Effect of Electrical Conductivity Through the Bulk Doping of the Product of Titanocene Dichloride and 2-Nitro-1,4-phenylenediamine
Abstract
:1. Introduction

2. Results and Discussion
2.1. General

2.2. Variation of Dissipation Factor and Dielectric Constant for Doped and Undoped Materials
2.3. Effect of Amount of Iodine
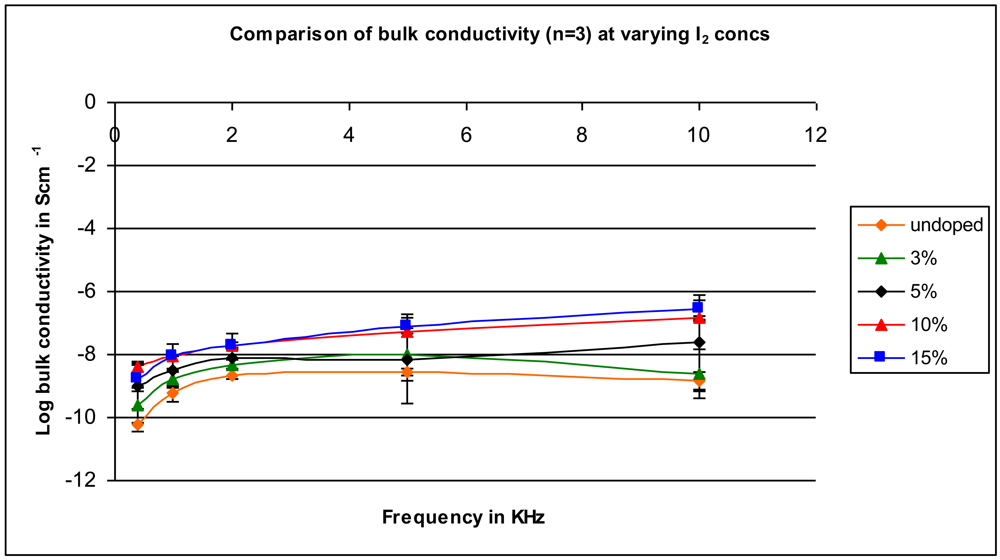
2.4. Effect of Heating





2.5. Physical Characterization
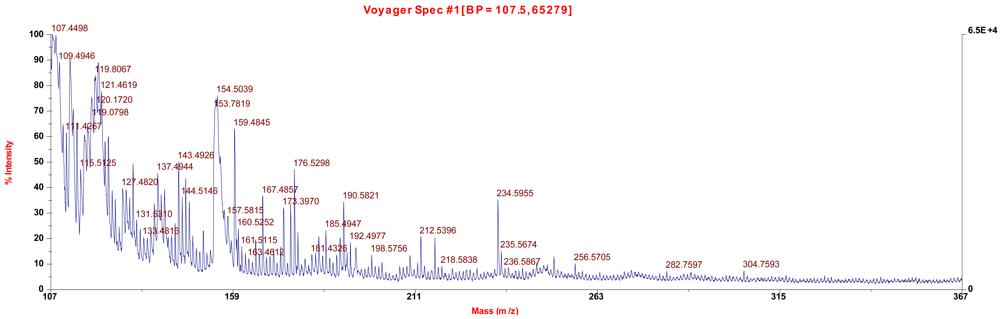

3. Experimental Section
3.1. Synthesis
3.2. Electrical Measurements
4. Conclusions
4.1. Future
References
- Chiang, C.K.; Druy, M.A.; Gau, S.C.; Heeger, A.J.; Louis, E.J.; MacDiarmid, A.G.; Park, Y.W.; Shirakawa, H. Synthesis of Highly Conducting Films of Derivatives of Polyacetylene, (CH)x. J. Am. Chem. Soc. 1978, 100, 1013–1015. [Google Scholar]
- Kaner, R.B.; MacDiarmid, A.G. Plastics That Conduct Electricity. Sci. Am. 1988, 106–111. [Google Scholar]
- MacDiarmid, A.G.; Epstein, A.J. “Synthetic Metals”: A Novel Role for Organic Polymers. Macromol. Chem. 1991, 51, 11–28. [Google Scholar]
- MacDiarmid, A.G.; Epstein, A.J. Science and Technology of Conducting Polymers. In Frontiers of Polymer Research; Prasad, P.N., Nigam, J.K., Eds.; Plenum Press: New York, NY, USA, 1991; p. 259. [Google Scholar]
- Hohnholz, D.; MacDiarmid, A.G. Line Patterning of Conducting Polymers: New Horizons for Inexpensive, Disposable Electronic Devices. Synth. Met. 2001, 121, 1327–1328. [Google Scholar]
- MacDiarmid, A.G. Twenty-five Years of Conducting Polymers. Chem. Comm. 2003, 1–4. [Google Scholar]
- Jozefiak, T.H.; Ginsburg, E.J.; Gorman, C.B.; Grubbs, R.H.; Lewis, N.S. Voltammetric Characterization of Soluble Polyacetylene Derivatives Obtained from the Ring-Opening Metathesis Polymerization (ROMP) of Substituted Cyclooctatetraenes. J. Am. Chem. Soc. 1993, 115, 4705–4713. [Google Scholar]
- Gorman, C.B.; Ginsburg, E.J.; Grubbs, R.H. Soluble, Highly Conjugated Derivatives of Polyacetylene from the Ring-Opening Metathesis Polymerization of Monosubstituted Cyclooctatetraenes: Synthesis and the Relationship between Polymer Structure and Physical Properties. J. Am. Chem. Soc. 1993, 115, 1397–1409. [Google Scholar]
- Langsdorf, B.L.; Zhou, X.; Lonergan, M.C. Kinetic Study of the Ring-Opening Metathesis Polymerization of Ionically Functionalized Cyclooctatetraenes. Macromolecules 2001, 34, 2450–2458. [Google Scholar]
- MacDiarmid, A.G. Nobel Lecture: Synthetic metals: A novel role for organic polymers. Revs. Mod. Phys. 2001, 73, 701–712. [Google Scholar]
- Shirakawa, H. Nobel Lecture: The discovery of polyacetylene film-the drawing of an era of conducting polymers. Revs. Mod. Phys. 2001, 73, 713–718. [Google Scholar]
- Carraher, C. Polymer Chemistry, 8th ed.; Taylor and Francis: New York, NY, USA, 2011. [Google Scholar]
- Carraher, C. Introduction to Polymer Chemistry, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Carraher, C.; Nwufoch, V.; Taylor, J.R. Electrical properties of palladium polyamines with respect to the theory of whole chain resonance. Polym. Mater. Sci. Eng. 1989, 60, 685–689. [Google Scholar]
- Carraher, C.; Venable, W.; Blaxall, H.; Sheats, J. Synthesis and characterization of antimony V-polycobalticinium esters. J. Macromol. Sci. Chem. 1980, A14, 571–579. [Google Scholar]
- Carraher, C.; Blaxall, H.; Schroeder, J.; Venable, W. Synthesis and Electrical, Thermal and Solution Characterization of Antimony V Polyesters. Org. Coat. Plast. Chem. 1978, 39, 549–554. [Google Scholar]
- Carraher, C.; Christensen, M.; Schroeder, J. Physical characterization of titanium polyferrocene oximes. J. Macromol. Sci. Chem. 1977, A11, 2021–2028. [Google Scholar]
- Carraher, C.; Lanz, L. Synthesis and physical characterization of group IVB metallocene polymers containing norfloxacin. J. Polym. Mater. 2005, 21, 51–60. [Google Scholar]
- Carraher, C.; Leahy, D.; Ailts, S. Initial Electrical Conductivity Measurements of Selected Organometallic Polymers. Org. Coat. Plast. Chem. 1977, 37, 201–204. [Google Scholar]
- Carraher, C.; Schroeder, J.; Venable, W.; McNeely, C. Synthesis and electrical, thermal and solution characterization of antimony V polyesters. Org. Coat. Plast. Chem. 1978, 38, 544–553. [Google Scholar]
- Carraher, C.; Schroeder, J.; Venable, W.; McNeely, C.; Giron, D.; Woelk, W.; Feddrson, M. Electrical, Solvent, Thermal and Fungal Properties of Organotin-Containing Poly(ethyleneimine). In Additives for Plastics; Academic Press: New York, NY, USA, 1978; Chapter 8; pp. 81–91. [Google Scholar]
- Carraher, C.; Manek, T.; Linville, R.; Taylor, J.R.; Torre, L.; Venable, W. Preliminary AC and DC Electrical Properties of Group IV B Metallocene Polyoximes. Org. Coat. Plast. Chem. 1980, 43, 753–757. [Google Scholar]
- Carraher, C.; Linville, R.; Manek, T.; Blaxall, H.; Taylor, J.R.; Torre, L. Electrical Properties of Group IV B Metallocene Polyoximes. In Electrical Properties of Polymers; Plenum Press: New York, NY, 1981. [Google Scholar]
- Battin, A.; Carraher, C. Effect of doping by exposure to iodine vapor on the electrical conductivity of the polyamine from titanocene dichloride and 2-nitro-p-phenylenediamine. J. Polym. Mater. 2008, 25, 23–33. [Google Scholar]
- Carraher, C. Synthesis of titanium polyesters. J. Polym. Sci. 1971, 9, 3661–3670. [Google Scholar]
- Carraher, C. Condensation metallocene polymers. J. Inorg. Organomet. Polym. Mater. 2005, 15, 121–145. [Google Scholar]
- Carraher, C. Fiber forming and thermal properties of polyesters of group IV metals. Chem. Tech. 1971, 741–744. [Google Scholar]
- Stewart, H.; Soldani, W.; Carraher, C.; Reckleben, L. Polymeric auxin plant growth hormones based on the condensation products of indole-3-butyric acid with bis(cyclopentadieneyl)titanium IV dichloride and dipyridine manganese II dichloride. In Inorganic and Metal-Containing Polymeric Materials; Plenum Press: New York, NY, 1991. [Google Scholar]
- Carraher, C.; Carraher, S.; Stewart, H. Metal-containing polymer structures for enhanced seed germination and plant growth. Adv. Environ. Bio. 2010, 4, 108–116. [Google Scholar]
- Carraher, C.; Foster, V.; Linville, R.; Stevison, D.; Venkatachalam, R. Organotin and organotitanium-containing polydyes for color permanence, reduction of laser damage and biological resistance to rot and mildew. In Adhesives, Sealants, and Coatings for Space and Harsh Environments; Plenum Press: New York, NY, USA, 1988. [Google Scholar]
- Roner, M.; Carraher, C.; Shahi, K.; Ashida, Y.; Barot, G. Ability of group IVB metallocene polyethers containing dienestrol to arrest the growth of selected cancer cell lines. BMC Caner 2009, 9, 358–369. [Google Scholar]
- Carraher, C.; Roner, M.; Shahi, K.; Ashida, Y.; Barot, G. Synthesis, structural characterization, and anti-cancer evaluation of group IVB-metallocene polyethers containing the synthetic estrogen diethylstibestrol. J. Polym. Mater. 2007, 24, 357–369. [Google Scholar]
- Carraher, C.; Lessek, P. Synthesis of titanium polyamines via the interfacial and aqueous solution techniques. Eur. Polym. J. 1972, 8, 1339–1345. [Google Scholar]
- Carraher, C.; Jorgensen, S. Study of Associated Reaction Variables in the Synthesis of Titanium (IV) Polyamines and a Comparison of Synthesis by Different Techniques. J. Polym. Sci. 1978, 16, 2965–2970. [Google Scholar]
- Inzelt, G. Conducting Polymers; Springer: New York, NY, USA, 2008. [Google Scholar]
- Bott, D. Electrically conducting polymers. Phys. Technol. 1985, 16, 121–126. [Google Scholar]
- Harum, M.; Saion, E.; Kassim, A.; Yahya, N.; Mahmud, E. Conjugated conducting polymers: A brief overview. JASA 2007, 63–68. [Google Scholar]
- Kumar, D.; Sharma, R.C. Advances in conductive polymers. Eur. Polym. J. 1998, 34, 1053–1060. [Google Scholar]
- Chan, W.K. Metal containing polymers with heterocyclic rigid main chains. Coord. Chem. Revs. 2007, 251, 2104–2118. [Google Scholar]
- Barford, W. Electronic and Optical Properties of Conjugated Polymers; Oxford University: Ithaca, NY, USA, 2009. [Google Scholar]
- Chaing, C.K. The bromine doping of polyacetlyene. Physica A 2003, 321, 139–151. [Google Scholar]
- Chaing, C.K.; Park, Y.; Heeger, A.; Shirakawa, H.; Louis, E.; MacDiarmid, A. Conducting polymers: Halogen-doped polyacetlyene. J. Chem. Phys. 1978, 69, 5098–6001. [Google Scholar]
- Bekkali, A.; Thurzo, I.; Kampen, T.; Zahn, D.; Dietrich, R. Impedance spectroscopy study of metal-organic-metal structures. Appl. Surf. Sci. 2004, 234, 149–154. [Google Scholar]
- Cotton, F.A.; Wilkerson, G. Advanced Inorganic Chemistry; Wiley: Hoboken, NY, USA, 1988. [Google Scholar]
- Silverstein, R.; Webster, F.; Kiemle, D. Spectrometric identification of organic compounds; Wiley: Hoboken, NY, USA, 2005. [Google Scholar]
- Rao, C.N.R. Chemical Application of Infrared Spectroscopy; Academic Press: New York, NY, USA, 1963. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Carraher, C.E., Jr.; Battin, A.J.; Roner, M.R. Effect of Electrical Conductivity Through the Bulk Doping of the Product of Titanocene Dichloride and 2-Nitro-1,4-phenylenediamine. J. Funct. Biomater. 2011, 2, 18-30. https://doi.org/10.3390/jfb2010018
Carraher CE Jr., Battin AJ, Roner MR. Effect of Electrical Conductivity Through the Bulk Doping of the Product of Titanocene Dichloride and 2-Nitro-1,4-phenylenediamine. Journal of Functional Biomaterials. 2011; 2(1):18-30. https://doi.org/10.3390/jfb2010018
Chicago/Turabian StyleCarraher, Charles E., Jr., Amitabh J. Battin, and Michael R. Roner. 2011. "Effect of Electrical Conductivity Through the Bulk Doping of the Product of Titanocene Dichloride and 2-Nitro-1,4-phenylenediamine" Journal of Functional Biomaterials 2, no. 1: 18-30. https://doi.org/10.3390/jfb2010018




