Poly(2-hydroxyethyl methacrylate)-quercetin Conjugate as Biomaterial in Ophthalmology: An “ab initio” Study
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Instruments
2.3. Synthesis of pHEMA-Q Conjugate
2.4. Ocular Tolerance Test (HET-CAM Test)
| Effect | Score | Inference |
|---|---|---|
| No visible hemorrhage | 0 | Non irritant |
| Just visible membrane discoloration | 1 | Mild irritant |
| Structures are covered partially due to membrane discoloration or hemorrhage | 2 | Moderately irritant |
| Structures are covered totally due to membrane discoloration or hemorrhage | 3 | Severe irritant |
2.5. Evaluation of Disposable Phenolic Groups by Folin-Ciocalteu Procedure
2.6. Hydrophilic Properties of Polymers
2.7. Determination of Scavenging Effect on the DPPH Radical
2.8. Determination of Total Antioxidant Activity
2.9. β-Carotene-Linoleic Acid Assay
2.10. Determination of Scavenging Properties on Peroxynitrite Anion
2.11. Scavenging Activity on Hydroxyl Radical
2.12. Anti-Inflammatory Effect
2.13. Statistical Analyses
3. Results and Discussion
3.1. Synthesis and Characterization of the pHEMA-Q Conjugate




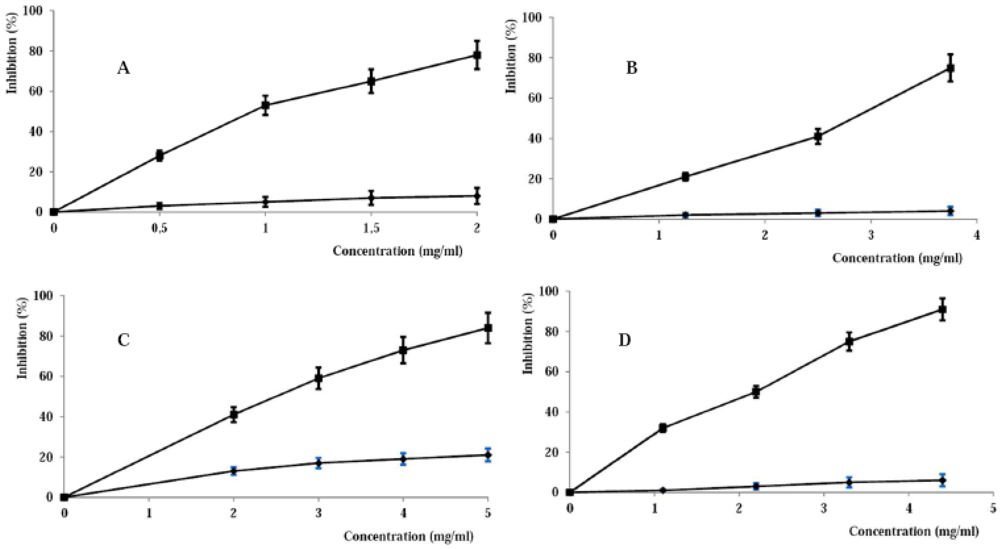
3.2. Evaluation of the Antioxidant and Anti Inflammatory Properties
4. Conclusions
Acknowledgments
References and Notes
- White, C.J.; Byrne, M.E. Molecularly imprinted therapeutic contact lenses. Exp. Opin. Drug Deliv. 2010, 7, 756–780. [Google Scholar]
- Horak, D.; Jayakrishnan, A.; Arshady, R. Poly(2-hydroxyethyl methacrylate) hydrogels: Preparation and properties. In Introduction to Polymeric Biomaterials; Arshady, R., Ed.; Kentus Books: London, UK, 2003; pp. 65–107. [Google Scholar]
- Andrade-Vivero, P.; Fernandez-Gabriel, E.; Alvarez-Lorenzo, C.; Concheiro, A. Improving the loading and release of NSAIDs from pHEMA hydrogels by copolymerization with functionalized monomers. J. Pharm. Sci. 2007, 96, 802–813. [Google Scholar]
- Venkata, S.J.A.; Narayanasamy, A.; Srinivasan, V.; Iyer, G.K.; Sivaramakrishnan, R.; Subramanian, M.; Mahadevan, R. Tear ascorbic acid levels and the total antioxidant status in contact lens wearers: A pilot study. Indian J. Ophthalmol. 2009, 57, 289–292. [Google Scholar]
- Rosa dos Santos, J.F.; Alvarez-Lorenzo, C.; Silva, M.; Balsa, L.; Couceiro, J.; Torres-Labandeira, J.J.; Concheiro, A. Soft contact lenses functionalized with pendant cyclodextrins for controlled drug delivery. Biomaterials 2009, 30, 1348–1355. [Google Scholar]
- Jones, L.W.; Jones, D.A. Non-inflammatory corneal complications of contact lens wear. Cont. Lens Anterior Eye 2001, 24, 73–79. [Google Scholar]
- Mandell, R.B. Symptomology and refitting. In Contact Lens Practice, 4th ed.; Mandell, R.B., Ed.; Thomas: Springfield, IL, USA, 1988; pp. 388–389. [Google Scholar]
- Richer, S.P.; Rose, R.C.; Gogia, R. An apparatus for ultraviolet B irradiation of small volumes of biological fluid under controlled oxygen tension. Optom. Vis. Sci. 1996, 73, 683–688. [Google Scholar]
- Augustin, A.J.; Spitznas, M.; Kaviani, N.; Meller, D.; Koch, F.H.; Grus, F.; Gobbels, M.J. Oxidative reactions in the tear fluid of patients suffering from dry eyes. Graefe's Arch. Clin. Exp. Ophthalmol. 1995, 233, 694–698. [Google Scholar]
- Ganea, E.; Harding, J.J. Glutathione-related enzymes and the eye. Curr. Eye Res. 2006, 31, 1–11. [Google Scholar]
- Vinson, J.A. Oxidative stress in cataracts. 2006, 13, 151–162. [Google Scholar]
- Saygili, E.I.; Aksoy, S.N.; Gurler, B.; Aksoy, A.; Erel, O.; Ozaslan, M. Oxidant/antioxidant status of patients with diabetic and senile cataract. Biotechnol. Biotechnol. Equip. 2010, 24, 1648–1652. [Google Scholar]
- Gupta, S.K.; Halder, N.; Srivastava, S.; Trivedi, D.; Joshi, S.; Varma, S.D. Green tea (Camellia sinensis) protects against selenite-induced oxidative stress in experimental cataractogenesis. Ophthal. Res. 2002, 34, 258–263. [Google Scholar]
- Zhang, J.; Wang, S. Topical use of Coenzyme Q10-loaded liposomes coated with trimethyl chitosan: Tolerance, precorneal retention and anti-cataract effect. Int. J. Pharm. 2009, 372, 66–75. [Google Scholar]
- Katalinić, V.; Možina, S.S.; Skroza, D.; Generalić, I.; Abramović, H.; Miloš, M.; Ljubenkov, I.; Piskernik, S.; Pezo, I.; Terpinc, P.; Boban, M. Polyphenolic profile, antioxidant properties and antimicrobial activity of grape skin extracts of 14 Vitis vinifera varieties grown in Dalmatia (Croatia). Food Chem. 2010, 119, 715–723. [Google Scholar]
- Poudel, P.R.; Tamura, H.; Kataoka, I.; Mochioka, R. Phenolic compounds and antioxidant activities of skin and seeds of five wild grapes and two hybrids native to Japan. J. Food Compos. Anal. 2008, 21, 622–625. [Google Scholar]
- Hakkinen, S. Flavonols and Phenolic Acids in Berries and Berry Products; Kuopio University Publications D, Medical Sciences: Kuopio, Finland, 2000; pp. 17–21. [Google Scholar]
- Dueňas, M.; González-Manzano, S.; González-Paramás, A.; Santos-Buelga, C. Antioxidant evaluation of O-methylated metabolites of catechin, epicatechin and quercetin. J. Pharm. Biomed. Anal. 2010, 51, 443–449. [Google Scholar]
- Di Carlo, G.; Mascolo, N.; Izzo, A.A.; Capasso, F. Flavonoids: Old and new aspects of a class of natural therapeutic drugs. Life Sci. 1999, 65, 337–353. [Google Scholar]
- Cirillo, G.; Kraemer, K.; Fuessel, S.; Puoci, F.; Curcio, M.; Spizzirri, U.G.; Altimari, I.; Iemma, F. Biological activity of a gallic acid-gelatin conjugate. Biomacromolecules 2010, 11, 3309–3315. [Google Scholar]
- Parisi, O.I.; Puoci, F.; Iemma, F.; De Luca, G.; Curcio, M.; Cirillo, G.; Spizzirri, U.G.; Picci, N. Antioxidant and spectroscopic studies of crosslinked polymers synthesized by grafting polymerization of ferulic acid. Polym. Adv. Technol. 2010, 21, 774–779. [Google Scholar]
- Casas, M.; Ferrero, C.; Jiménez-Castellanos, M.R. Graft tapioca starch copolymers as novel excipients for controlled-release matrix tablets. Carbohyd Polym. 2010, 80, 71–77. [Google Scholar]
- Curcio, M.; Puoci, F.; Iemma, F.; Parisi, O.I.; Cirillo, G.; Spizzirri, U.G.; Picci, N. Covalent insertion of antioxidant molecules on chitosan by free radical grafting procedure. J. Agric. Food Chem. 2009, 57, 5933–5938. [Google Scholar]
- Kook, D.; Wolf, A.H.; Yu, A.L.; Neubauer, A.S.; Priglinger, S.G.; Kampik, A.; Welge-Lussen, A.G. The protective effect of quercetin against oxidative stress in the human RPE in vitro. Invest. Ophthalmol. Vis. Sci. 2008, 49, 1712–1720. [Google Scholar]
- Venkat Ratnam, D.; Ankola, D.D.; Bhardwaj, V.; Sahana, D.K.; Ravi Kumar, M.N.V. Role of antioxidants in prophylaxis and therapy: A pharmaceutical perspective. J. Control Rel. 2006, 113, 189–207. [Google Scholar]
- Taylor, A. Cataract: Relationship between nutrition and oxidation. J. Am. Coll. Nutr. 1993, 12, 138–146. [Google Scholar]
- Careri, M.; Corradini, C.; Elviri, L.; Nicoletti, I.; Zagnoni, I. Direct HPLC analysis of quercetin and trans-Resveratrol in red wine, grape, and winemaking byproducts. J. Agric. Food Chem. 2003, 51, 5226–5231. [Google Scholar]
- Jimenez, N.; Galan, J.; Vallet, A.; Egea, M.A.; Garcia, M.L. Methyl trypsin loaded poly(D,L-lactide-coglycolide) nanoparticles for contact lens care. J. Pharm. Sci. 2010, 99, 1414–1426. [Google Scholar]
- Spielmann, H.; Liebsch, M.; Moldenhauer, F.; Holzhütter, H.G.; Bagley, D.M.; Lipman, J.M.; Pape, W.J.; Miltenburger, H.; de Silva, O.; Hofer, H.; Steiling, W. IRAG working group 2. CAM-based assays. Interagency Regulatory Alternatives Group. Food Chem. Toxicol. 1997, 35, 39–66. [Google Scholar]
- Pan, Y.; Zhu, J.; Wang, H.; Zhang, X.; Zhang, Y.; He, C.; Ji, X.; Li, H. Antioxidant activity of ethanolic extract of Cortex fraxini and use in peanut oil. Food Chem. 2007, 103, 913–918. [Google Scholar]
- Ardestani, A.; Yazdanparast, R. Antioxidant and free radical scavenging potential of Achillea santolina extracts. Food Chem. 2007, 104, 21–29. [Google Scholar]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal Biochem. 1999, 269, 337–341. [Google Scholar]
- Amin, I.; Zamaliah, M.M.; Chin, W.F. Total antioxidant activity and phenolic content in selected vegetables. Food Chem. 2004, 87, 581–586. [Google Scholar]
- Robaszkiewicz, A.; Bartosz, G. Estimation of antioxidant capacity against peroxynitrite and hypochlorite with fluorescein. Talanta 2010, 80, 2196–2198. [Google Scholar]
- Bartosz, M.; Kedziora, J.; Bartosz, G. Antioxidant and prooxidant properties of captopril and enalapril. Free. Radic. Biol. Med. 1997, 23, 729–735. [Google Scholar]
- Awah, F.M.; Uzoegwu, P.N.; Oyugi, J.O.; Rutherford, J.; Ifeonu, P.; Yao, X.J.; Fowke, K.R.; Eze, M.O. Free radical scavenging activity and immunomodulatory effect of Stachytarpheta angustifolia leaf extract. Food Chem. 2010, 119, 1409–1416. [Google Scholar]
- Al-Malaika, S.; Suharty, N. Reactive processing of polymers: Mechanisms of grafting reactions of functional antioxidants on polyolefins in the presence of a coagent. Polym. Degrad. Stabil. 1995, 49, 77–89. [Google Scholar]
- Iemma, F.; Puoci, F.; Curcio, M.; Parisi, O.I.; Cirillo, G.; Spizzirri, U.G.; Picci, N. Ferulic acid as a comonomer in the synthesis of a novel polymeric chain with biological properties. J. Appl. Polym. Sci. 2010, 115, 784–789. [Google Scholar]
- Kitagawa, M.; Tokiwa, Y. Polymerization of vinyl sugar ester using ascorbic acid and hydrogen peroxide as a redox reagent. Carbohydr. Polym. 2006, 64, 218–223. [Google Scholar]
- Vinardell, M.P.; Mitjans, M. Alternative methods for eye and skin irritation tests: An overview. J. Pharm. Sci. 2008, 97, 46–59. [Google Scholar]
- Spielmann, H. Ocular irritation. In In Vitro Methods in Pharmaceutical Research; Castle, J.V., Gomez, M.J., Eds.; Academic Press: San Diego, CA, USA, 1997; pp. 265–287. [Google Scholar]
- Barile, F.A. Validating and troubleshooting ocular in vitro toxicology tests. J. Pharmacol. Toxicol. Methods 2010, 61, 136–145. [Google Scholar]
- Valdes, T.; Kreutzer, D.; Moussy, F. The chick chorioallantoic membrane as a novel in vivo model for the testing of biomaterials. J. Biomed. Mater. Res. 2002, 62, 273–282. [Google Scholar]
- Araújo, J.; Vega, E.; Lopes, C.; Egea, M.A.; Garcia, M.L.; Souto, E.B. Effect of polymer viscosity on physicochemical properties and ocular tolerance of FB-loaded PLGA nanospheres. Coll. Surf. B: Biointerf. 2009, 72, 48–56. [Google Scholar]
- Huang, Y.S.; Ho, S.C. Polymethoxy flavones are responsible for the anti-inflammatory activity of citrus fruit peel. Food Chem. 2010, 119, 868–873. [Google Scholar]
- Heijnen, C.G.; Haenen, G.R.M.M.; Vekemans, J.A.J.M.; Bast, A. Peroxynitrite scavenging of flavonoids: Structure activity relationship. Environ. Toxicol. Pharmacol. 2001, 10, 199–206. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Curcio, M.; Cirillo, G.; Parisi, O.I.; Iemma, F.; Spizzirri, U.G.; Altimari, I.; Picci, N.; Puoci, F. Poly(2-hydroxyethyl methacrylate)-quercetin Conjugate as Biomaterial in Ophthalmology: An “ab initio” Study. J. Funct. Biomater. 2011, 2, 1-17. https://doi.org/10.3390/jfb2010001
Curcio M, Cirillo G, Parisi OI, Iemma F, Spizzirri UG, Altimari I, Picci N, Puoci F. Poly(2-hydroxyethyl methacrylate)-quercetin Conjugate as Biomaterial in Ophthalmology: An “ab initio” Study. Journal of Functional Biomaterials. 2011; 2(1):1-17. https://doi.org/10.3390/jfb2010001
Chicago/Turabian StyleCurcio, Manuela, Giuseppe Cirillo, Ortensia Ilaria Parisi, Francesca Iemma, Umile Gianfranco Spizzirri, Ilaria Altimari, Nevio Picci, and Francesco Puoci. 2011. "Poly(2-hydroxyethyl methacrylate)-quercetin Conjugate as Biomaterial in Ophthalmology: An “ab initio” Study" Journal of Functional Biomaterials 2, no. 1: 1-17. https://doi.org/10.3390/jfb2010001






