.; Vrecek, P. Determination of Pb-210 and Po-210 in environmental samples. Acta Chim. Slov. 2001, 48, 199–213. [Google Scholar]
- Vrecek, P.; Benedik, L.; Pihlar, B. Determination of Pb-210 and Po-210 in sediment and soil leachates and in biological materials using a Sr-resin column and evaluation of column reuse. Appl. Radiat. Isotopes 2004, 60, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Godoy, J.M.; Schuttelkopf, H.S. A Radiochemical Procedure for the Determination of Po-210 in Environmental Samples; Kernforschungszentrum Karlsruhe: Karlsruhe, Germany, 1980. [Google Scholar]

| pH | 1st Measurement | 2nd Measurement |
|---|---|---|
| 1 | 64 ± 2 | 66 ± 4 |
| 1.5 | 66.3 ± 3 | 66.5 ± 3 |
| 2.5 | 62.2 ± 7 | 63.8 ± 7 |
| 3 | 64.4 ± 2 | 64 ± 1 |
| 5.5 | 44.4 ± 3 | 45 ± 2 |
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babatunde, B.B. Optimization of Plating Conditions for the Determination of Polonium Using Copper Foils. Challenges 2016, 7, 18. https://doi.org/10.3390/challe7020018
Babatunde BB. Optimization of Plating Conditions for the Determination of Polonium Using Copper Foils. Challenges. 2016; 7(2):18. https://doi.org/10.3390/challe7020018
Chicago/Turabian StyleBabatunde, Bolaji Benard. 2016. "Optimization of Plating Conditions for the Determination of Polonium Using Copper Foils" Challenges 7, no. 2: 18. https://doi.org/10.3390/challe7020018






