The Dichotomy of the Poly(ADP-Ribose) Polymerase-Like Thermozyme from Sulfolobus solfataricus
Abstract
:1. Introduction
2. The Poly(ADP-Ribose)Polymerase-Like Enzyme in S. solfataricus
3. PARPSso and the World of PARPs
4. PARPSso Belongs to the DING Protein Family
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Woese, C.R.; Kandler, O.; Wheelis, M.L. Towards a natural system of organisms: Proposal for the domains Archaea, Bacteria and Eucarya. Proc. Natl. Acad. Sci. USA 1990, 87, 4576–4579. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, P.; Thode, S.; Hancke, J.; Vielmetter, W. Mechanisms of overlap formation in non homologous DNA end joining. Mol. Cell. Biol. 1994, 14, 888–895. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, M.; Gambacorta, A.; Bu’lock, J.D. Extremely thermophilic acidophilic bacteria convergent with Sulfolobus acidocaldarius. J. Gen. Microbiol. 1975, 86, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Singh, Q.; Confalonieri, R.K.; Zivanovic, Y.; Allard, G.; Awayez, M.J.; Chan-Weiher, C.C.; Clausen, I.G.; Curtis, B.A.; De Moors, A.; Erauso, G.; et al. The complete genome of the Crenarchaeon Sulfolobus solfataricus P2. Proc. Natl. Acad. Sci. USA 2001, 98, 7835–7840. [Google Scholar]
- Kessel, M.; Klink, F. Archaebacterial elongation factor is ADP-ribosylated by diphtheria toxin. Nature 1980, 287, 250–251. [Google Scholar] [CrossRef] [PubMed]
- Honjo, T.; Nishizuka, Y.; Hayaishi, O.; Kato, I. Diphtheria Toxin-dependent Adenosine Diphosphate Ribosylation of Aminoacyl Transferase II and Inhibition of Protein Synthesis. J. Biol. Chem. 1968, 243, 3553–3555. [Google Scholar] [PubMed]
- Quesada, P.; Faraone-Mennella, M.R.; De Rosa, M.; Gambacorta, A.; Nicolaus, B.; Farina, B. ADP-Ribosylating Activity in Sulfolobus solfataricus. In ADP-Ribose Transfer Reactions; Jacobson, M.K., Jacobson, E., Eds.; Springer: New York, NY, USA, 1989; pp. 101–104. [Google Scholar]
- Faraone-Mennella, M.R.; De Lucia, F.; Quesada, P.; De Rosa, M.; Gambacorta, A.; Nicolaus, B.; Farina, B. Heterogeneity of ADP-ribosylation Reaction in Sulfolobus solfataricus. In ADP-Ribosylation Reactions; Poirier, G.G., Moreau, P., Eds.; Springer: New York, NY, USA, 1992; pp. 369–372. [Google Scholar]
- Faraone-Mennella, M.R.; De Lucia, F.; De Maio, A.; Gambacorta, A.; Quesada, P.; De Rosa, M.; Nicolaus, B.; Farina, B. ADP-ribosylation reactions in Sulfolobus solfataricus, a thermoacidophilic archaeon. Biochim. Biophys. Acta 1995, 1246, 151–159. [Google Scholar] [CrossRef]
- Gambacorta, A.; Gliozzi, A.; De Rosa, M. Archaeal lipids and their biotechnological applications. World J. Microbiol. Biotechnol. 1995, 11, 115–131. [Google Scholar] [CrossRef] [PubMed]
- Nicolaus, B.; Trincone, A.; Lama, L.; Romano, I.; Marsiglia, F.; Gambacorta, A. Adaptation of Sulfolobus solfataricus on minimal media. Biotechnol. Lett. 1991, 13, 667–670. [Google Scholar] [CrossRef]
- Faraone-Mennella, M.R.; Gambacorta, A.; Nicolaus, B.; Farina, B. Purification and biochemical characterization of a poly(ADP-ribose) polymerase-like enzyme from the thermophilic archaeon Sulfolobus solfataricus. Biochem. J. 1998, 335, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Faraone-Mennella, M.R.; Gambacorta, A.; Nicolaus, B.; Farina, B. Immunochemical detection of ADP-ribosylating enzymes in the archaeon Sulfolobus solfataricus. FEBS Lett. 1996, 378, 199–201. [Google Scholar] [CrossRef]
- De Maio, A.; Porzio, E.; D’Angelo, R.; Rotondo, S.; Bianchi, A.R.; Confalone, E.; Raucci, R.; Natale, E.; Faraone-Mennella, M.R. A Glycosyltransferase from Sulfolobus solfataricus MT-4 Exhibits Poly(ADP-ribose) Glycohydrolase Activity. Curr. Proteom. 2015, 12, 253–263. [Google Scholar] [CrossRef]
- Faraone-Mennella, M.R.; De Lucia, F.; De Maio, A.; Gambacorta, A.; Nicolaus, B.; Farina, B. ADPribosylation reaction by free ADPribose in Sulfolobus solfataricus, a thermophilic archaeon. J. Cell. Biochem. 1997, 66, 37–42. [Google Scholar] [CrossRef]
- Faraone-Mennella, M.R.; Castellano, S.; De Luca, P.; Discenza, A.; Gambacorta, A.; Nicolaus, B.; Farina, B. Comparison of the ADP-ribosylatingthermozyme from Sulfolobus solfataricus and the mesophilic poly(ADP-ribose) polymerases. FEMS Microbiol. Lett. 2000, 192, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Faraone-Mennella, M.R.; Discenza, A.; Gambacorta, A.; Nicolaus, B.; Farina, B. Purification of the ADP-ribosylating enzyme from S. solfataricus by SDS-polyacrylamide gel electrophoresis and electroelution. Prep. Biochem. Biotechnol. 2000, 30, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Faraone-Mennella, M.R.; De Luca, P.; Giordano, A.; Gambacorta, A.; Nicolaus, B.; Farina, B. High stability binding of poly(ADPribose) polymerase-like thermozyme from S. solfataricus with circular DNA. J. Cell. Biochem. 2002, 85, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Faraone-Mennella, M.R.; Farina, B. In the thermophilic archaeon Sulfolobus solfataricus a DNA-binding protein is in vitro (ADP-ribosyl)ated. Biochem. Biophys. Res. Commun. 1995, 208, 55–62. [Google Scholar]
- Reeve, J.N. Archaeal chromatin and transcription. Mol. Microbiol. 2003, 48, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Baumann, H.; Knapp, S.; Lundback, T.; Ladenstein, R.; Hard, T. Solution structure and DNA-binding properties of a thermostable protein from the archaeon Sulfolobus solfataricus. Nat. Struct. Biol. 1994, 1, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Guagliardi, A.; Cerchia, L.; Rossi, M. The Sso7d protein of Sulfolobus solfataricus: In Vitro relationship among different activities. Archaea 2002, 1, 87–93. [Google Scholar] [CrossRef] [PubMed]
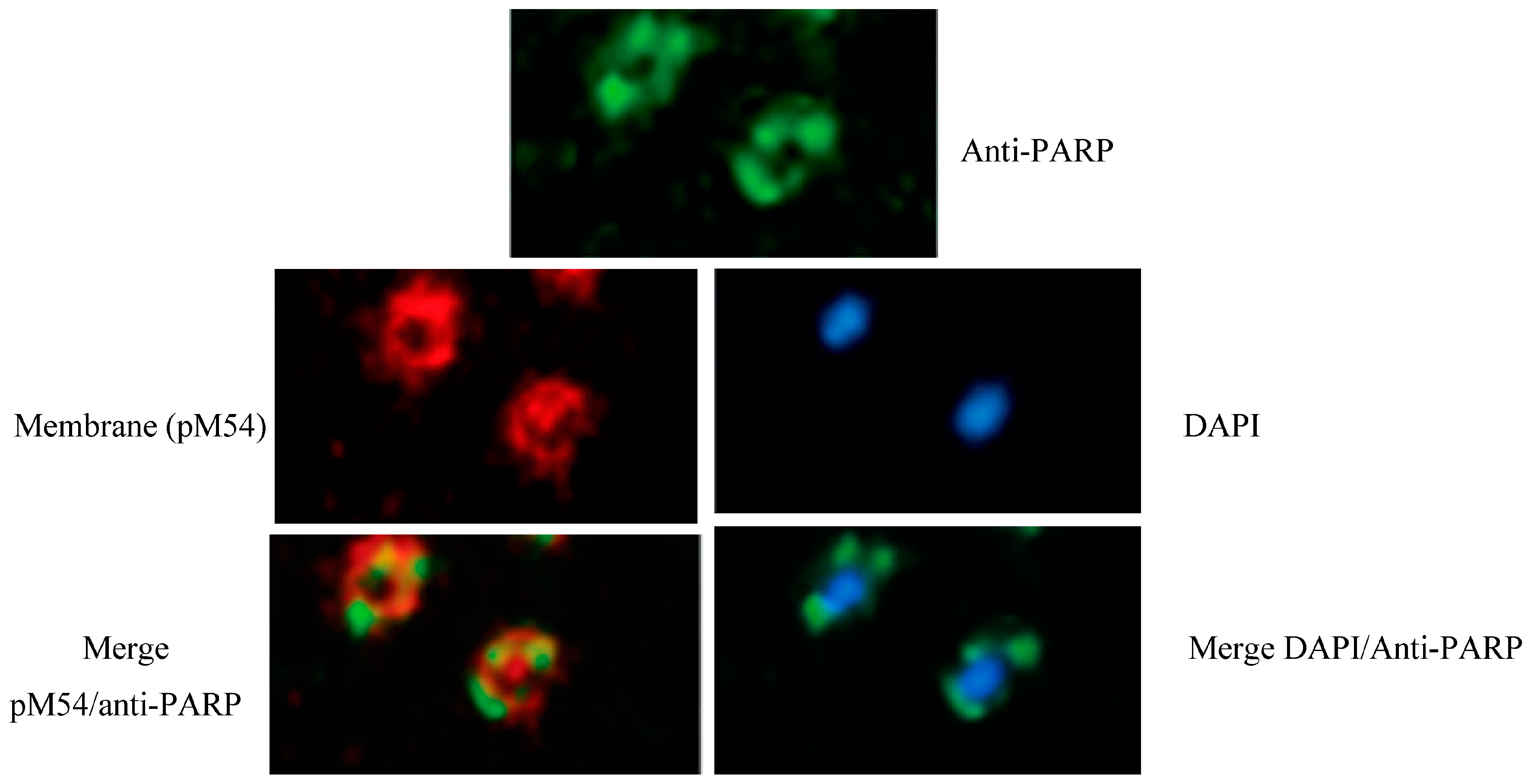
- Porzio, E.; Bianchi, A.R.; Baccigalupi, L.; Isticato, R.; Faraone Mennella, M.R. The DINGGG thermoprotein is membrane bound in the Crenarchaeon Sulfolobus solfataricus. Chem. Biol. Technol. Agric. 2016, 3. [Google Scholar] [CrossRef]
- Doly, J.; Mandel, P. Demonstration of the biosynthesis in vivo of a compound polymer, polyadenosine diphosphoribose in the nucleus of the liver of chickens. C. R. Acad. Sci. Hebd. Seances Acad. Sci. D 1967, 264, 2687–2690. [Google Scholar] [PubMed]
- Zahradka, P.; Ebisuzaki, K. Poly(ADP-ribose) polymerase is a zinc metalloenzyme. Eur. J. Biochem. 1984, 142, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Mazen, A.; Menissier-de Murcia, J.; Molinete, M.; Simonin, F.; Gradwohl, G.; Poirier, G.; de Murcia, G. Poly(ADP-ribose)polymerase: A novel finger protein. Nucleic Acids Res. 1989, 17, 4689–4698. [Google Scholar] [CrossRef] [PubMed]
- Amé, J.C.; Rolli, V.; Schreiber, V.; Niedergang, C.; Apiou, F.; Decker, P.; Muller, S.; Höger, T.; Ménissier-de Murcia, J.; de Murcia, G. PARP-2, A novel mammalian DNA damage-dependent poly(ADP-ribose) polymerase. J. Biol. Chem. 1999, 274, 17860–17868. [Google Scholar] [CrossRef] [PubMed]
- Hottiger, M.O.; Hassa, P.O.; Luscher, B.; Schuler, H.; Koch-Nolte, F. Toward a unified nomenclature for mammalian ADP-ribosyltransferases. Trends Biochem. Sci. 2010, 35, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Citarelli, M.; Teotia, S.; Lamb, R.S. Evolutionary history of the poly(ADP-ribose) polymerase gene family in eukaryotes. BMC Evol. Biol. 2010, 10, 308. [Google Scholar] [CrossRef] [PubMed]
- Helge, O.; Reche, P.A.; Bazan, F.; Dittmar, K.; Haag, F.; Koch-Nolte, F. In Silico characterization of the family of PARP-like poly(ADP-ribosyl)transferases (pARTs). BMC Genomics 2005, 6, 139. [Google Scholar] [CrossRef]
- Perina, D.; Mikoč, A.; Ahel, J.; Ćetković, H.; Žaja, R.; Ahel, I. Distribution of protein poly(ADP-ribosyl)ation systems across all domains of life. DNA Repair (Amst) 2014, 23, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Velasco de Castro Oliveira, J.; de Melo, F.L.; Malta Romano, C.; Iamarino, A.; Sampaio Rizzi, T.; Peres Yeda, F.; Hársi, C.M.; Caldas Wolff, J.L.; Marinho de Andrade Zanotto, P. Structural and phylogenetic relationship of ORF 31 from the Anticarsiagemmatalis MNPV to poly (ADP-ribose) polymerases (PARP). Virus Genes 2008, 37, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Semighini, C.P.; Savoldi, M.; Goldman, G.H.; Harris, S.D. Functional characterization of the putative Aspergillus nidulans poly(ADP-ribose) polymerase homolog PrpA. Genetics 2006, 173, 87–98. [Google Scholar] [CrossRef] [PubMed]
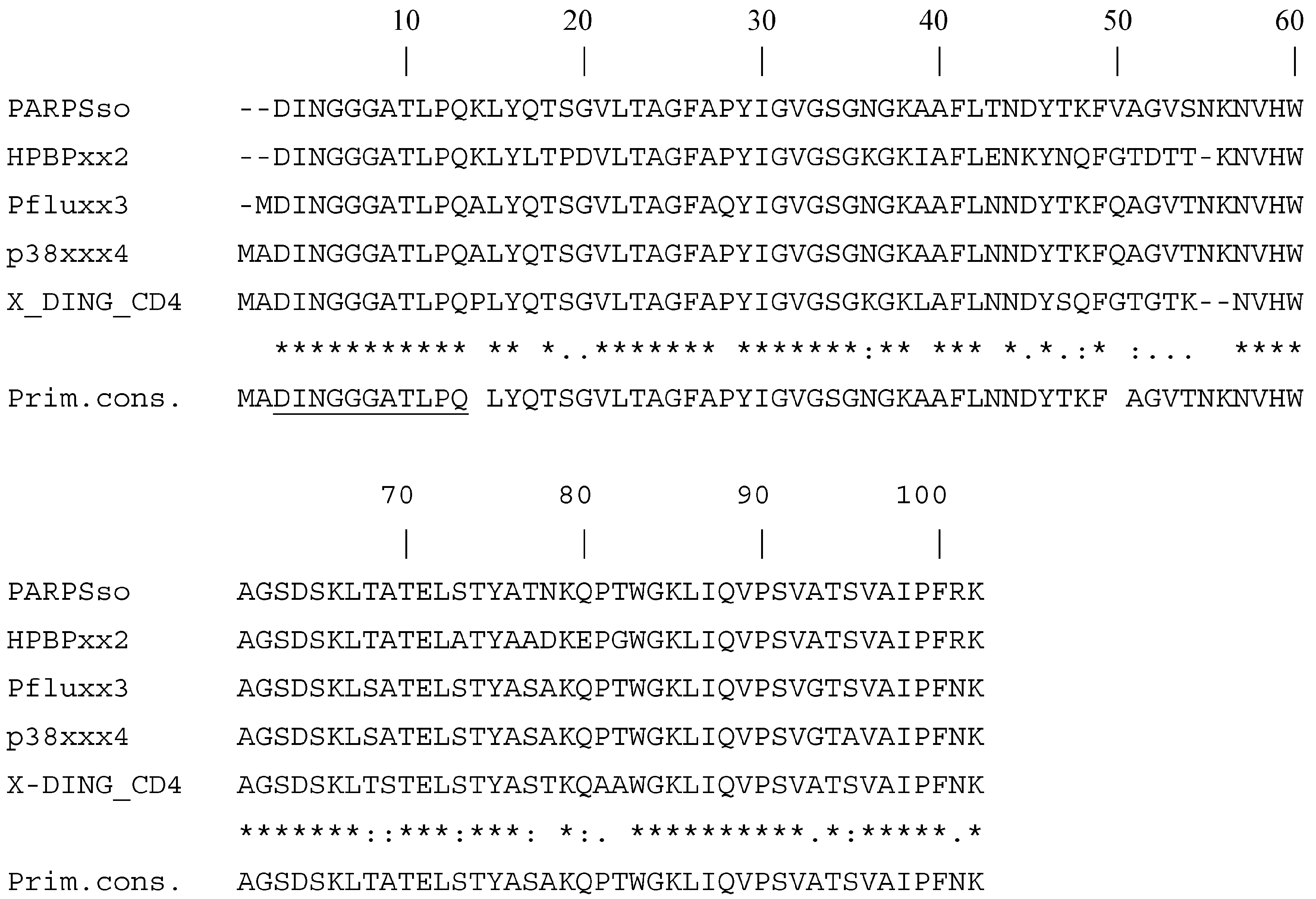
- Di Maro, A.; De Maio, A.; Castellano, S.; Parente, A.; Farina, B.; Faraone-Mennella, M.R. The ADPribosylatingthermozyme from Sulfolobus solfataricus is a DING protein. Biol. Chem. 2009, 390, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Berna, A.; Scott, K.; Chabrière, E.; Bernier, F. The DING family of proteins: Ubiquitous in eukaryotes, but where are the genes? Bioessays 2009, 31, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Bernier, F. DING proteins: Numerous functions, elusive genes, a potential for health. Cell. Mol. Life Sci. 2013, 70, 3045–3056. [Google Scholar] [CrossRef] [PubMed]
- Porzio, E. The Relationship of the ADP-Ribosylating Enzyme from S. solfataricus with DING Proteins and Its Intracellular Localization. Ph.D. Thesis, University of Naples “Federico II”, Naples, Italy, 2010. [Google Scholar]
- Porzio, E.; De Maio, A.; Ricciardi, T.; Mistretta, C.; Manco, G.; Faraone-Mennella, M.R. Comparison of the archaeal DING protein from the archaeon Sulfolobus solfataricus with Human Phosphate Binding Protein and Pseudomonas fluorescence DING counterparts. Extremophiles 2018. [Google Scholar] [CrossRef] [PubMed]
- Faraone-Mennella, M.R.; Scarpa, R.; Petrella, A.; Manguso, F.; Peluso, R.; Farina, B. Detecting clinical activity in systemic lupus erythematosus with an archaeal poly(ADP-ribose) polymerase-like thermozyme: A pivotal study. Biomarkers 2009, 14, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, R.; Darbinian, N.; Khalili, K.; Amini, S.; Gonzalez, D.; Djeghader, A.; Chabriére, E.; Suh, A.; Scott, K.; Simm, M. DING Proteins from Phylogenetically Different Species Share High Degrees of Sequence and Structure Homology and Block Transcription of HIV-1 LTR Promoter. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faraone Mennella, M.R. The Dichotomy of the Poly(ADP-Ribose) Polymerase-Like Thermozyme from Sulfolobus solfataricus. Challenges 2018, 9, 5. https://doi.org/10.3390/challe9010005
Faraone Mennella MR. The Dichotomy of the Poly(ADP-Ribose) Polymerase-Like Thermozyme from Sulfolobus solfataricus. Challenges. 2018; 9(1):5. https://doi.org/10.3390/challe9010005
Chicago/Turabian StyleFaraone Mennella, Maria Rosaria. 2018. "The Dichotomy of the Poly(ADP-Ribose) Polymerase-Like Thermozyme from Sulfolobus solfataricus" Challenges 9, no. 1: 5. https://doi.org/10.3390/challe9010005




