The Current Role of Image Compression Standards in Medical Imaging
Abstract
:1. Introduction
2. Characteristics of Medical Imaging Data Sets
2.1. Digital Radiography and Computed Tomography
2.2. Magnetic Resonance Imaging
2.3. Ultrasound
2.4. Nuclear Imaging
2.5. Digital Pathology
3. Image Compression Standards
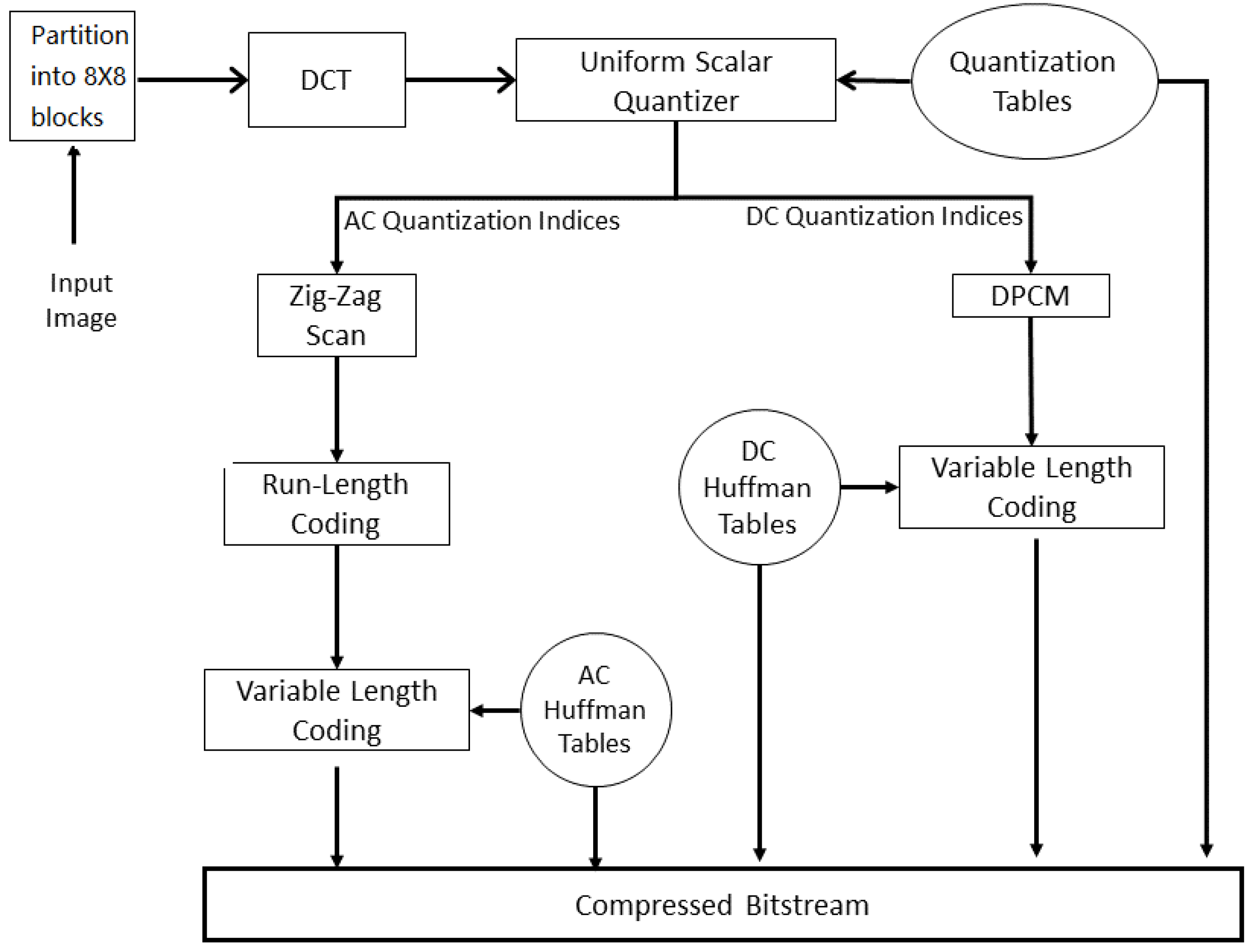
3.1. JPEG
3.2. JPEG2000
3.3. JPEG-LS
3.4. JPEG-XR
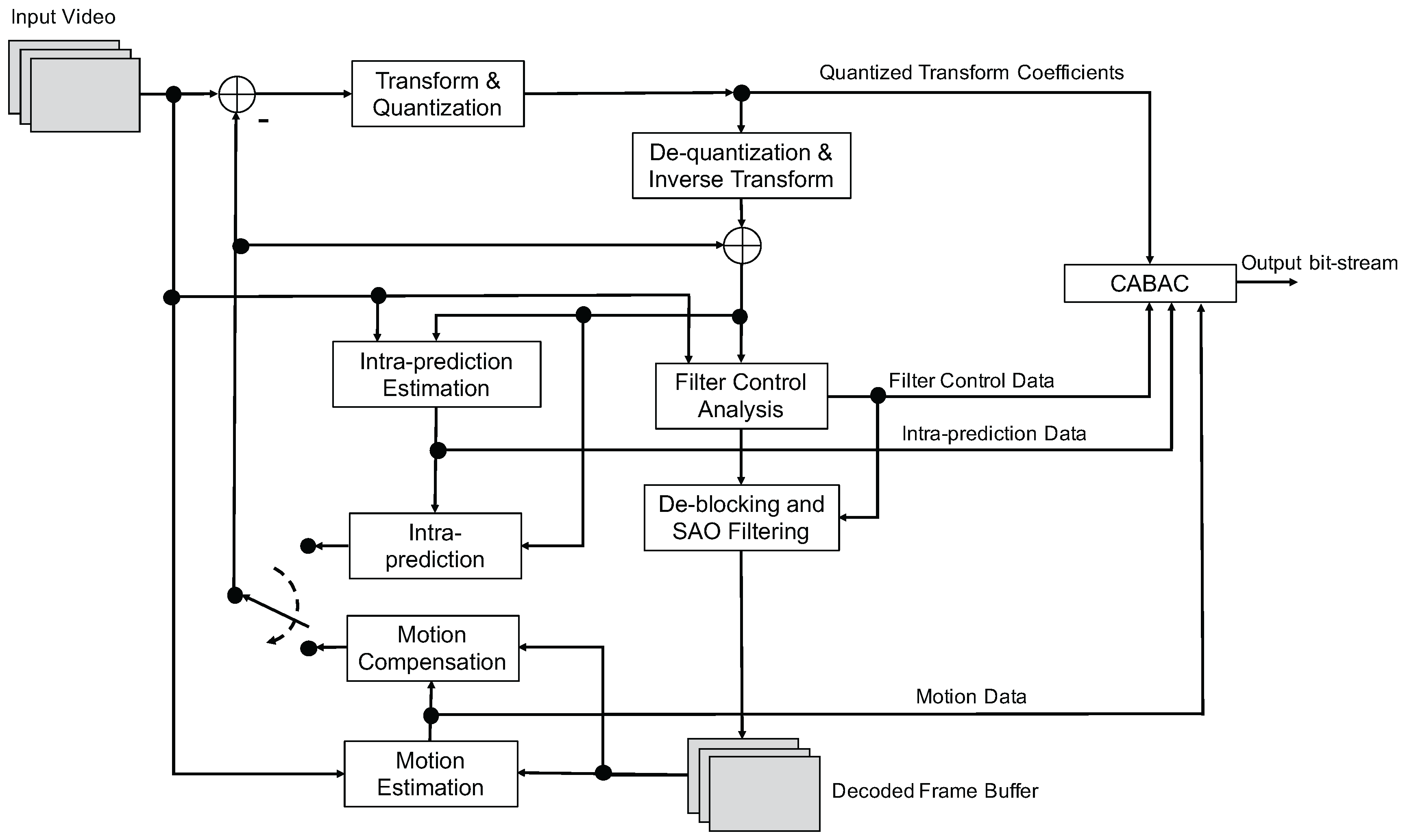
3.5. H.265
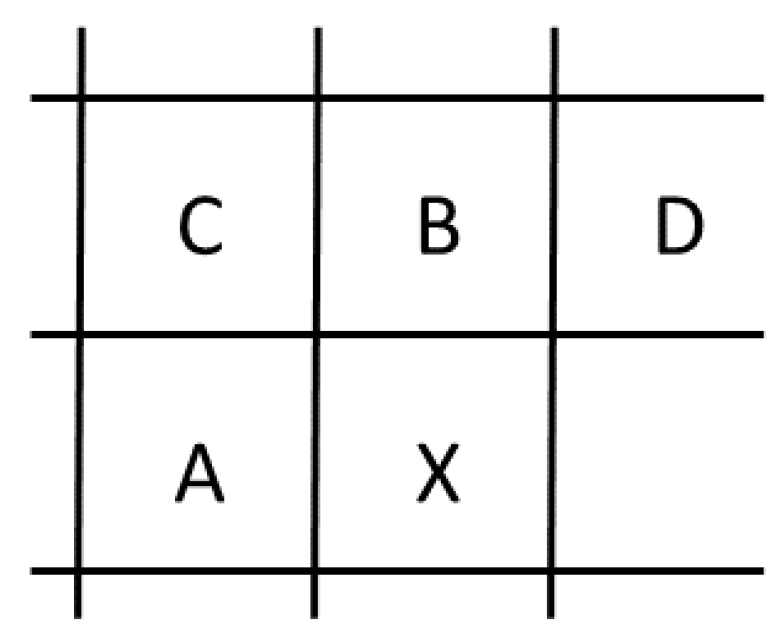
- 35 prediction modes, with 33 angular modes, one DC and one planar mode;
- adaptive smoothing of the reference samples; and
- filtering of the prediction block boundary samples.
- improved motion compensation with quarter-sample precision for motion vectors (MVs), and 7-tap or 8-tap filters for interpolation of fractional sample positions;
- multiple reference pictures that allows transmitting one or two MVs for each block, resulting in unipredictive or bipredictive coding, respectively;
- advanced motion vector prediction, which includes derivation of several most probable candidate MVs based on data from adjacent blocks and the reference frame; and
- sample adaptive offset (SAO), which is a nonlinear amplitude mapping used after the deblocking filter with the objective to better reconstruct the original signal amplitudes.
4. Standards in Medical Image Communications
5. Legal and Regulatory Environment
6. Compression Performance of Image Compression Standards on Medical Data Sets
6.1. Lossless Compression
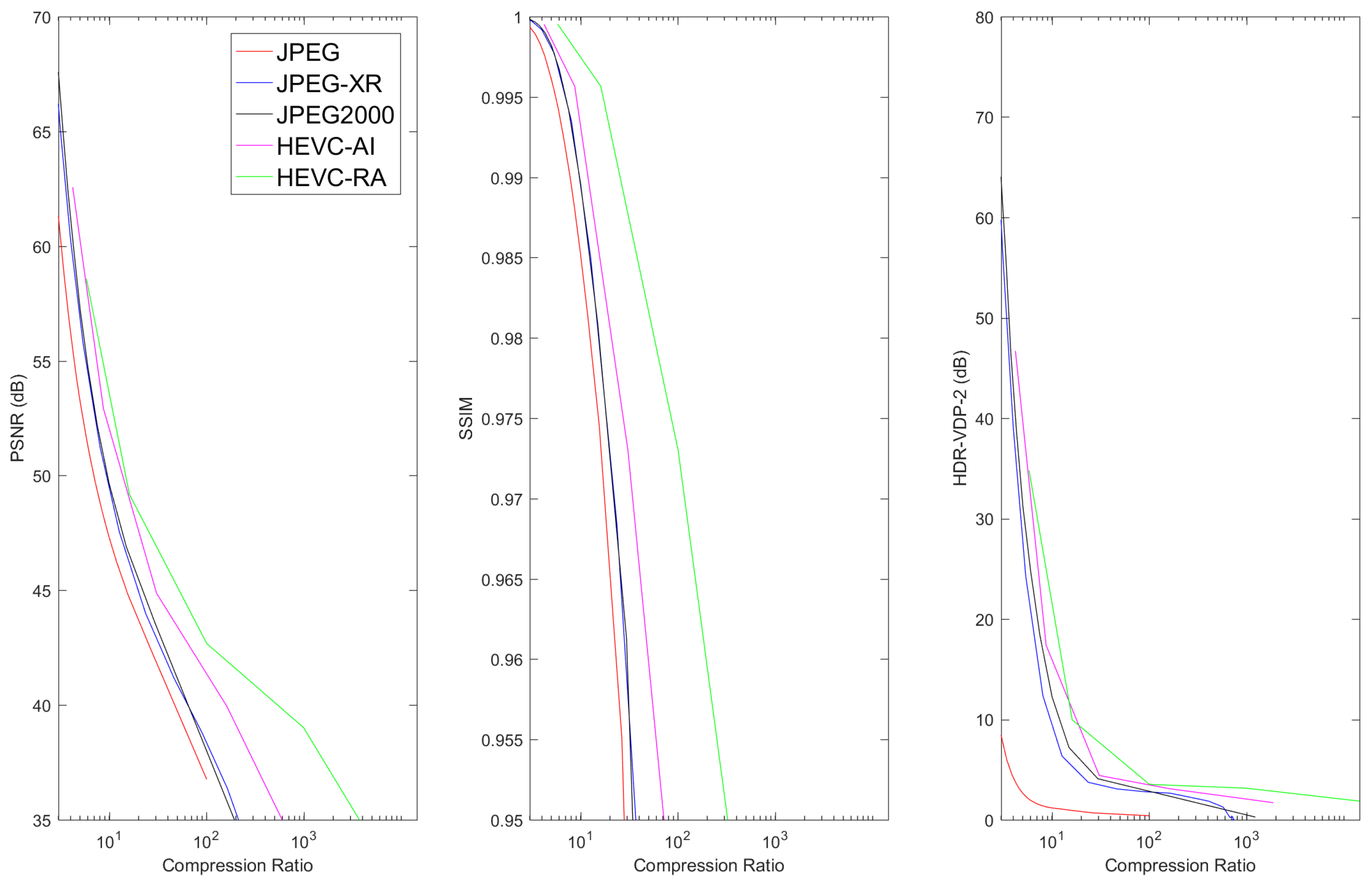
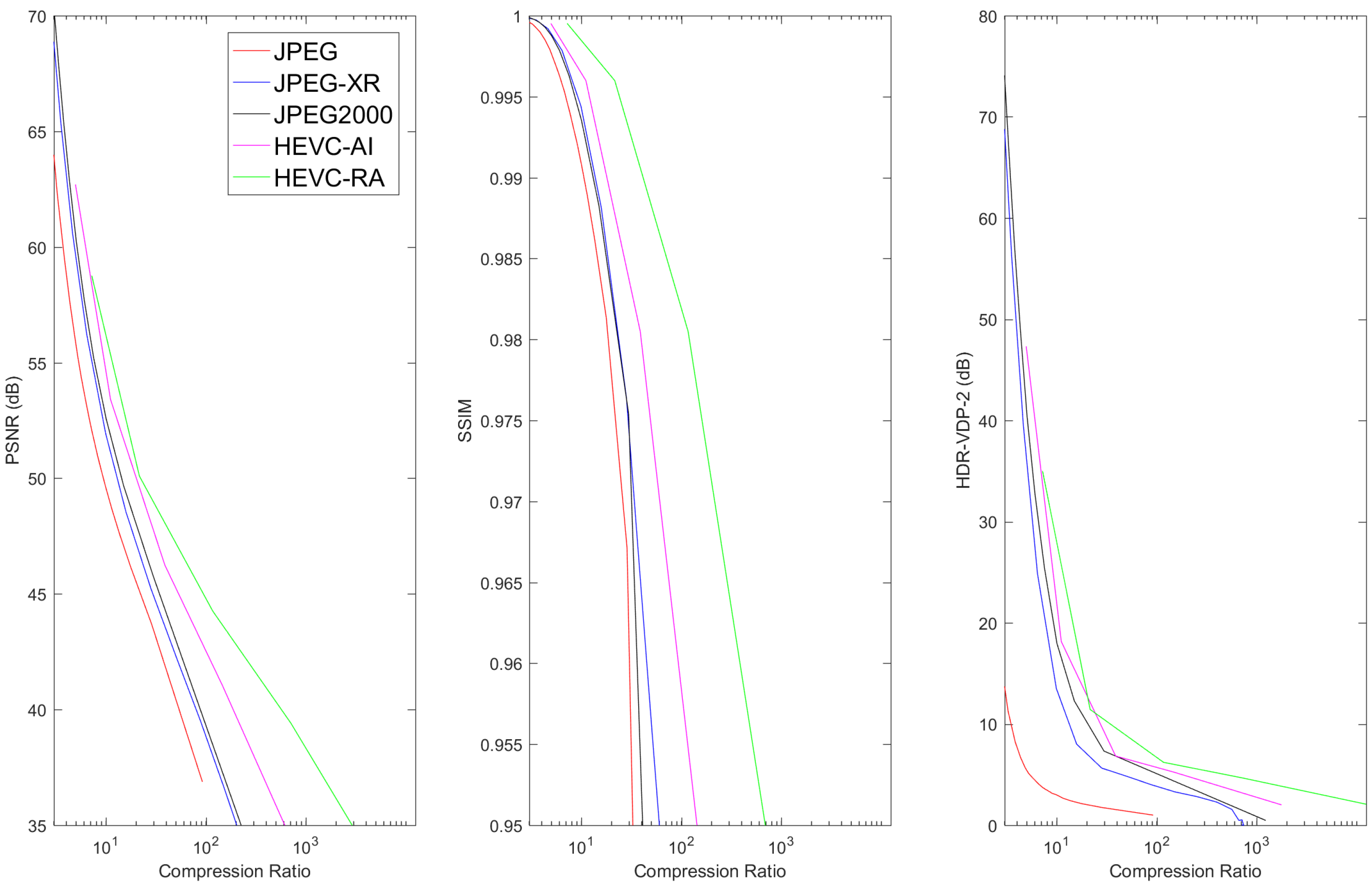
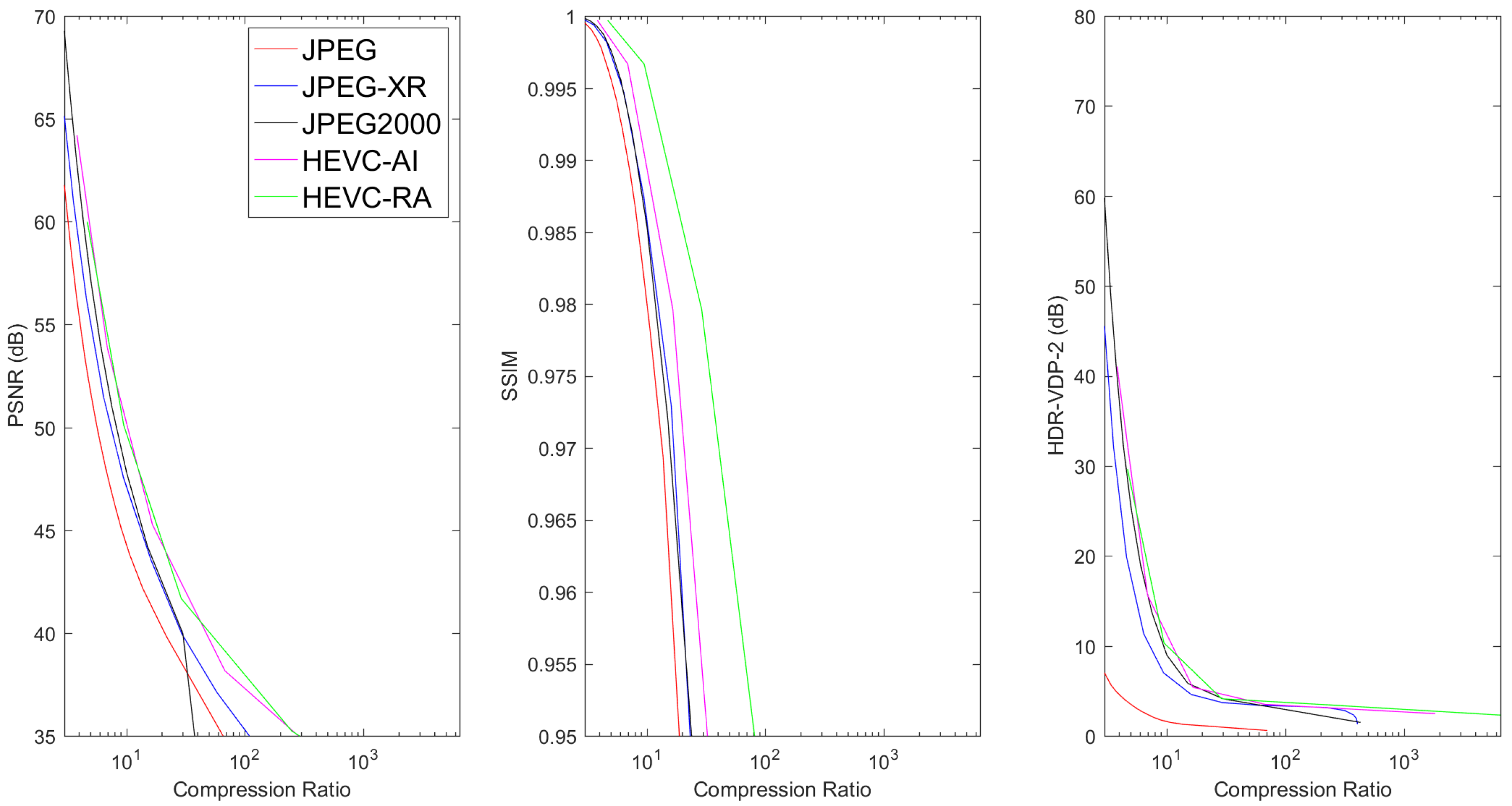
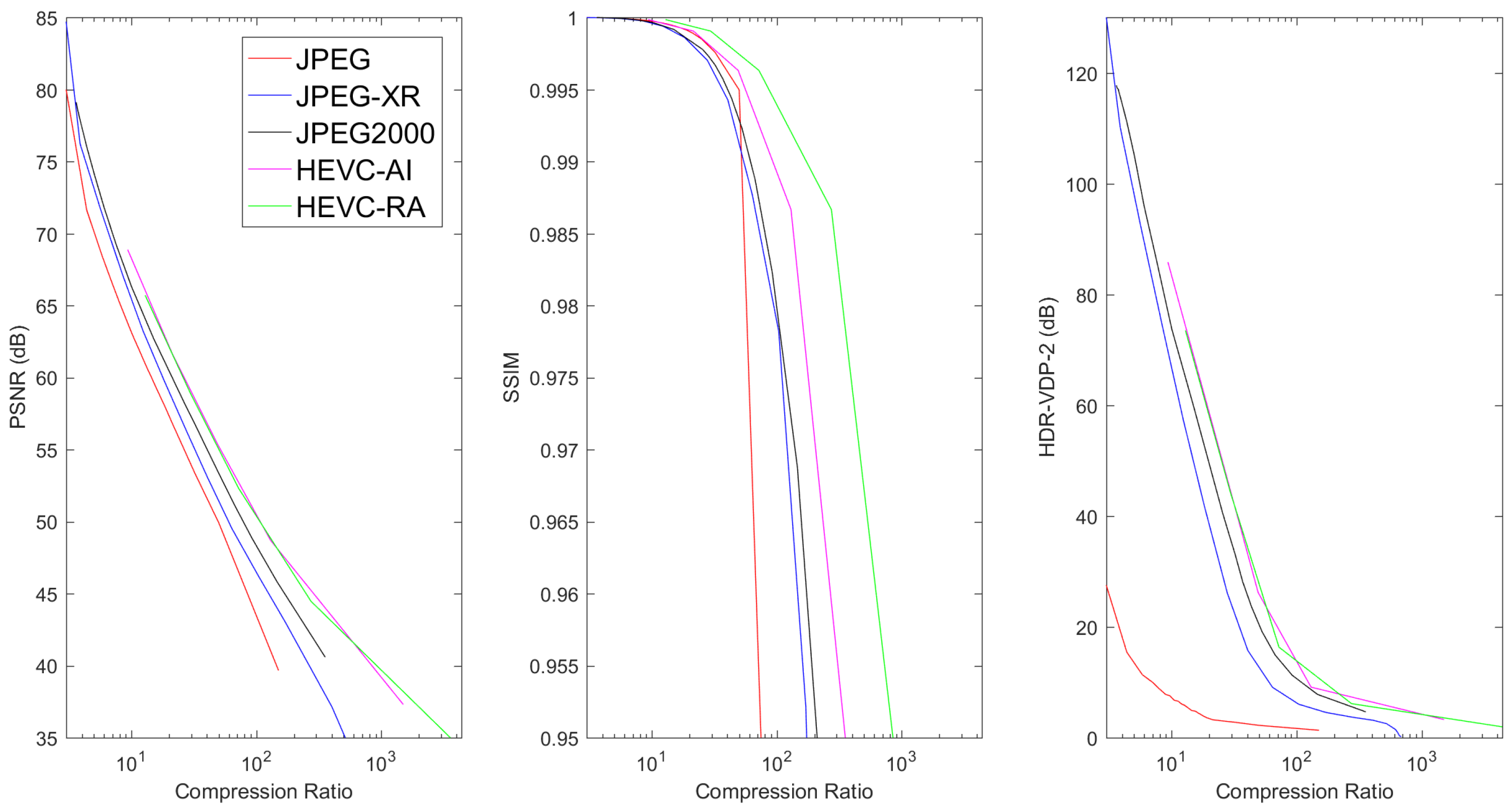
6.2. Lossy Compression
7. Conclusions and Future Directions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Duszak, R. Medical imaging: Is the growth boom over? In the Neiman Report; Harvey L. Neiman Health Policy Institute: Reston, VA, USA, 2012. [Google Scholar]
- Dodoo, M.S.; Richard, R.D., Jr.; Hughes, D.R. Trends in the Utilization of Medical Imaging From 2003 to 2011: Clinical Encounters Offer a Complementary Patient-Centered Focus. J. Am. Coll. Radiol. 2013, 10, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Van Aken, I.W.; Reijns, G.L.; De Valk, J.P.J.; Nijhof, J.A.M.; Dwyer, S.J., III; Schneider, R.H. Compressed Medical Images and Enhanced Fault Detection within an ARC-NEMA Compatible Picture Archiving and Communications System. Proc. SPIE 1987. [Google Scholar] [CrossRef]
- Clunie, D.A. What is Different About Medical Image Compression. IEEE COMSOC MMTC E-Lett. 2011, 6, 31–37. [Google Scholar]
- Koff, D.A.; Shulman, H. An overview of digital compression of medical images: Can we use lossy image compression in radiology? Can. Assoc. Radiol. J. 2006, 57, 211. [Google Scholar] [PubMed]
- Erickson, B.J. Irreversible compression of medical images. J. Digit. Imaging 2002, 15, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Menegaz, G. Trends in medical image compression. Curr. Med. Imaging Rev. 2006, 2, 165–185. [Google Scholar] [CrossRef]
- Bhavani, S.; Thanushkodi, K. A survey on coding algorithms in medical image compression. Int. J. Comput. Sci. Eng. 2010, 2, 1429–1434. [Google Scholar]
- Sridevi, S.; Vijayakuymar, V.R.; Anuja, R. A survey on various compression methods for medical images. Int. J. Intell. Syst. Appl. 2012, 4, 13. [Google Scholar]
- Banu, N.M.M.; Sujatha, S. 3D Medical Image Compression: A Review. Indian J. Sci. Technol. 2015, 8, 1. [Google Scholar]
- Sahu, N.K.; Kamargaonkar, C. A survey on various medical image compression techniques. Int. J. Sci. Eng. Technol. Res. 2012, 2, 501–506. [Google Scholar]
- Ukrit, M.F.; Umamageswari, A.; Suresh, G.R. A survey on lossless compression for medical images. Int. J. Comput. Appl. 2011, 31, 47–50. [Google Scholar]
- Sridhar, V. A Review of the Effective Techniques of Compression in Medical Image Processing. Int. J. Comput. Appl. 2014, 97. [Google Scholar] [CrossRef]
- Nait-Ali, A.; Cavaro-Ménard, C. Compression of Biomedical Images and Signals, 1st ed.; Wiley-ISTE: London, UK, 2008. [Google Scholar]
- Cavaro-MéNard, C.; NaïT-Ali, A.; Tanguy, J.Y.; Angelini, E.; Bozec, C.L.; Jeune, J.J.L. Specificities of Physiological Signals and Medical Images. In Compression of Biomedical Images and Signals; Nait-Ali, A., Cavaro-MéNard, C., Eds.; Wiley-ISTE: London, UK, 2008; Chapter 4; pp. 266–290. [Google Scholar]
- Kak, A.C.; Slaney, M. Principles of Computerized Tomography; IEEE Press: Piscataway, NJ, USA, 1988. [Google Scholar]
- Helvie, M.A. Digital Mammography Imaging: Breast Tomosynthesis and Advanced Applications. Radiol. Clin. N. Am. 2010, 48, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Clunie, D.A. Lossless Compression of Breast Tomosynthesis: Objects to Maximize DICOM Transmission Speed and Review Performance and Minimize Storage Space; Radiological Society of North America 2012 Sicentific Assembly and Annual Meeting: Chicago, IL, USA, 2012. [Google Scholar]
- Liang, Z.P.; Lauterbur, P.C. Principles of Magnetic Resonance Imaging: A Signal Processing Perspective; Wiley-IEEE Press: New York, NY, USA, 1999. [Google Scholar]
- Gudbjartsson, H.; Patz, S. The Rician distribution of noisy MRI data. Magn. Reson. Med. 1995, 34, 910–914. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.R.; Bamber, J.C.; ter Haar, G.R. Physical Principles of Medical Ultrasonics; John Wiley and Sons, Ltd.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Wagner, R.F.; Smith, S.W.; Sandrik, J.M.; Lopez, H. Statistics of Speckle in Ultrasound B-Scans. IEEE Trans. Sonics Ultrason. 1983, 30, 156–163. [Google Scholar] [CrossRef]
- Cherry, S.R.; Sorenson, J.A.; Phelps, M.E. Physics in Nuclear Medicine, 4th ed.; Elsevier/Saunders: Philadelphia, PA, USA, 2012. [Google Scholar]
- Teymurazyan, A.; Riauka, T.; Jans, H.S.; Robinson, D. Properties of Noise in Positron Emission Tomography Images Reconstructed with Filtered-Backprojection and Row-Action Maximum Likelihood Algorithm. J. Digit. Imaging 2013, 26, 447–456. [Google Scholar] [CrossRef] [PubMed]
- May, M. A better lens on disease. Sci. Am. 2010, 302, 74–77. [Google Scholar] [CrossRef] [PubMed]
- BigTIFF. Available online: http://bigtiff.org/ (accessed on 20 February 2017).
- Supplement 145: Whole Slide Microscopic Image IOD and SOP Classes. Available online: ftp://medical.nema.org/MEDICAL/Dicom/Final/sup145_ft.pdf (accessed on 15 October 2017).
- JPEG 2000 Image Coding System: Interactivity Tools, APIs and Protocols; ISO/IEC IS 15444-9; ISO: Geneva, Switzerland, 2005.
- Digital Compression and Coding of Continuous-Tone Still Images, Part I: Requirements and Guidelines; ISO/IEC IS 10918-1; ISO: Geneva, Switzerland, 1994.
- Pennebaker, W.B.; Mitchell, J.L. JPEG Still Image Data Compression Standard, 1st ed.; Kluwer Academic Publishers: Norwell, MA, USA, 1992. [Google Scholar]
- Digital Compression and Coding of Continuous-Tone Still Images: Compliance Testing; ISO/IEC IS 109182; ISO: Geneva, Switzerland, 1995.
- Digital Compression and Coding of Continuous-Tone Still Images: Extensions; ISO/IEC IS 10918-3; ISO: Geneva, Switzerland, 1997.
- Digital Compression and Coding of Continuous-Tone Still Images: Registration Of JPEG Profiles, SPIFF Profiles, SPIFF Tags, SPIFF Colour Spaces, APPn Markers, SPIFF Compression Types and Registration Authorities (REGAUT); ISO/IEC IS 10918-4; ISO: Geneva, Switzerland, 1999.
- Digital Compression and Coding of Continuous-Tone Still Images: JPEG File Interchange Format (JFIF); ISO/IEC IS 10918-5; ISO: Geneva, Switzerland, 2013.
- Digital Compression and Coding of Continuous-Tone Still Images: Application to Printing Systems; ISO/IEC IS 10918-6; ISO: Geneva, Switzerland, 2013.
- Independent JPEG Group. Available online: http://www.ijg.org (accessed on 20 May 2016).
- JPEG 2000 Image Coding System: Core Coding System; ISO/IEC IS 15444-1; ISO: Geneva, Switzerland, 2004.
- JPEG 2000 Image Coding System: Extensions for Three-Dimensional Data; ISO/IEC IS 15444-10; ISO: Geneva, Switzerland, 2011.
- Taubman, D.S.; Marcellin, M.W. JPEG 2000: Image Compression Fundamentals, Standards and Practice; Kluwer Academic Publishers: Norwell, MA, USA, 2001. [Google Scholar]
- Tzannes, A. Compression of 3-Dimensional Medical Image Data Using Part 2 of JPEG 2000. Available online: http://dicom.nema.org/dicom/minutes/WG-04/2004/2004-02-18/3D_compression_RSNA_2003_ver2.pdf (accessed on 20 February 2017).
- Siegel, E.; Siddiqui, K.; Johnson, J.; Crave, O.; Wu, Z.; Dagher, J.; Bilgin, A.; Marcellin, M.; Nadar, M.; Reiner, B. Compression of multislice CT: 2D vs. 3D JPEG2000 and effects of slice thickness. Proc. SPIE 2005, 5748, 162–170. [Google Scholar]
- Lee, H. Advantage in image fidelity and additional computing time of JPEG2000 3D in comparison to JPEG2000 in compressing abdomen CT image datasets of different section thicknesses. Med. Phys. 2010, 37, 4238–4248. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J. JPEG2000 2D and 3D Reversible Compressions of Thin-Section Chest CT Images: Improving Compressibility by Increasing Data Redundancy Outside the Body Region. Radiology 2011, 259, 271–277. [Google Scholar] [CrossRef] [PubMed]
- JPEG 2000 Part 2 Multi-Component Transfer Syntaxes. Available online: ftp://medical.nema.org/medical/dicom/final/sup105_ft.pdf (accessed on 15 October 2017).
- Askelöf, J.; Carlander, M.L.; Christopoulos, C. Region of interest coding in {JPEG} 2000. Signal Proc. Image Commun. 2002, 17, 105–111. [Google Scholar] [CrossRef]
- Reference JPEG2000 Implementations. Available online: https://jpeg.org/jpeg2000/software.html (accessed on 20 May 2016).
- OpenJPEG JPEG2000 Implementation. Available online: http://www.openjpeg.org/ (accessed on 20 February 2017).
- Jasper JPEG2000 Implementation. Available online: https://www.ece.uvic.ca/~frodo/jasper/ (accessed on 20 February 2017).
- JJ2000 JPEG2000 Implementation. Available online: https://code.google.com/p/jj2000/ (accessed on 20 February 2017).
- Kakadu JPEG2000 Implementation. Available online: http://www.kakadusoftware.com/ (accessed on 20 May 2016).
- Lossless and Near-Lossless Compression of Continuous-Tone Still Images—Baseline; ISO/IEC IS 14495-1; ISO: Geneva, Switzerland, 1999.
- Weinberger, M.J.; Seroussi, G.; Sapiro, G. LOCO-I: A low complexity, context-based, lossless image compression algorithm. In Proceedings of the Data Compression Conference (DCC’96), Snowbird, UT, USA, 31 March–3 April 1996; pp. 140–149. [Google Scholar]
- Weinberger, M.J.; Seroussi, G.; Sapiro, G. The LOCO-I lossless image compression algorithm: Principles and standardization into JPEG-LS. IEEE Trans. Image Process. 2000, 9, 1309–1324. [Google Scholar] [CrossRef] [PubMed]
- HP on Mars: Labs Technology Used to Send Images. Available online: http://www.hpl.hp.com/news/2004/jan-mar/hp_mars.html (accessed on 4 February 2017).
- Lossless and Near-Lossless Compression of Continuous-Tone Still Images: Extensions; ISO/IEC IS 14495-2; ISO: Geneva, Switzerland, 2003.
- Pountain, D. Run-length encodings. IEEE Trans. Inf. Theory 1966, 12, 399–401. [Google Scholar]
- CharLS, A JPEG-LS Library. Available online: https://github.com/team-charls/charls (accessed on 20 May 2016).
- Libjpeg. Available online: https://github.com/thorfdbg/libjpeg (accessed 20 May 2016).
- LOCO-I/JPEG-LS Download Area. Available online: http://www.hpl.hp.com/research/info_theory/loco/locodown.htm (accessed on 4 February 2017).
- JPEG-LS Software. Available online: http://www.dclunie.com/jpegls.html (accessed on 4 February 2017).
- JPEG-LS Public Domain Code. Available online: http://www.stat.columbia.edu/jakulin/jpeg-ls/mirror.htm (accessed on 4 February 2017).
- JPEG XR Image Coding System—System Architecture; ISO/IEC IS 29199-1; ISO: Geneva, Switzerland, 2011.
- Srinivasan, S.; Tu, C.; Regunathan, S.L.; Sullivan, G.J. HD Photo: A new image coding technology for digital photography. Proc. SPIE 2007, 6696, 66960A. [Google Scholar]
- JPEG XR Image Coding System—Image Coding Specification; ISO/IEC IS 29199-2; ISO: Geneva, Switzerland, 2012.
- JPEG XR Image Coding System—Motion JPEG XR; ISO/IEC IS 29199-3; ISO: Geneva, Switzerland, 2010.
- JPEG XR Image Coding System—Conformance Testing; ISO/IEC IS 29199-4; ISO: Geneva, Switzerland, 2010.
- JPEG XR Image Coding System—Reference Software; ISO/IEC IS 29199-5; ISO: Geneva, Switzerland, 2012.
- Ohm, J.R.; Sullivan, G.J.; Schwarz, H.; Tan, T.K.; Wiegand, T. Comparison of the Coding Efficiency of Video Coding Standards—Including High Efficiency Video Coding (HEVC). IEEE Trans. Circuits Syst. Video Technol. 2012, 22, 1669–1684. [Google Scholar] [CrossRef]
- Sullivan, G.J.; Topiwala, P.N.; Luthra, A. The H.264/AVC advanced video coding standard: Overview and introduction to the fidelity range extensions. In Proceedings of the SPIE 49th Annual Meeting on Optical Science and Technology, Denver, CO, USA, 2–6 August 2004; pp. 454–474. [Google Scholar]
- Barroux, G. Lossless Coding for Still Pictures with HEVC. 2016. Available online: http://dicom.nema.org/Dicom/News/March2016/docs/sups/sup195-slides.pdf (accessed on 15 October 2017).
- Cai, Q.; Song, L.; Li, G.; Ling, N. Lossy and lossless intra coding performance evaluation: HEVC, H.264/AVC, JPEG 2000 and JPEG LS. In Proceedings of the 2012 Asia Pacific Signal and Information Processing Association Annual Summit and Conference, Hollywood, CA, USA, 3–6 December 2012; pp. 1–9. [Google Scholar]
- Zhou, M.; Gao, W.; Jiang, M.; Yu, H. HEVC Lossless Coding and Improvements. IEEE Trans. Circuits Syst. Video Technol. 2012, 22, 1839–1843. [Google Scholar] [CrossRef]
- Chen, H.; Braeckman, G.; Satti, S.M.; Schelkens, P.; Munteanu, A. HEVC-based video coding with lossless region of interest for telemedicine applications. In Proceedings of the 20th International Conference on Systems, Signals and Image Processing (IWSSIP), Bucharest, Romania, 7–9 July 2013; pp. 129–132. [Google Scholar]
- Gao, W.; Jiang, M.; Yu, H. On lossless coding for HEVC. SPIE 2013, 8666, 866609. [Google Scholar]
- Choi, J.; Ho, Y. Differential Pixel Value Coding for HEVC Lossless Compression. Adv. Video Coding Next-Gener. Multimed. Serv. 2012, 3–19. [Google Scholar] [CrossRef]
- Tan, Y.H.; Yeo, C.; Li, Z. Residual DPCM for lossless coding in HEVC. In Proceedings of the IEEE International Conference on Acoustics, Speech and Signal Processing, Vancouver, BC, Canada, 26–31 May 2013; pp. 2021–2025. [Google Scholar]
- Sanchez, V.; Aulí-Llinàs, F.; Bartrina-Rapesta, J.; Serra-Sagristà, J. HEVC-based lossless compression of Whole Slide pathology images. In Proceedings of the IEEE Global Conference on Signal and Information Processing (GlobalSIP), Atlanta, GA, USA, 3–5 December 2014; pp. 297–301. [Google Scholar]
- Sanchez, V.; Llinàs, F.A.; Rapesta, J.B.; Sagristà, J.S. Improvements to HEVC Intra Coding for Lossless Medical Image Compression. In Proceedings of the 2014 Data Compression Conference, Snowbird, UT, USA, 26–28 March 2014; p. 423. [Google Scholar]
- Sanchez, V.; Bartrina-Rapesta, J. Lossless compression of medical images based on HEVC intra coding. In Proceedings of the IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Florence, Italy, 4–9 May 2014; pp. 6622–6626. [Google Scholar]
- Sanchez, V.; Aulí-Llinàs, F.; Vanam, R.; Bartrina-Rapesta, J. Rate control for lossless region of interest coding in HEVC intra-coding with applications to digital pathology images. In Proceedings of the 2015 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), South Brisbane, Australia, 19–24 April 2015; pp. 1250–1254. [Google Scholar]
- Sanchez, V. Sample-based edge prediction based on gradients for lossless screen content coding in HEVC. In Proceedings of the 2015 Picture Coding Symposium (PCS), Cairns, Australia, 31 May–3 June 2015; pp. 134–138. [Google Scholar]
- Flynn, D.; Marpe, D.; Naccari, M.; Nguyen, T.; Rosewarne, C.; Sharman, K.; Sole, J.; Xu, J. Overview of the Range Extensions for the HEVC Standard: Tools, Profiles, and Performance. IEEE Trans. Circuits Syst. Video Tech. 2016, 26, 4–19. [Google Scholar] [CrossRef]
- ACR-NEMA Standards Publication No. 300-1985-DICOM-Digital Imaging and Communications. 1985. Available online: ftp://medical.nema.org/medical/dicom/1985/ACR-NEMA_300-1985.pdf (accessed on 20 May 2016).
- ACR-NEMA Standards Publication No. 300-1988-DICOM-Digital Imaging and Communications. 1988. Available online: ftp://medical.nema.org/medical/dicom/1988/ACR-NEMA_300-1988.pdf (accessed on 20 May 2016).
- ACR/NEMA Standards Publication No. PS 3.1. 1993. Available online: ftp://medical.nema.org/medical/dicom/1992-1995/ (accessed on 20 May 2016).
- DICOM PS3.1 2016b. 2016. Available online: http://dicom.nema.org/MEDICAL/Dicom/current/output/chtml/part01/PS3.1.html (accessed on 20 May 2016).
- DICOM PS3.5 2016b—Data Structures and Encoding. 2016. Available online: http://dicom.nema.org/medical/Dicom/current/output/chtml/part05/PS3.5.html (accessed on 20 May 2016).
- PS2-1989 ACR-NEMA Data Compression Standard. 1989. Available online: ftp://medical.nema.org/MEDICAL/Dicom/1989/PS2_1989.pdf (accessed on 20 February 2017).
- Information Technology—Generic Coding of Moving Pictures and Associated Audio Information: Systems; ISO/IEC IS 13818-1; ISO: Geneva, Switzerland, 2000.
- Information Technology—Coding of Audio-Visual Objects—Part 10: Advanced Video Coding; ISO/IEC IS 14496-10; ISO: Geneva, Switzerland, 2003.
- Proposal for New Work Item from WG13 on HEVC/H.265 Video Coding. Available online: ftp://medical.nema.org/MEDICAL/Dicom/Overviews-CPs-Sups-WIs/Work-Items/2015-12-A-HEVC-H265-v.2.docx (accessed on 15 October 2017).
- Digital Imaging and Communications in Medicine (DICOM) Supplement 195: HEVC/H.265 Transfer Syntax. Available online: ftp://medical.nema.org/medical/dicom/supps/PC/sup195_pc2.pdf (accessed on 15 October 2017).
- MINUTES of DICOM STANDARDS COMMITTEE on December 2, 2010. Available online: http://dicom.nema.org/dicom/minutes/committee/2010/2010-12-02/DICOM_2010-12-02_Min-Rev1.doc (accessed on 15 October 2017).
- Usability of irreversible image compression in radiological imaging. A position paper by the European Society of Radiology (ESR). Insights Imaging 2011, 2, 103–115.
- ACR–AAPM–SIIM Technical Standard for Electronic Practice of Medical Imaging—Resolution 39. 2014. Available online: http://www.acr.org/~/media/AF1480B0F95842E7B163F09F1CE00977.pdf (accessed on 20 May 2016).
- Guidance for the content and review of 510(K) Notifications for Picture Archiving and Communications Systems (PACS) and Related Devices; Food and Drug Administration: Rockville, MD, USA, 1983.
- Wong, S.; Zaremba, L.; Gooden, D.; Huang, H.K. Radiologic image compression—A review. Proc. IEEE 1995, 83, 194–219. [Google Scholar] [CrossRef]
- Gooden, D.S. Legal Aspects of Image Compression. In Proceedings of the American Association of Physicists in Medicine (AAPM) 35th Annual Meeting, Washington, DC, USA, 8–12 August 1993. [Google Scholar]
- Bak, P.R.G. Will the Use of Irreversible Compression become a Standard of Practice? Newsl. Soc. Comput. Appl. Radiol. 2006, 18, 10–11. [Google Scholar]
- Mammography Quality Standards Act (MQSA). 2004. Available online: https://www.fda.gov/Radiation-EmittingProducts/MammographyQualityStandardsActandProgram/Regulations/ucm110823.htm (accessed on 20 May 2016).
- Mammography Quality Standards Act (MQSA) Policy Guidance Help System—Recordkeeping. 2014. Available online: http://www.fda.gov/Radiation-EmittingProducts/MammographyQualityStandardsActandProgram/Guidance/PolicyGuidanceHelpSystem/ucm052108.htm (accessed on 20 February 2017).
- The Adoption of Lossy Image Data Compression for the Purpose of Clinical Interpretation. Available online: https://www.rcr.ac.uk/sites/default/files/docs/radiology/pdf/IT_guidance_LossyApr08.pdf. (accessed on 15 October 2017).
- CAR Standards for Irreversible Compression in Digital Diagnostic Imaging within Radiology. 2011. Available online: http://www.car.ca/uploads/standards%20guidelines/Standard_Lossy_Compression_EN.pdf (accessed on 20 May 2016).
- Koff, D.; Bak, P.; Brownrigg, P.; Hosseinzadeh, D.; Khademi, A.; Kiss, A.; Lepanto, L.; Michalak, T.; Shulman, H.; Volkening, A. Pan-Canadian Evaluation of Irreversible Compression Ratios (“Lossy” Compression) for Development of National Guidelines. J. Digit. Imaging 2008, 22, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Loose, R.; Braunschweig, R.; Kotter, E.; Mildenberger, P.; Simmler, R.; Wucherer, M. Compression of Digital Images in Radiology—Results of a Consensus Conference. Fortschr. Röntgenstr. 2009, 181, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Foos, D.H.; Muka, E.; Slone, R.M.; Erickson, B.J.; Flynn, M.J.; Clunie, D.A.; Hildebrand, L.; Kohm, K.S.; Young, S.S. JPEG 2000 compression of medical imagery. In Proceedings of the Medical Imaging 2000: PACS Design and Evaluation: Engineering and Clinical Issues, San Diego, USA, 18 May 2000; pp. 85–96. [Google Scholar]
- Clunie, D.A. Lossless compression of grayscale medical images: Effectiveness of traditional and state-of-the-art approaches. Med. Imaging 2000, 3980, 74–84. [Google Scholar]
- Visser, R.; Oostveen, L.; Karssemeijer, N. Lossless compression of digital mammograms. In International Workshop on Digital Mammography; Springer: Berlin, Germany, 2006; pp. 533–540. [Google Scholar]
- Ait-Aoudia, S.; Benhamida, F.; Yousfi, M. Lossless compression of volumetric medical data. In International Symposium on Computer and Information Sciences; Springer: Berlin, Germany, 2006; pp. 563–571. [Google Scholar]
- Starosolski, R. Performance evaluation of lossless medical and natural continuous tone image compression algorithms. In Proceedings of the SPIE International Conference on Medical Imaging, San Diego, CA, USA, 12–17 February 2005; pp. 116–127. [Google Scholar]
- Siddiqui, K.M.; Johnson, J.P.; Reiner, B.I.; Siegel, E.L. Discrete cosine transform JPEG compression vs. 2D JPEG2000 compression: JNDmetrix visual discrimination model image quality analysis. Med. Imaging 2005, 5748, 202–207. [Google Scholar]
- Parikh, S.; Ruiz, D.; Kalva, H.; Fernandez-Escribano, G.; Adzic, V. High Bit-Depth Medical Image Compression with HEVC. IEEE J. Biomed. Health Inf. 2017. [Google Scholar] [CrossRef] [PubMed]
- Lucas, L.; Rodrigues, N.; Cruz, L.; Faria, S. Lossless Compression of Medical Images Using 3D Predictors. IEEE Trans. Med. Imaging 2017, PP, 1. [Google Scholar] [CrossRef] [PubMed]
- Cancer Image Archive. Available online: https://wiki.cancerimagingarchive.net (accessed on 6 February 2017).
- The Clinical Image Analysis Laboratory—The Ohio State University. Available online: http://bmi.osu.edu/cialab (accessed on 20 February 2017).
- Image Data Used in the Simulations of “The Role of Image Compression Standards in Medical Imaging: Current Status and Future Trends”. Available online: http://dx.doi.org/10.7937/K9/TCIA.2016.xHsOkCdc (accessed on 6 February 2017).
- Digital Pathology Image Data. Available online: http://www.ece.arizona.edu/~bilgin/HE_Samples.zip (accessed on 6 February 2017).
- JPEG XR Reference Codec. Available online: https://jpeg.org/downloads/jpegxr/jpegxr-ref.zip (accessed on 20 May 2016).
- HEVC Reference Software HM-16.4. Available online: https://hevc.hhi.fraunhofer.de/svn/svn_HEVCSoftware/tags/HM-16.4/ (accessed on 20 May 2016).
- Barrett, H.H.; Yao, J.; Rolland, J.P.; Myers, K.J. Model observers for assessment of image quality. Proc. Natl. Acad. Sci. USA 1993, 90, 9758–9765. [Google Scholar] [CrossRef] [PubMed]
- Pu, L.; Marcellin, M.W.; Bilgin, A.; Ashok, A. Image compression based on task-specific information. In Proceedings of the 2014 IEEE International Conference on Image Processing (ICIP), Paris, France, 27–30 October 2014; pp. 4817–4821. [Google Scholar]
- Zhang, L.; Cavaro-Menard, C.; Callet, P.L.; Tanguy, J.Y. A Perceptually Relevant Channelized Joint Observer (PCJO) for the Detection-Localization of Parametric Signals. IEEE Trans. Med. Imaging 2012, 31, 1875–1888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Zhang, L.; Yuan, W.; Yang, G.; Yang, J.; Xu, T.; Shu, H.; Luo, L.; Feng, Q. Extended PCJO for the detection-localization of hypersignals and hyposignals in CT images. IEEE Access. 2017, PP, 1. [Google Scholar] [CrossRef]
- Zhang, Y.; Pham, B.T.; Eckstein, M.P. Automated optimization of JPEG 2000 encoder options based on model observer performance for detecting variable signals in X-ray coronary angiograms. IEEE Trans. Med. Imaging 2004, 23, 459–474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pham, B.T.; Eckstein, M.P. Task-based model/human observer evaluation of {SPIHT} wavelet compression with human visual system-based quantization. Acad. Radiol. 2005, 12, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Bovik, A.C.; Sheikh, H.R.; Simoncelli, E.P. Image quality assessment: From error visibility to structural similarity. IEEE Trans. Image Proc. 2004, 13, 600–612. [Google Scholar] [CrossRef]
- Mantiuk, R.; Kim, K.J.; Rempel, A.G.; Heidrich, W. HDR-VDP-2: A calibrated visual metric for visibility and quality predictions in all luminance conditions. ACM Trans. Graph. 2011, 30, 40. [Google Scholar] [CrossRef]
- Fidler, A.; Skaleric, U.; Likar, B. The impact of image information on compressibility and degradation in medical image compression. Med. Phys. 2006, 33, 2832–2838. [Google Scholar] [CrossRef] [PubMed]
- Chandler, D.M.; Dykes, N.L.; Hemami, S.S. Visually lossless compression of digitized radiographs based on contrast sensitivity and visual masking. SPIE 2005, 5749, 359–372. [Google Scholar]
- Liu, Z.; Karam, L.J.; Watson, A.B. JPEG2000 encoding with perceptual distortion control. IEEE Trans. Image Proc. 2006, 15, 1763–1778. [Google Scholar]
- Oh, H.; Bilgin, A.; Marcellin, M.W. Visually lossless encoding for JPEG2000. IEEE Trans. Image Proc. 2013, 22, 189–201. [Google Scholar]
- Penedo, M.; Lado, M.J.; Tahoces, P.G.; Souto, M.; Vidal, J.J. Effects of JPEG2000 data compression on an automated system for detecting clustered microcalcifications in digital mammograms. IEEE Trans. Inf. Technol. Biomed. 2006, 10, 354–361. [Google Scholar] [CrossRef] [PubMed]
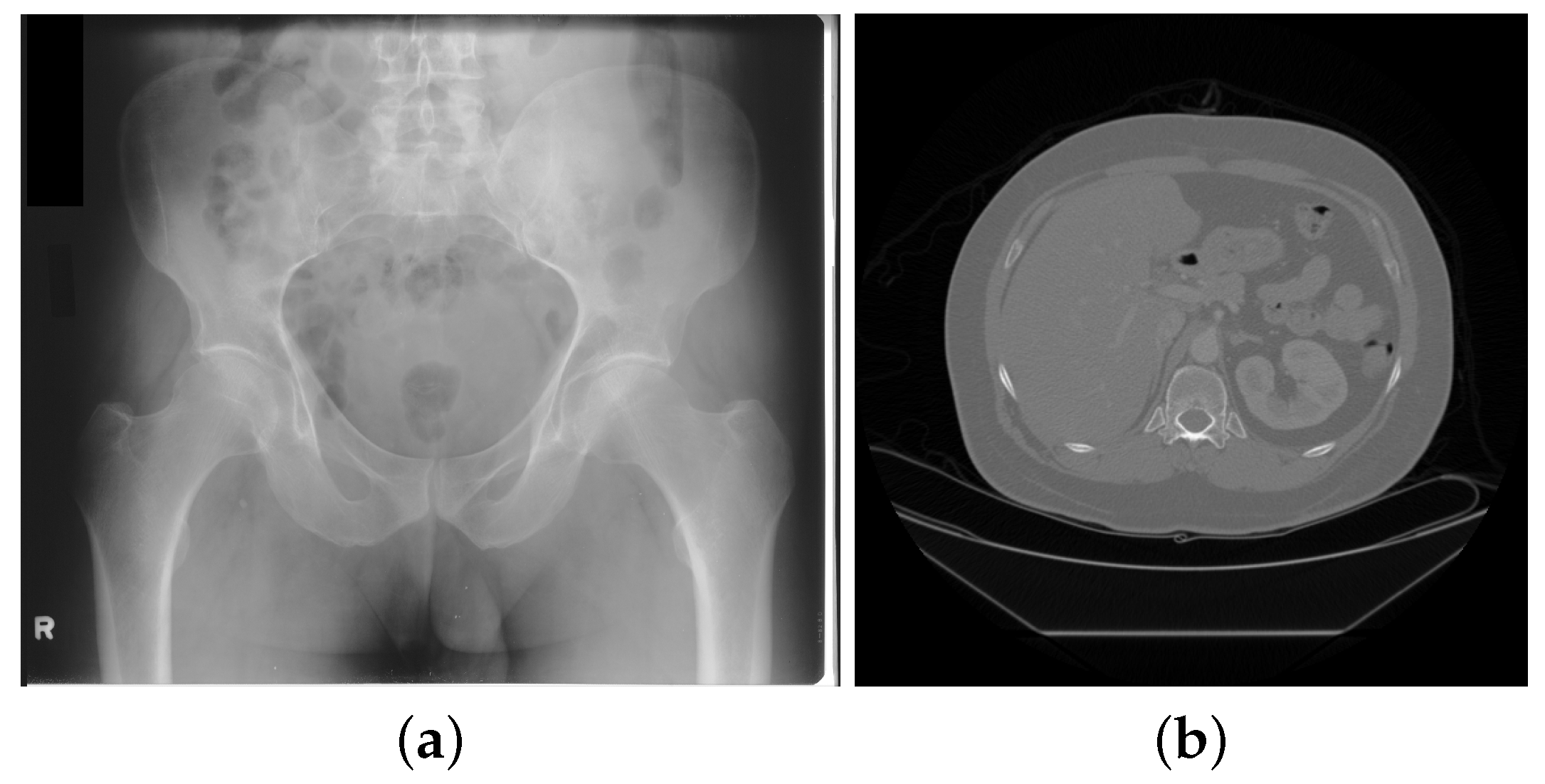
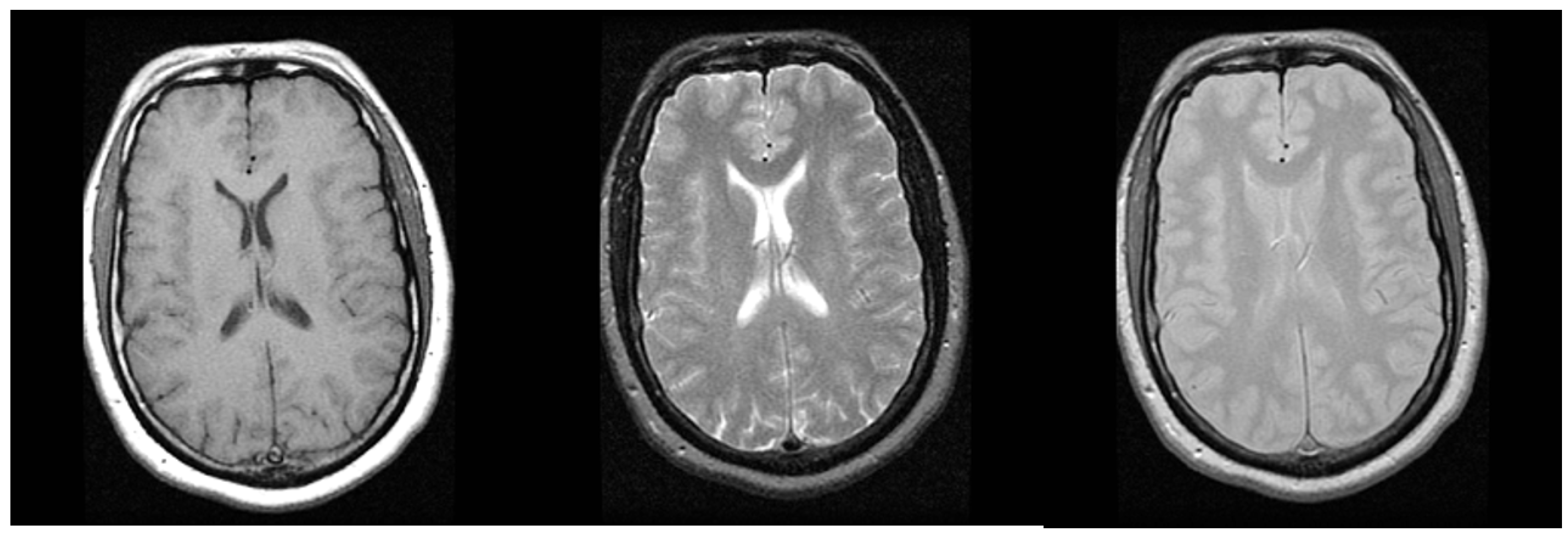
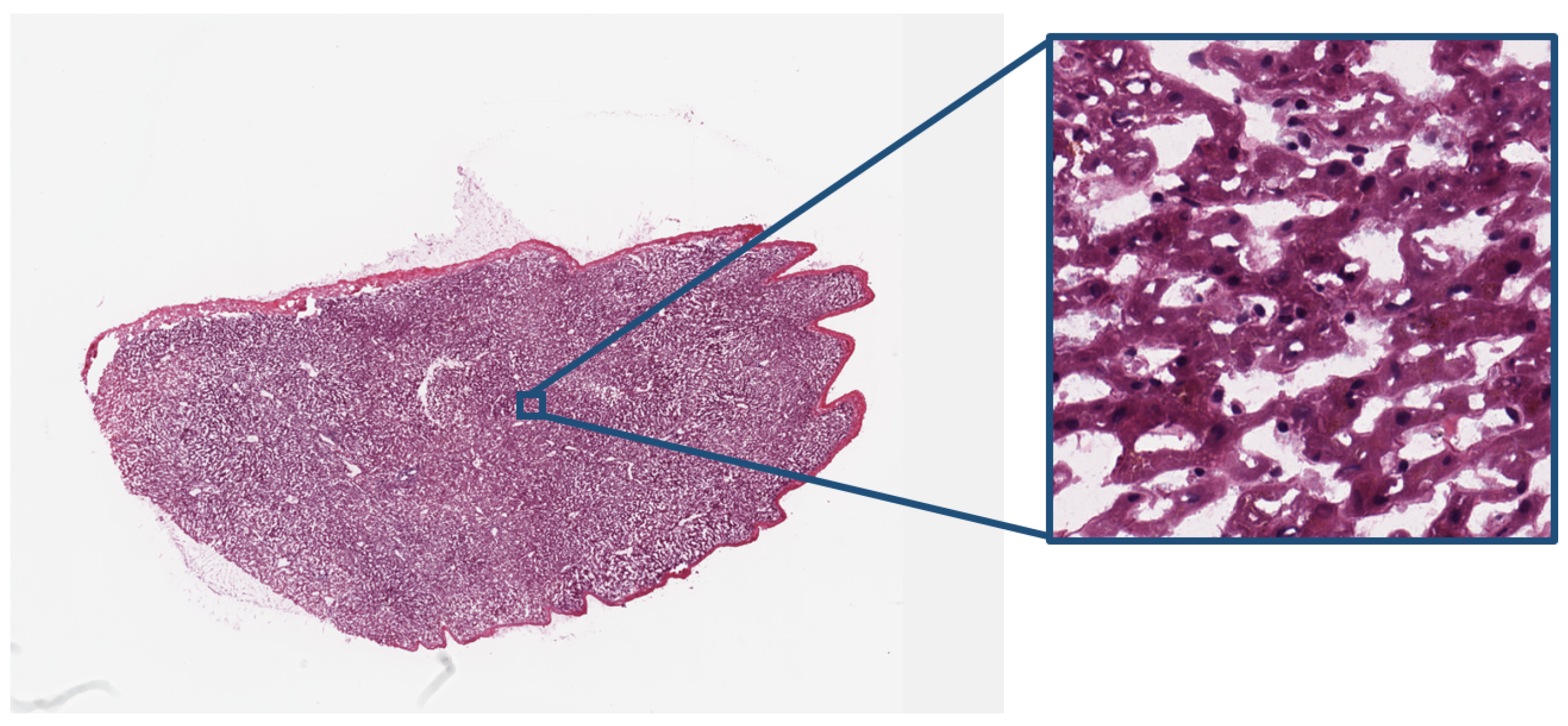


| Modality | Anatomy | Image Dimensions (x, y, z, t) | Bit Depth | Uncompressed File Size |
|---|---|---|---|---|
| Radiography | Chest | (2000, 2500, -, -) | 10–16 bits | 10 MB |
| CT | Abdomen | (512, 512, 500, -) | 12–16 bits | 250 MB |
| Brain | (512, 512, 300, -) | 12-16 bits | 150 MB | |
| Heart | (512, 512, 100, 20) | 12-16 bits | 1 GB | |
| Breast Tomosynthesis | Breast | (2457, 1890, 50, -) | 10–16 bits | 0.4 GB |
| MRI | Abdomen | (512, 512, 100, -) | 12–16 bits | 50 MB |
| Brain | (512, 512, 200, -) | 12–16 bits | 100 MB | |
| Heart | (256, 256, 20, 25) | 12–16 bits | 250 MB | |
| Ultrasound | Heart | (512, 512, -, 50)/s | 24 bits (color) | 38 MB/s |
| PET | Brain | (256, 256, 50, -) | 16 bits | 6 MB |
| Heart | (128, 128, 40, 16) | 16 bits | 1 MB | |
| Digital Pathology | Cells | (30,000, 30,000, -, -) | 24 bits (color) | 2.5 GB |
| Modality | Compression Ratio |
|---|---|
| Mammography | 20:1 |
| Chest Radiography | 10:1 |
| Skeletal Radiography | 10:1 |
| Ultrasound | 10:1 |
| Digital Angiography | 10:1 |
| CT (all areas) | 5:1 |
| Magnetic Resonance | 5:1 |
| Radiotherapy CT | No lossy compression |
| Modality | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anatomical | CR/DR | CT | CT | US | MR | NM | ||||||
| Region | JPEG | J2K | JPEG | J2K | JPEG | J2K | JPEG | J2K | JPEG | J2K | JPEG | J2K |
| Angio | - | - | 15:1 | 15:1 | - | - | - | - | 24:1 | 24:1 | 11:1 | 11:1 |
| Body | 30:1 | 30:1 | 15:1 | 10:1 | 12:1 | 12:1 | 12:1 | 12:1 | 24:1 | 24:1 | 11:1 | 11:1 |
| Breast | 25:1 | 25:1 | - | - | - | - | 12:1 | 12:1 | 24:1 | 24:1 | 11:1 | 11:1 |
| Chest | 30:1 | 30:1 | 15:1 | 15:1 | 12:1 | 12:1 | - | - | 24:1 | 24:1 | 11:1 | 11:1 |
| MSK | 30:1 | 20:1 | 15:1 | 15:1 | 12:1 | 12:1 | 12:1 | 12:1 | 24:1 | 24:1 | 11:1 | 11:1 |
| Neuro | - | - | 12:1 | 8:1 | 12:1 | 12:1 | - | - | 24:1 | 24:1 | 11:1 | 11:1 |
| Pediatrics | 30:1 | 30:1 | 15:1 | 15:1 | - | - | 12:1 | 12:1 | 24:1 | 24:1 | 11:1 | 11:1 |
| Modality | Compression Ratio |
|---|---|
| CR/DR (mammography) | 15:1 |
| CR/DR (all areas except mammography) | 10:1 |
| CT (all areas except brain) | 8:1 |
| CT (brain) | 5:1 |
| Magnetic Resonance | 7:1 |
| X-ray Angiography | 6:1 |
| Radio Fluoroscopy | 6:1 |
| Database | Modality | Image Dimensions (x, y, z) | Bit Depth |
|---|---|---|---|
| TCIA-COLONOGRAPY | CT Colonography | (512, 512, 426–566) | 12 |
| LIDC-IDRI | CT Lung | (512, 512, 133–280) | 12 |
| TCGA-BRCA | MR Mammography | (256, 256, 148–160) | 12 |
| TCGA-GBM | MR T2 Flair Axial | (384, 512, 25–29) | 12 |
| - | H&E Stained Digital Pathology | (6000, 6000, -) | 24 bits (color) |
| Modality | Compression Standard | Compression Ratio |
|---|---|---|
| CT Colonography | JPEG-LS | 1.89 ± 0.1089 |
| Lossless JPEG-XR | 1.79 ± 0.0869 | |
| Lossless JPEG2000 | 1.86 ± 0.1042 | |
| HEVC AI | 1.64 ± 0.0897 | |
| HEVC RA | 1.87 ± 0.1315 | |
| CT Lung | JPEG-LS | 2.08 ± 0.3195 |
| Lossless JPEG-XR | 1.94 ± 0.2571 | |
| Lossless JPEG2000 | 2.02 ± 0.3160 | |
| HEVC AI | 1.84 ± 0.2739 | |
| HEVC RA | 2.10 ± 0.3217 | |
| MR Mammography | JPEG-LS | 2.06 ± 0.2520 |
| Lossless JPEG-XR | 1.72 ± 0.1401 | |
| Lossless JPEG2000 | 2.01 ± 0.2406 | |
| HEVC AI | 1.77 ± 0.2357 | |
| HEVC RA | 2.03 ± 0.2504 | |
| MR T2 Flair Axial | JPEG-LS | 2.75 ± 0.2267 |
| Lossless JPEG-XR | 2.85 ± 0.2206 | |
| Lossless JPEG2000 | 2.91 ± 0.2463 | |
| HEVC AI | 2.26 ± 0.1818 | |
| HEVC RA | 2.51 ± 0.1955 | |
| Digital Pathology | JPEG-LS | 1.72 ± 0.0786 |
| Lossless JPEG-XR | 1.72 ± 0.0497 | |
| Lossless JPEG2000 | 1.76 ± 0.0674 | |
| HEVC AI | 1.49 ± 0.0905 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Hernandez-Cabronero, M.; Sanchez, V.; Marcellin, M.W.; Bilgin, A. The Current Role of Image Compression Standards in Medical Imaging. Information 2017, 8, 131. https://doi.org/10.3390/info8040131
Liu F, Hernandez-Cabronero M, Sanchez V, Marcellin MW, Bilgin A. The Current Role of Image Compression Standards in Medical Imaging. Information. 2017; 8(4):131. https://doi.org/10.3390/info8040131
Chicago/Turabian StyleLiu, Feng, Miguel Hernandez-Cabronero, Victor Sanchez, Michael W. Marcellin, and Ali Bilgin. 2017. "The Current Role of Image Compression Standards in Medical Imaging" Information 8, no. 4: 131. https://doi.org/10.3390/info8040131





