Binet’s Error: Developmental Change and Individual Differences in Intelligence Are Related to Different Mechanisms
Abstract
:1. A Brief History of the Conceptual and Measurement Basis of Intelligence in Children and Adults
2. Binet’s Genius
3. The g-Factor
4. The Information Processing Basis of g
5. Executive Functioning and Intelligence
6. Development of Speed of Processing (SoP)
7. The Development of Executive Functioning
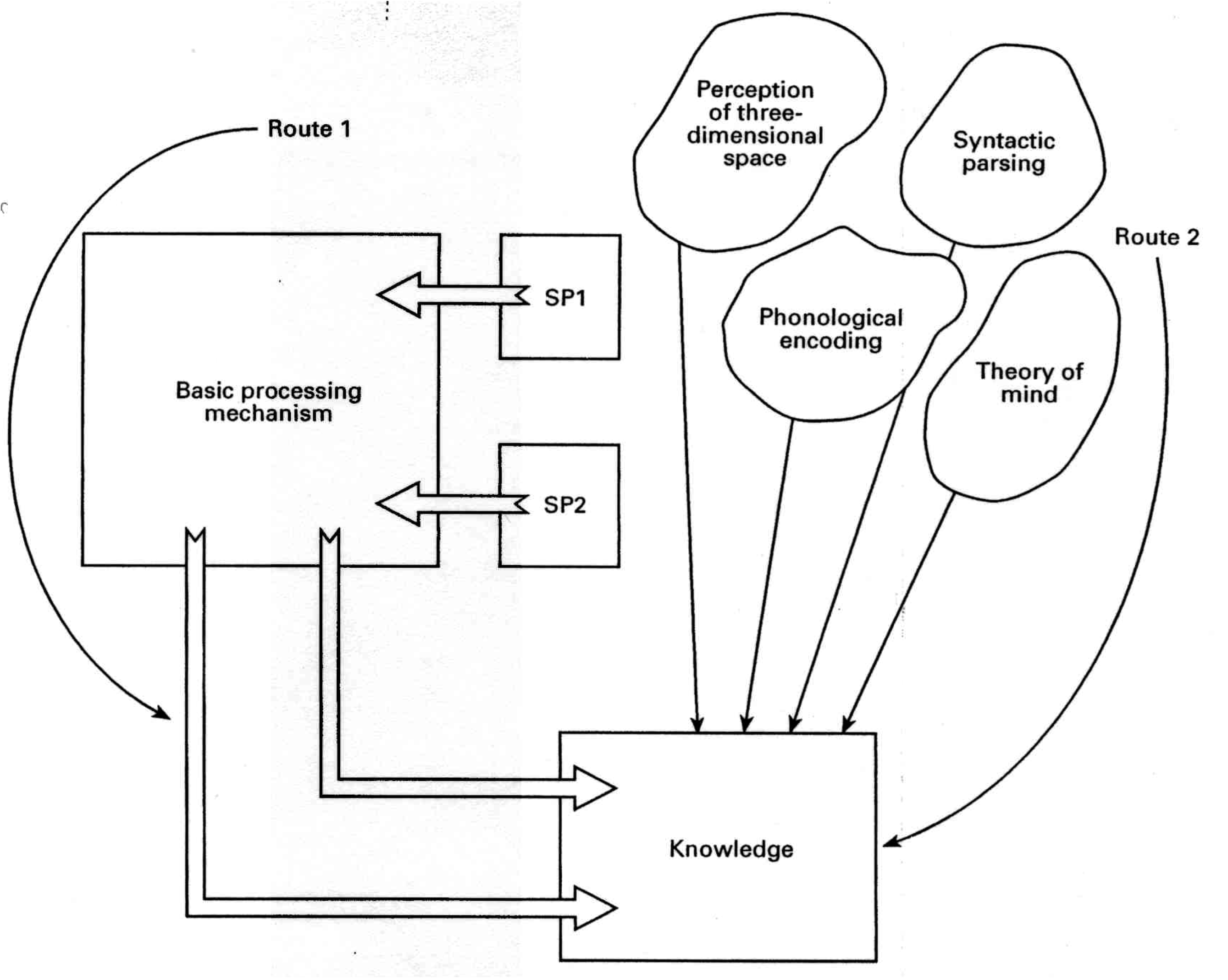
8. The Theory of the Minimal Cognitive Architecture (MCA) of Intelligence and Development
9. The Differentiation of Abilities
10. Where to from here?
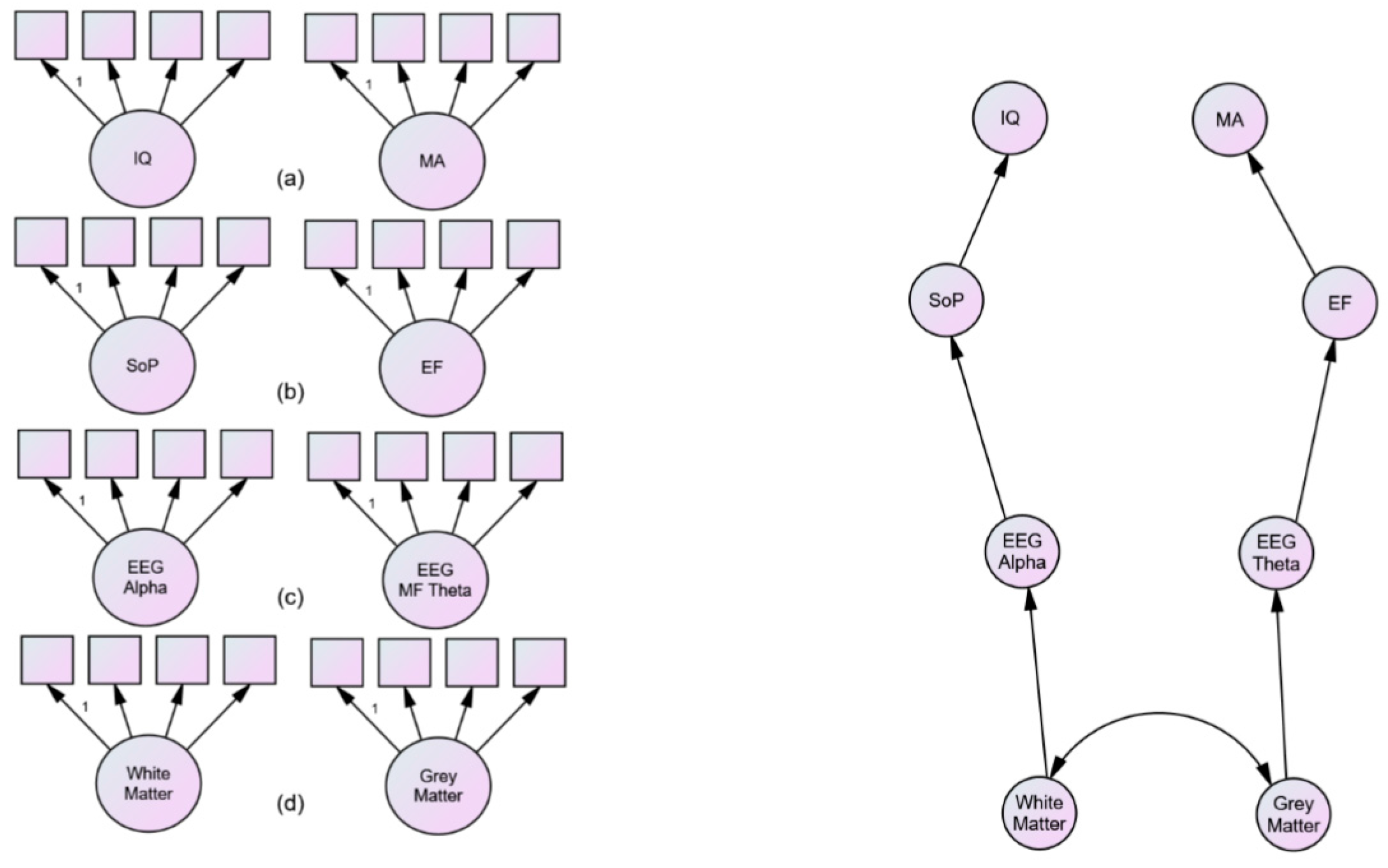
11. Duncan’s Multiple Demand Theory of Fluid g
12. A Neurodevelopmental Speculation
13. Conclusions
Acknowledgments
Conflicts of Interest
References
- Miller, G.A. The Test. Science 1984, 5, 55–60. [Google Scholar]
- Stern, W. Die Psychologische Methoden der Intelligenzprufung; Barth: Leipzig, Germany, 1912. [Google Scholar]
- Spearman, C. “General intelligence”, objectively determined and measured. Am. J. Psychol. 1904, 15, 201–293. [Google Scholar] [CrossRef]
- Gould, S.J. The Mismeasure of Man, 2nd ed.; W.W. Notorn & Company: New York, NY, USA, 1996. [Google Scholar]
- Carroll, J.B. Reflections of Stephan Jay Gould’s the mismeasure of man (1981): A retrospective review. Intelligence 1995, 21, 121–134. [Google Scholar] [CrossRef]
- Van der Maas, H.L.; Dolan, C.V.; Grasman, R.P.; Wicherts, J.M.; Huizenga, H.M.; Raijmakers, M.E. A Dynamical model of general intelligence: The positive manifold of intelligence by mutualism. Psychol. Rev. 2006, 113, 842–861. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.B. Human Cognitive Abilities: A Survey of Factor-Analytic Studies; Cambridge University Press: New York, NY, USA, 1993. [Google Scholar]
- Horn, J.L.; Cattell, R.B. Refinement and Test of the Theory of Fluid and Crystallized General Intelligences. J. Educ. Psychol. 1966, 57, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Cattell, R.B. Theory of fluid and crystallised intelligence: A critical experiment. J. Educ. Psychol. 1963, 54, 1–22. [Google Scholar] [CrossRef]
- Gustafsson, J.E. A unifying model for the structure of mental abilities. Intelligence 1984, 8, 179–203. [Google Scholar] [CrossRef]
- Anderson, M. Intelligence and Development: A Cognitive Theory, 1st ed.; Blackwell: Oxford, UK, 1992. [Google Scholar]
- Anderson, M. An unassailable defense of g but a siren-song for theories of intelligence. Psycoloquy 2000, 11, 28. [Google Scholar]
- Anderson, M. Conceptions of intelligence. J. Child Psychol. Psychiatry 2001, 42, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.R. Reaction time and psychometric g. In A Model for Intelligence; Eysenck, H.J., Ed.; Springer: Berlin/Heidelberg, Germany, 1982; pp. 93–132. [Google Scholar]
- Rabbitt, P.M.A. Age, information processing speed, and intelligence. Q. J. Exp. Psychol. A Hum. Exp. Psychol. 1994, 47, 741. [Google Scholar] [CrossRef]
- Schmiedek, F.; Oberauer, K.; Wilhelm, O.; Süß, H.W.; Wittmann, W.W. Individual Differences in Components of Reaction Time Distributions and their Relations to Working Memory and Intelligence. J. Exp. Psychol. Gen. 2007, 136, 414–429. [Google Scholar] [CrossRef] [PubMed]
- Nettelbeck, T. Inspection time and intelligence. In Speed of Information-Processing and Intelligence; Vernon, P.A., Ed.; Ablex Publishing: Westport, CT, USA, 1987; pp. 295–346. [Google Scholar]
- Kranzler, J.H.; Jensen, A.R. Inspection time and intelligence—A meta-analysis. Intelligence 1989, 13, 329–347. [Google Scholar] [CrossRef]
- Sheppard, L.D.; Vernon, P.A. Intelligence and speed of information-processing: A review of 50 years of research. Intelligence 2008, 44, 535–551. [Google Scholar] [CrossRef]
- Mackenzie, B.; Bingham, E. IQ, inspection time, and response strategies, in a university population. Aust. J. Psychol. 1985, 37, 257–268. [Google Scholar] [CrossRef]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The Unity and Diversity of EFs and Their Contributions to Complex “Frontal Lobe” Tasks: A Latent Variable Analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef] [PubMed]
- Friedman, N.P.; Miyake, A.; Corley, R.P.; Young, S.E.; DeFries, J.C.; Hewitt, J.K. Not All Executive Functions Are Related to Intelligence. Psychol. Sci. 2006, 17, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Demetriou, A.; Spanoudis, G.; Shayer, M.; van der Ven, S.; Brydges, C.; Kroesbergen, E.; Podjarny, G.; Swanson, H.L. Relations between speed, working memory, and intelligence from preschool to adulthood: Structural equation modeling of 14 studies. Intelligence 2014, 46, 107–121. [Google Scholar] [CrossRef]
- Fairweather, H.; Hutt, S.J. On the rate of gain of information in children. J. Exp. Child Psychol. 1978, 26, 216–229. [Google Scholar] [CrossRef]
- Keating, D.P.; Bobbitt, B.L. Individual and developmental differences in cognitive processing components of mental ability. Child Dev. 1978, 49, 155–167. [Google Scholar] [CrossRef]
- Nettelbeck, T.; Wilson, C. A cross-sequential analysis of developmental differences in speed of visual information processing. J. Exp. Child Psychol. 1985, 40, 1–22. [Google Scholar] [CrossRef]
- Kail, R. Sources of age differences in speed of processing. Child Dev. 1986, 57, 969–987. [Google Scholar] [CrossRef] [PubMed]
- Kail, R. Processing time declines exponentially during childhood and adolescence. Dev. Psychol. 1991, 27, 259–266. [Google Scholar] [CrossRef]
- Anderson, M. Inspection time, information processing and the development of intelligence. Br. J. Dev. Psychol. 1988, 6, 43–57. [Google Scholar] [CrossRef]
- Kail, R. Developmental change in speed of processing during childhood and adolescence. Psychol. Bull. 1991, 109, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Kail, R. Evidence for global developmental change is intact. J. Exp. Child Psychol. 1992, 54, 308–314. [Google Scholar] [CrossRef]
- Kail, R.; Park, Y.-S. Global developmental change in processing time. Merrill-Palmer Q. 1992, 4, 525–541. [Google Scholar]
- Kail, R.; Salthouse, T.A. Processing speed as a mental capacity. Acta Psychol. 1994, 86, 199–225. [Google Scholar] [CrossRef]
- Hale, S. A global developmental trend in cognitive processing speed. Child Dev. 1990, 61, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Salthouse, T.A. A Cognitive Theory of Aging; Springer: Berlin, Germany, 1985. [Google Scholar]
- Salthouse, T.A. Theoretical Perspectives in Cognitive Aging; Erlbaums: Hillsdale, NJ, USA, 1991. [Google Scholar]
- Salthouse, T.A. The processing-speed theory of adult age-differences in cognition. Psychol. Rev. 1996, 103, 403–428. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M. Inspection time and IQ in young children. Personal. Individ. Differ. 1986, 7, 677–686. [Google Scholar] [CrossRef]
- Anderson, M. Evidence for a single global factor of developmental change—Too good to be true? Aust. J. Psychol. 1995, 47, 18–24. [Google Scholar] [CrossRef]
- Anderson, M.; Reid, C.; Nelson, J. Developmental changes in inspection time: What a difference a year makes. Intelligence 2001, 29, 475–486. [Google Scholar] [CrossRef]
- Anderson, M.; Nettelbeck, T.; Barlow, J. Using Reaction time measures of speed of information processing: Speed of response selection increases with age but speed of stimulus categorisation does not. Br. J. Dev. Psychol. 1997, 15, 145–157. [Google Scholar] [CrossRef]
- Anderson, M. The effect of attention on developmental differences in inspection time. Personal. Individ. Differ. 1989, 10, 559–563. [Google Scholar] [CrossRef]
- Anderson, M. Inspection time and the relationship between stimulus encoding and response selection factors in development. In Human Information Processing Measures, Mechanisms and Models; Vickers, D., Smith, P.L., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1989; pp. 509–516. [Google Scholar]
- Spitz, H.H. Intellectual extremes, mental age, and the nature of human intelligence. Merrill-Palmer Q. 1982, 28, 167–192. [Google Scholar]
- Pascual-Leone, J. A mathematical model for the transition rule in Piaget’s developmental stages. Acta Psychol. 1970, 32, 301–345. [Google Scholar] [CrossRef]
- Halford, G.S. Analogical reasoning and conceptual complexity in cognitive development. Hum. Dev. 1992, 35, 193–217. [Google Scholar] [CrossRef]
- Demetriou, A.; Spanoudis, D.; Shayer, M. Developmental intelligence: From empirical to hidden constructs. Intelligence 2013, 41, 744–749. [Google Scholar] [CrossRef]
- Bjorklund, D.F.; Harnishfeger, K.K. The resources construct in cognitive development: Diverse sources of evidence and a theory of inefficient inhibition. Dev. Rev. 1990, 10, 48–71. [Google Scholar] [CrossRef]
- Michel, F.; Anderson, M. Using the antisaccade task to investigate the relationship between the development of inhibition and the development of intelligence. Dev. Sci. 2009, 12, 272–288. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.; Anderson, M. Developmental and individual differences in fluid intelligence: Evidence against the unidimensional hypothesis. Br. J. Dev. Psychol. 2001, 19, 181–206. [Google Scholar] [CrossRef]
- Fodor, J.A. The Modularity of Mind; Cambridge, a Bradford Book; MIT Press: Cambridge, MA, USA, 1983. [Google Scholar]
- Anderson, M. The concept and development of general intellectual ability. In Child Neuropsychology: Concepts, Theory and Practice; Reed, J., Warner-Rogers, J., Eds.; Wiley: Chichester, UK, 2008; pp. 112–135. [Google Scholar]
- Anderson, M. What can autism and dyslexia tell us about intelligence? Q. J. Exp. Psychol. 2008, 61, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.; Reid, C. Theoretical insights from neuroscience in early childhood research. In The SAGE Handbook of Early Childhood Research; Farrell, A., Kagan, S.L., Tisdall, E.K.M., Eds.; Sage: London, UK, 2016; pp. 148–162. [Google Scholar]
- Pylyshyn, Z.W. Computation and cognition: Issues in the foundations of cognitive science. Behav. Brain Sci. 1980, 3, 111–169. [Google Scholar] [CrossRef]
- Anderson, M.; Miller, K.L. Modularity, mental retardation, and speed of processing. Dev. Sci. 1998, 1, 239–245. [Google Scholar] [CrossRef]
- Brydges, C.; Reid, C.; Fox, A.; Anderson, M. A Unitary Executive Function Predicts Intelligence in Children. Intelligence 2012, 40, 458–469. [Google Scholar] [CrossRef]
- Brydges, C.; Fox, A.; Reid, C.; Anderson, M. Predictive validity of the N2 and P3 ERP components to executive functioning in children: A latent-variable analysis. Front. Hum. Neurosci. 2014, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Brydges, C.; Anderson, M.; Reid, C.; Fox, A. Maturation of cognitive control: Delineating response inhibition and interference suppression. PLoS ONE 2013, 8, e69826. [Google Scholar] [CrossRef] [PubMed]
- Demetriou, A.; Spanoudis, D. On the structure and development of executive functions in middle and late childhood: Remodelling and commentary on Brydges, Fox, Reid and Anderson. Intelligence 2015, 50, 131–134. [Google Scholar] [CrossRef]
- Anderson, M.; Nelson, J. Individual differences and cognitive models of the mind:using the differentiation hypothesis to distinguish general and specific cognitive processes. In Measuring the Mind: Speed, Control and Age; Duncan, J., Phillips, L., McLeod, P., Eds.; Oxford Univerity Press: Oxford, UK, 2005. [Google Scholar]
- Tourva, A.; Spanoudis, G.; Demetriou, A. Cognitive correlated of developing intelligence: The contribution of working memory, processing speed and attention. Intelligence 2016, 54, 136–146. [Google Scholar] [CrossRef]
- Baughman, F.D.; Thomas, M.S.C.; Anderson, M.; Reid, C. Common mechanisms in intelligence and development: A study of ability profiles in mental-age matched primary school children. Intellgience 2016, 56, 99–107. [Google Scholar] [CrossRef]
- Anderson, M.; Reid, C. Don’t forget about levels of explanation. Cortex J. Devoted Stud. Nerv. Syst. Behav. 2009, 45, 560–561. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J. Attention, intelligence, and the frontal lobes. In The Cognitive Neurosciences; Gazzaniga, M.S., Ed.; MIT Press: Cambridge, MA, USA, 1995; pp. 721–733. [Google Scholar]
- Duncan, J.; Emslie, H.; Williams, P.; Johnson, R.; Freer, C. Intelligence and the frontal lobe: The organization of goal-directed behavior. Cogn. Psychol. 1996, 30, 257–303. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.; Owen, A.M. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000, 23, 475–483. [Google Scholar] [CrossRef]
- Duncan, J. The Structure of Cognition: Attentional Episodes in Mind and Brain. Neuron 2013, 80, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J. The multiple-demand (MD) system of the primate brain: Mental programs for intelligent behaviour. Trends Cogn. Sci. 2010, 14, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Anokhin, A.; Vogel, F. EEG Alpha rhythm frequency and intelligence in normal adults. Intelligence 1996, 23, 1–14. [Google Scholar] [CrossRef]
- Klimesch, W. EEG-alpha rhythms and memory processes. Int. J. Psychophysiol. 1997, 26, 319–340. [Google Scholar] [CrossRef]
- Doppelmayr, M.; Klimesch, W.; Sausang, P.; Hodlmoser, K.; Stadler, W. Hanslmayr Intelligence related differences in EEG-bandpower. Neurosci. Lett. 2005, 381, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Grandy, T.H.; Werkle-Bergner, M.; Chicherio, C.; Lövdén, M.; Schmiedek, F.; Lindenberger, U. Individual Alpha Peak Frequency is Related to Latent Factors of General Cognitive Abilities. NeuroImage 2013, 79, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Hanslmayr, S.; Klimesch, W.; Sauseng, P.; Gruber, W.; Doppelmayr, M.; Freunberger, R.; Pecherstorfer, T. Visual discrimination performance is related to decreased alpha amplitude but increased phase locking. Neurosci. Lett. 2005, 375, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W.; Sauseng, P.; Hanslmayr, S. EEG alpha oscillations: The inhibition–timing hypothesis. Brain Res. Rev. 2007, 53, 63–88. [Google Scholar] [CrossRef] [PubMed]
- Doppelmayr, M.; Klimesch, W.; Stadler, W.; Pollhuber, D.; Heine, C. EEG alpha power and intelligence. Intelligence 2002, 30, 289–302. [Google Scholar] [CrossRef]
- Hsieh, L.; Ranganath, C. Frontal Midline Theta Oscillations during Working Memory Maintenance and Episodic Encoding and Retrieval. NeuroImage 2014, 85, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Anokhin, A.P.; Lutzenberger, W.; Birbaumer, N. Spatiotemporal organization of brain dynamics and intelligence: An EEG study in adolescents. Int. J. Psychophysiol. 1999, 33, 259–273. [Google Scholar] [CrossRef]
- Roberts, G.; Anderson, M. Task structure complexity and goal neglect in typically developing children. J. Exp. Child Psychol. 2014, 120, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.; Parr, A.; Woolgar, A.; Thompson, R.; Bright, P.; Cox, S.; Bishop, S.; Nimmo-Smith, I. Goal neglect and Spearman’s g: Competing parts of a complex task. J. Exp. Psychol. Gen. 2008, 137, 131–148. [Google Scholar] [CrossRef] [PubMed]
- Koenis, M.M.G.; Brouwer, R.M.; van den Heuvel, M.P.; Mandl, R.C.W.; van Soelen, I.L.C.; Kahn, R.S.; Boomsma, D.I.; Hulshoff Pol, H.E. Development of the brain’s structural network efficiency in early adolescence: A longitudinal DTI twin study. Hum. Brain Mapp. 2015, 36, 4938–4953. [Google Scholar] [CrossRef] [PubMed]
- Penke, L.; Maniega, S.M.; Murray, C.; Gow, A.J.; Hernández, M.C.V.; Clayden, J.D. A general factor of brain white matter integrity predicts information processing speed in healthy older people. J. Neurosci. 2010, 30, 7569–7574. [Google Scholar] [CrossRef] [PubMed]
- Giedd, J.N.; Rapoport, J.L. Structural MRI of pediatric brain development: What have we learned and where are we going? Neuron 2010, 67, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.W.; Laurent, P.; Stocco, A. Rapid instructed task learning: A new window into the human brain’s unique capacity for flexible cognitive control. Cogn. Affect. Behav. Neurosci. 2013, 13, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Cannon, T.D.; Keller, M.C. Endophenotypes in the genetic analyses of mental disorders. Annu. Rev. Clin. Psychol. 2006, 2, 267–290. [Google Scholar] [CrossRef] [PubMed]
- Kievit, R.A.; Davis, S.W.; Griffiths, J.; Correia, M.C.; Henson, R.N. A watershed model of individual differences in fluid intelligence. Neuropsychologia 2016, 91, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Bishop, D.V.M.; Anderson, M.; Reid, C.; Fox, A.M. Auditory development between 7 and 11 years: An event-related potential (ERP) study. PLoS ONE 2011, 6, e18993. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anderson, M. Binet’s Error: Developmental Change and Individual Differences in Intelligence Are Related to Different Mechanisms. J. Intell. 2017, 5, 24. https://doi.org/10.3390/jintelligence5020024
Anderson M. Binet’s Error: Developmental Change and Individual Differences in Intelligence Are Related to Different Mechanisms. Journal of Intelligence. 2017; 5(2):24. https://doi.org/10.3390/jintelligence5020024
Chicago/Turabian StyleAnderson, Mike. 2017. "Binet’s Error: Developmental Change and Individual Differences in Intelligence Are Related to Different Mechanisms" Journal of Intelligence 5, no. 2: 24. https://doi.org/10.3390/jintelligence5020024




