Is Macroporosity Absolutely Required for Preliminary in Vitro Bone Biomaterial Study? A Comparison Between Porous Materials and Flat Materials
Abstract
:1. Introduction
2. Experimental Section
2.1. Material Synthesis
2.2. Material Characterization and Sterilization
2.3. Cell Subculturing
2.4. RT-qPCR with Cells from Porous Materials and Flat Materials
2.5. ALP Enzymatic Assay with Different Cell Plating Densities
2.6. SEM of Cells on Scaffolds
2.7. DAPI Staining
2.8. Statistical Analysis
3. Results
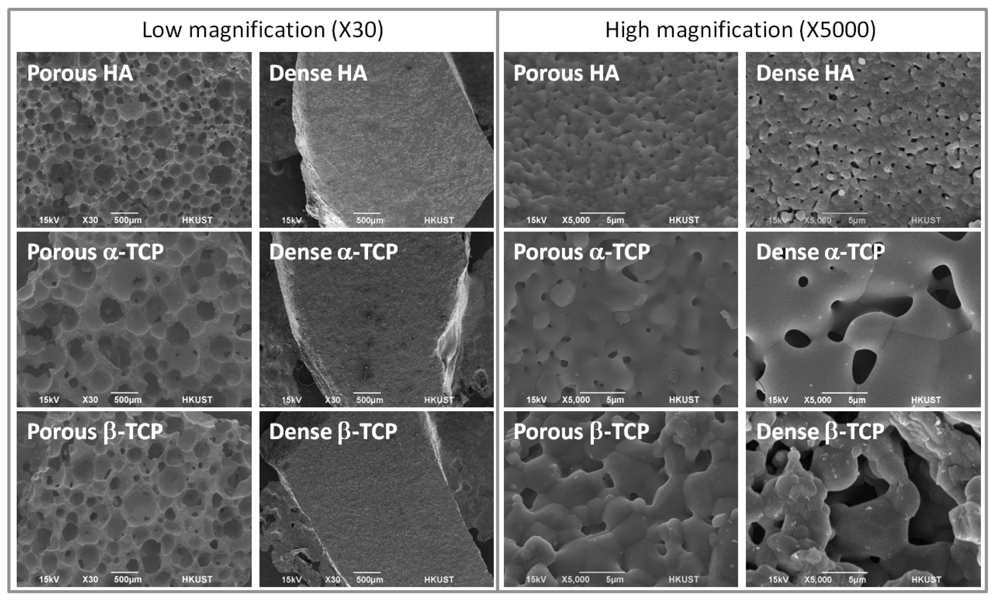
3.1. Material Characterization
| Porous HA | Porous α-TCP | Porous β-TCP | |
|---|---|---|---|
| Surface porosity a (%) | 67.3 ± 1.7 | 68.1 ± 5.8 | 66.5 ± 1.5 |
| Crater size b (μm) | 203 ± 88 | 342 ± 134 | 269 ± 111 |

3.2. Yield of RNA from Cells on Materials
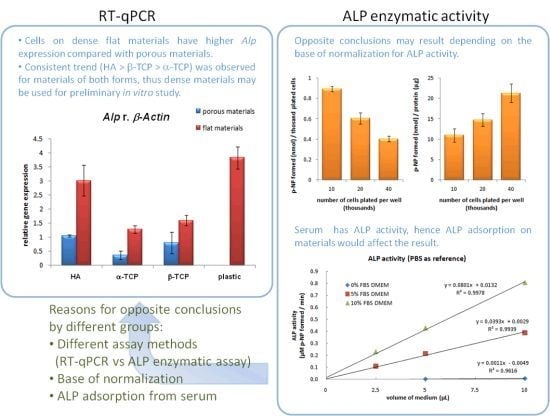
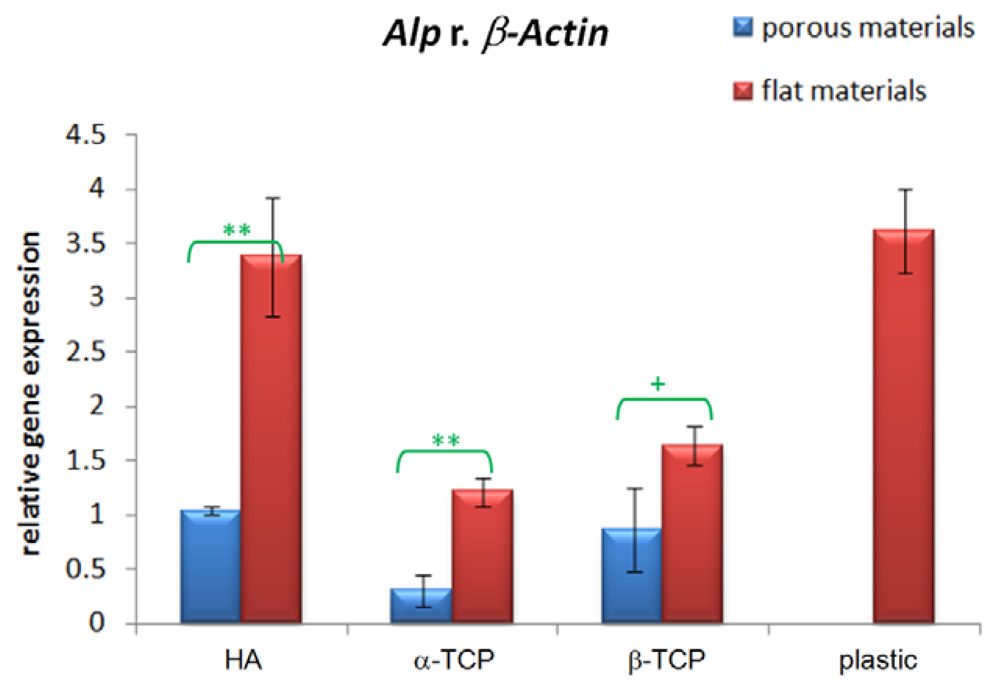
3.3. Alp Gene Expression
3.4. Effect of Cell Density

3.5. Distribution and Morphology of Cells in Porous Scaffolds

4. Discussion
4.1. Effect of Macroporosity of Calcium Phosphate Discs
4.2. ALP Activity Assay

| Number of cells plated per well of a 96-well plate | 10,000 | 20,000 | 40,000 |
|---|---|---|---|
| Cell plating density (thousand cells/sq cm) | 31.3 | 62.5 | 125 |
| p-NP formed (nmol) | 8.92 (±0.26) | 12.06 (±1.10) | 16.08 (±1.10) |
| Normalized by initial cell number (nmol/thousand cells) | 0.89 (±0.03) | 0.60 (±0.05) | 0.40 (±0.03) |
| Normailzed by protein (nmol/mg protein) | 10.91 (±1.66) | 14.68 (±1.53) | 21.25 (±2.26) |
| Normalized by area covered (nmol/sq cm) | 27.87 (±0.81) | 37.68 (±3.43) | 50.25 (±3.43) |
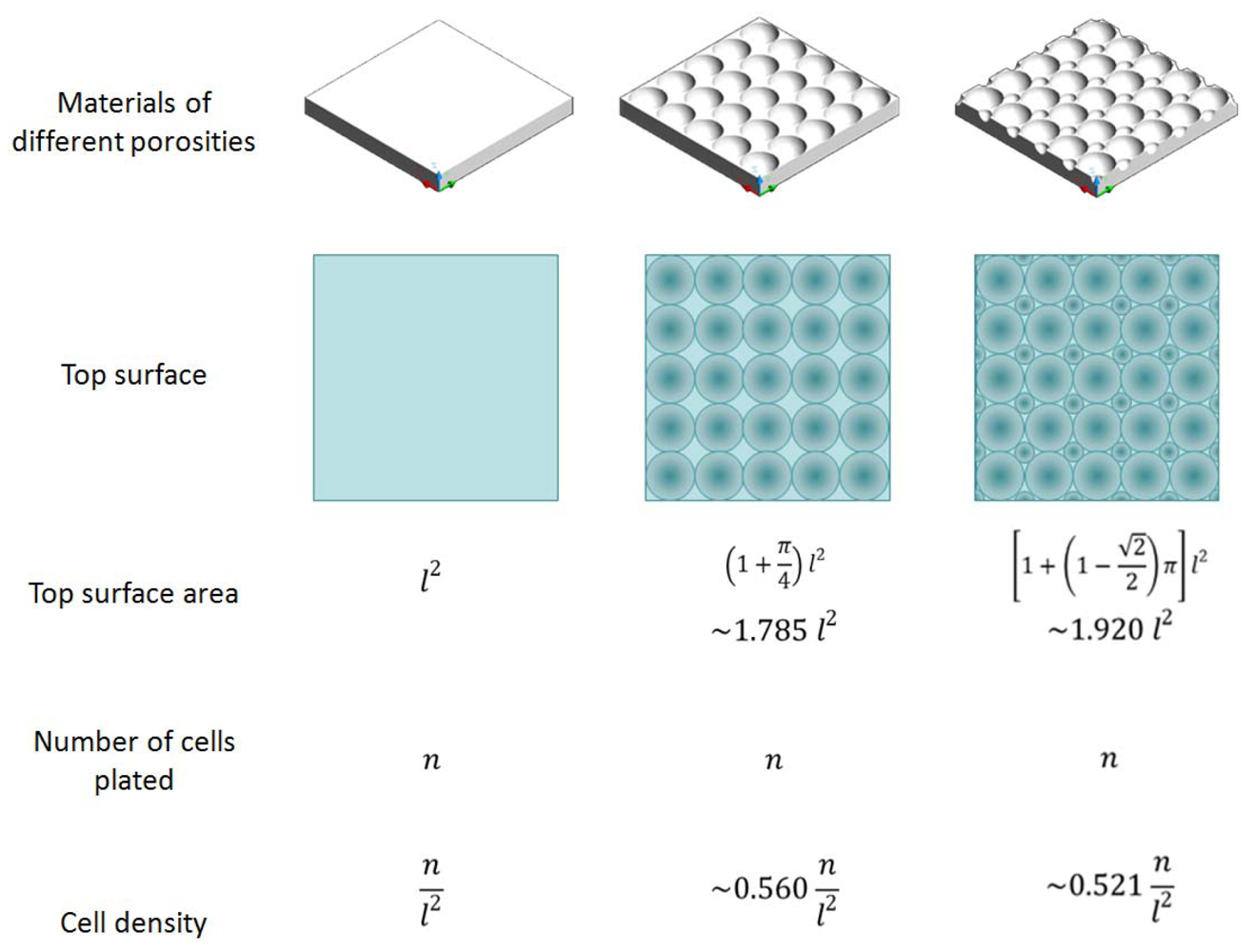
4.3. Tradeoff between Higher Clinical Relevance and Lower Variation

5. Conclusions
Acknowledgments
Appendix
| Material a | Implant site | Porosity (%) | Macropore size (μm) | Specific surface area (m2/g) | Assessments b | Conclusion a,b | Reference |
|---|---|---|---|---|---|---|---|
| BCP | In femurs of rabbits | 40, 50% | 300, 565 | / | Histology | Macroporous biphasic calcium phopshate implants with 565 μm pore diameter and 40% macroporosity represented the optimal association for homogeneous and abundant bone ingrowth. | [62] |
| BCP | Intramuscularly in goats | macroporosity: 58%; microporosity: 4–24% | <∼400 | 0.2–9.7 | Histology and histomorphometry | The increased specific surface area led to more surface reactivity, which is hypothesized to be essential for osteoinductivity by biomaterials. | [63] |
| BCP | Intramuscularly in goats | 70, 75% | / | 0.2, 1.5 | Histology and histomorphometry | The BCP scaffold with 75% porosity performed better than the one with 70% porosity in both the amount and rate of bone formation. | [64] |
| β-TCP | In drill hole defects in cancellous bone of sheep | / | 150, 260, 510, 1220 | / | Macroscopy, histology and histomorphometry | Samples with an intermediate macropore size (510 μm) were resorbed significantly faster than samples with smaller macropore sizes (150 and 260 μm). However, this fast resorption was associated with a lower bone content and a higher soft tissue content. | [65] |
| β-TCP | Subcutaneously in mice | 25, 65, 75% | / | 0.12, 0.18, 0.3 | Specific ALP activity, histology | A higher porosity of TCP scaffolds does not necessarily mean a higher ALP activity in vivo. The distribution and size of the pores, as well as the surface structure, might play an important role for osteogenic differentiation in vivo. | [66] |
| HA | Intramuscularly in goats | 60, 70% | 400, 800 | / | Histology and histomorphometry | The microporous HA scaffolds with 70% porosity and 800 μm pore size yielded more bone than did HA scaffolds with 60% porosity and 400 μm. | [67] |
| HA | Subcutaneously in mice | 65, 80% | / | 0.87, 1.63 | Histology | The porosity and pore interconnection of osteoconductive scaffolds can influence the overall amount of bone deposition, the pattern of blood vessels invasion and finally the kinetics of the bone neoformation process. | [68] |
| HA | Subcutaneously in rats | 30, 50, 70% | / | / | Histology, ALP acitivity and OCN content | Expanded bone formation was observed earlier for constructs with higher porosity. The ALP activity and OCN production also increased with increasing porosity. | [34] |
| HA | Subcutaneously in rats | 70% | 106–600 | 0.024 | Histology, ALP acitivity and OCN content | The ALP activities at 2 wk and the OCN contents at 4 wk after implantation was the highest in the ceramics implants with pore size of 300-400 μm. | [69] |
| HA | Subcutaneously in rats | Tunnels of 0.7mm or 3mm in diameter | / | / | Histology, Ca and Type II collagen content | The “vasculature-inducing geometry” of the carrier as an extracellular matrix is crucially important for osteogenesis. | [70] |
| HA, BCP | Intramuscularly in goats | 46.5–54.3% | 243.9–380.2 | 0.07–1.60 | Histology and histomorphometry | Histomorphometrical results showed that the presence of micropores within macropore walls is necessary to make a material osteoinductive. | [71] |
| HA, TCP | In cancellous bone of rabbits | 60% | 50–100 and 200–400 | / | Histology | For TCP, bone and tissue ingrowth and implant resorption occurred at a higher rate in the smaller-pored materials compared with the larger-pored ones. For HA, the smaller-pored materials was totally infiltrated by bone or bone marrow after four months but not for the larger-pored ones. | [72] |
| Ni-Ti alloy | In femurs of rats | 46.6, 59.2, 66.1 | 259, 272, 505 | / | Histology and histomorphometry | The porosity of 66.1% showed best bone contact (51%) of the porosities tested. | [73] |
| PCL | Subcutaneously in mice | / | 350, 550, 800 | / | Histology, μCT and mechanical testing | Pore sizes between 350 and 800 μm play a limited role in bone regeneration in this tissue engineering model. | [74] |
| PPF | In cranial defects in rabbits | 57–75% | 300–500, 600–800 | / | Light microscopy, histological scoring analysis and histomorphometric analysis | Scaffold porosity and scaffold pore size were not found to significantly affect the observed tissue response. | [75] |
| Ti6Al4V | In femurs of rabbits | / | 100, 200, 300 | / | Histology and histomorphometry | 200 μm may be the optimal pore size for laser-textured Ti6Al4V implants. | [76] |
| Ti6Al4V | In femurs of rats | 3, 11, 25% | / | / | Ca concentration | 25% porosity samples showed the highest amount of calcium concentration within the pores, suggesting a faster rate of tissue generation and integration compared to samples with lower pore volume. | [77] |
| Material a | Cell | Porosity (%) | Macropore size (μm) | Specific surface area (m2/g) | Assessments b | Conclusion a,b | Reference |
|---|---|---|---|---|---|---|---|
| β-TCP | Human mesenchymal stem cells | 25, 65, 75% | / | 0.12, 0.18, 0.3 | Protein production, specific ALP activity | In vitro porosity was beneficial for protein production, but did not influence osteogenic differentiation. | [66] |
| CO3Ap-collagen sponges | MC3T3-E1 cells | 48.9, 72.6, 79.2% | 50–300 | / | Histology | There was no significant difference in the invading osteoblast quantity for composite of apatite and collagen with pores ranging from 50–300 μm and porosities of 49–79%. | [78] |
| COL-CG | MC3T3-E1 cells | / | 85–325 | 0.00221–0.00845 μm−1 | Cell adhesion and infiltration | Scaffolds with a mean pore size of 325 μm were deemed optimal for bone tissue engineering. | [79] |
| HA | Rat primary bone marrow mesenchymal stem cells | 30, 50, 70% | / | / | ALP acitivity and OCN content | The ALP activity and OCN production increased with increasing porosity. | [34] |
| HA | Primary human osteoblast-like cells | 67-76% | / | / | SEM | The introduction of microporosity has no evident effect on cellular morphology at later time points but it seems to play a role in initial cellular anchorage and attachment. | [80] |
| HA | Rat bone marrow cells | <5, 15, 30% | / | / | Cell attachment, proliferation, total protein content, ALP activity and bone-like nodule formation | The intermediary and final events such as proliferation, protein synthesis, ALP activity, and bone-like nodule formation favored surfaces with a more regular topography, such as that presents in HA with 15% or less of microporosity. | [81] |
| HA | MC3T3-E1 cells, L132 cells | 0.2, 18.3, 25, 80% | 1–10, 10–50, 500–600 | / | Cell proliferation, viability, SEM, cytochemical staining | The micro-porous HA (internal pore size of 1–10 μm) induced the highest cell growth. | [82] |
| HA | Autologous human mesenchymal stem cells | 75, 88% | 200, 500 | / | DNA content, ALP activity, histology, SEM and RT-qPCR | The 200-μm pore scaffolds exhibited a faster rate of osteogenic differentiation than did the 500-μm pore scaffolds. | [53] |
| PCL | Rat marrow stromal cells | 84-89% | 20–45 | / | Cell attachment, spreading and histology | Increasing the thickness of the nanofiber layer (also increasing the pore size) reduced the infiltration of cells into the scaffolds. | [83] |
| PET | Mesenchymal stem cells | 92.9-97.0% | / | / | Cell proliferation, ALP activity, OCN content and SEM | The attachment, proliferation and bone differentiation of MSC was influenced by the fiber diameter and porosity of non-woven fabrics as the scaffold. | [33] |
| TiO2 | Human bone-derived cells | / | 0.4, 13, 49 | / | Cell proliferation | Smaller pores (0.4 and 13 μm) in TiO2 films enhanced the proliferation of human cells trypsinised from bone in contrast to larger pores (49 μm). | [84] |
| HA | α-TCP | β-TCP | |||
|---|---|---|---|---|---|
| Temp. (°C) | Duration (min) | Temp. (°C) | Duration (min) | Temp. (°C) | Duration (min) |
| 0–400 | 160 | 0–400 | 160 | 0–400 | 160 |
| 400 | 120 | 400 | 120 | 400 | 120 |
| 400–800 | 160 | 400–800 | 160 | 400–800 | 160 |
| 800 | 240 | 800 | 240 | 800 | 240 |
| 800–1100 | 120 | 800–1250 | 180 | 800–1,100 | 120 |
| 1100 | 120 | 1250 | 360 | 1,100–900 | 240 |
| normal cooling | quick cooling | 900 | 360 | ||
| normal cooling | |||||
| Related cell type | Cell | 2D material | 3D material a | Comparison between 2D and 3D a | Reference |
|---|---|---|---|---|---|
| Cancer cell | C4-2B cells | Collagen-coated tissue culture plastic | Electrospun collagen membrane | The cells on electrospun substrates were more resistant to both antineoplastic agents, docetaxel, and camptothecin compared to the cells grown on standard collagen-coated tissue culture polystyrene. | [85] |
| Chondrocyte | Rat bone marrow cells | Monolayer | 3D chitosan or chitosan/gelatin scaffolds | The chitosan scaffolds caused a reduction in alkaline phosphatase production and an increase in the collagen concentration indicating phenotypic changes in the cells after the addition of a chondrogenic medium. | [86] |
| Chondrocyte | Human articular chondrocytes (HAC) | Monolayer on culture plates | Type II collagen sponges | Three-dimensional expansion of HAC on the scaffolds, as compared with 2D expansion for the same number of doublings, better maintained the chondrocytic phenotype of the expanded cells mRNA) but did not enhance their accumulation of glycosaminoglycan (GAG) following chondrogenic culture. Besides, increasing the HAC seeding density in the scaffolds (from 25 × 103 to 66 × 103 cells/mm3) significantly improved chondrogenesis (up to 3.3-fold higher GAG accumulation and up to 9.3-fold higher type II collagen mRNA). | [87] |
| Chondrocyte | Autologous chondrocytes and mesenchymal stem cells (MSCs) | Monolayer | 3D pellet or fibrin-sealant construct | There was a proliferative effect for MSCs exposed to PRP in monolayer culture and an increase in the expression of chondrogenic markers when cells are exposed to a 3D environment. | [88] |
| Chondrocyte | Bovine chondrocytes | Monolayer in flasks | 3D Minusheet | The Minusheet cultures usually showed a markedly higher mRNA expression than monolayer cultures. Besides, the ratio of type-I to type-II collagen or aggrecan to type-I collagen remained higher in Minusheet 3D cultures than in monolayer cultures. | [89] |
| Endothelial cell | Primary human artery-derived fibroblasts and human umbilical vein endothelial cells | Monolayer cell sheets | Self-assembled cell-based microtissues | The microtissue has significant enhancement of ECM expression and maturation. | [90] |
| Fibroblast | Fibroblast cell line GD25b1 | Monolayer | 3D matrices derived mouse embryo sections or naturally deposited ECM | The relative content of unsaturated fatty acids, which serve as targets of oxidative attack, was observed to be higher in major phospholipids in plasma membranes of 3D cells. | [91] |
| Fibroblast | Human foreskin fibroblasts | Glass cover slips coated with different proteins or 3D matrix compressed to form localised 2D matrix | 3D matrices derived either from detergent-extracted mouse embryo sections or naturally deposited 3D ECM | 3D-matrix adhesions differ from focal and fibrillar adhesions characterized on 2D substrates in their content of α5β1 and αvβ3 integrins, paxillin, other cytoskeletal components, and tyrosine phosphorylation of focal adhesion kinase (FAK). Relative to 2D substrates, 3D-matrix interactions also display enhanced cell biological activities and narrowed integrin usage. | [92] |
| Fibrochondrocyte | Primary fibrochondrocytes | tailored biomimetic surface (C6S surface) on glass cover slip | tailored biomimetic surface on PLGA scaffolds | Human fibrochondrocyte redifferentiation was enhanced by hypoxia in the 3D cultures, independent of hypoxia inducible factor (HIF) transcriptional activity and was shown to potentially involve the transcriptional activation of Sox-9. | [93] |
| Hepatocyte | Primary rat hepatocytes | Collagen-coated polymeric substrates | 3D polymeric scaffold coated with collagen | Albumin and urea assays demonstrated that hepatocytes cultured in the 3D scaffold maintained higher levels of liver specific function over a period of 6 days as compared to the monolayer control. These results may be attributed to the high local concentration of soluble factors within the scaffold, which is important for maintaining the hepatocyte phenotype. | [94] |
| Hepatocyte | Hepatocyte cell line HepG2 | Monolayer | Agar/gelatin sponge | The results showed that the agar–gelatin hybrid sponges induced the formation of 3D HepG2 spheroids with significant liver-specific functions. These spheroids exhibited higher amounts of albumin and urea synthesis than the control monolayer culture. These 3D spheroids were found to be more sensitive to the drug than the control monolayer. | [95] |
| Myoblast | Rat skeletal myoblasts | Culture plate | PLGA-collagen composite scaffolds | Tensile strain induces higher and faster integrin β1 and ILK expression in 3D cultured rat skeletal myoblasts than in 2D cultures. | [96] |
| Osteoblast | Rat bone marrow cells | Monolayer | 3D homogenized pellet and 3D organotypic explant | The 3D organotypic marrow explant culture resulted in the greatest level of ossification with plate-like bone formations. | [97] |
| Osteoblast | Osteoblast-like cells MG-63 | Monolayer on culture plates | Solid PLGA microspheres | The microspheres give stronger cell attachment, better phenotypic characteristics, higher cell viability and mineralization levels but lower cell count compared with 2D monolayer cell culture over 28 day cell culture studies. | [98] |
| Tenocyte | Human primary tenocytes | Monolayer | PLGA scaffolds or high-density cultures | Compared to native tendon, decorin and COMP were reduced in 2D and increased in 3D culture almost to ex vivo level. | [99] |
| Tenocyte | Rat dermal fibroblasts | Monolayer | 3D resorbable polyester scaffolds | Direct comparisons between the 2D and 3D studies are difficult, given the differences in experimental variables (i.e. surface chemistry, topography, serum content, seeding density, etc.). | [100] |
A1. Surface Porosity Estimation from SEM Photos
A1.1. Principle of Estimation
A1.2. Photo Selection
- Low magnification (30× photos for our case, to ensure there is a sufficient number of pores/craters in the photos).
- No charging of the materials in the photos (charge build up during SEM examination would cause bright spots on the photos which may result in extremely high intensity values that does not reflect the true topography).
- Good contrast and brightness (the photos should have appropriate contrast so that the intensity of each pixel of the photos truly represents the surface topography).
A1.3. Modifications
- The lowest z-value mapped from our photos is not always 0. Thus we made a slight modification in the method to accurately estimate the porosity for our porous materials. In addition to obtaining the fmax value, we also incorporated fmin into the calculation. Considering a surface represented by a function f(x,y),
- Surface porosity
- The labels on the photos were cropped away. Otherwise, they would be treated as topography features.
- When the photos in TIFF format were mapped into the pixel intensity values using OriginPro 8.5.1 (Image > Conversion > Convert to Data), the first and last x/y values may be extremely small. In such case, the value needs to be reset according to the matrix dimension (Matrix > Set Dimension and Labels). Otherwise, the Integral values obtained may become 0 since extremely small.
- Contour maps were produced to check whether the numerical values representing the surface topography is consistent with the SEM photos (Plot > Contour > Color Fill).
A1.4. Note
A2. ALP Activity Due to Adsorbed ALP from Serum

A3. Base of Normalization for ALP Enzymatic Assay

References
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar]
- Peng, Q.; Jiang, F.; Huang, P.; Zhou, S.; Weng, J.; Bao, C.; Zhang, C.; Yu, H. A novel porous bioceramics scaffold by accumulating hydroxyapatite spherules for large bone tissue engineering in vivo. I. Preparation and characterization of scaffold. J. Biomed. Mater. Res. Part A 2010, 93, 920–929. [Google Scholar]
- Yun, H.-S.; Kim, S.-E.; Park, E.K. Bioactive glass-poly (ε-caprolactone) composite scaffolds with 3 dimensionally hierarchical pore networks. Mater. Sci. Eng. C 2011, 31, 198–205. [Google Scholar]
- Blakeney, B.A.; Tambralli, A.; Anderson, J.M.; Andukuri, A.; Lim, D.-J.; Dean, D.R.; Jun, H.-W. Cell infiltration and growth in a low density, uncompressed three-dimensional electrospun nanofibrous scaffold. Biomaterials 2011, 32, 1583–1590. [Google Scholar]
- Tang, P.-F.; Li, G.; Wang, J.-F.; Zheng, Q.-J.; Wang, Y. Development, characterization, and validation of porous carbonated hydroxyapatite bone cement. J. Biomed. Mater. Res. Part B 2009, 90, 886–893. [Google Scholar]
- Jamuna-Thevi, K.; Zakaria, F.A.; Othman, R.; Muhamad, S. Development of macroporous calcium phosphate scaffold processed via microwave rapid drying. Mater. Sci. Eng. C 2009, 29, 1732–1740. [Google Scholar]
- Cruz, D.M.G.; Gomes, M.; Reis, R.L.; Moratal, D.; Salmerón-Sánchez, M.; Ribelles, J.L.G.; Mano, J.F. Differentiation of mesenchymal stem cells in chitosan scaffolds with double micro and macroporosity. J. Biomed. Mater. Res. Part A 2010, 95, 1182–1193. [Google Scholar] [Green Version]
- Li, Y.; Xiong, J.; Hodgson, P.D.; Wen, C. Effects of structural property and surface modification of Ti6Ta4Sn scaffolds on the response of SaOS2 cells for bone tissue engineering. J. Alloys Compd. 2010, 494, 323–329. [Google Scholar]
- Wang, S.; Jain, H. High surface area nanomacroporous bioactive glass scaffold for hard tissue engineering. J. Am. Ceram Soc. 2010, 93, 3002–3005. [Google Scholar]
- Oliveira, J.M.; Silva, S.S.; Malafaya, P.B.; Rodrigues, M.T.; Kotobuki, N.; Hirose, M.; Gomes, M.E.; Mano, J.F.; Ohgushi, H.; Reis, R.L. Macroporous hydroxyapatite scaffolds for bone tissue engineering applications: Physicochemical characterization and assessment of rat bone marrow stromal cell viability. J. Biomed. Mater. Res. Part A 2009, 91, 175–186. [Google Scholar] [Green Version]
- Teixeira, S.; Rodriguez, M.A.; Pena, P.; de Aza, A.H.; de Aza, S.; Ferraz, M.P.; Monteiro, F.J. Physical characterization of hydroxyapatite porous scaffolds for tissue engineering. Mater. Sci. Eng. C 2009, 29, 1510–1514. [Google Scholar]
- Whited, B.M.; Whitney, J.R.; Hofmann, M.C.; Xu, Y.; Rylander, M.N. Pre-osteoblast infiltration and differentiation in highly porous apatite-coated PLLA electrospun scaffolds. Biomaterials 2011, 32, 2294–304. [Google Scholar]
- Jones, G.L.; Walton, R.; Czernuszka, J.; Griffiths, S.L.; El Haj, A.J.; Cartmell, S.H. Primary human osteoblast culture on 3D porous collagen-hydroxyapatite scaffolds. J. Biomed. Mater. Res. Part A 2010, 94, 1244–1250. [Google Scholar]
- Roldán, J.C.; Detsch, R.; Schaefer, S.; Chang, E.; Kelantan, M.; Waiss, W.; Reichert, T.E.; Gurtner, G.C.; Deisinger, U. Bone formation and degradation of a highly porous biphasic calcium phosphate ceramic in presence of BMP-7, VEGF and mesenchymal stem cells in an ectopic mouse model. J. Cranio-Maxillofac. Surg. 2010, 38, 423–430. [Google Scholar]
- Hing, K.A. Bioceramic bone graft substitutes: Influence of porosity and chemistry. Int. J. Appl. Ceram. Technol. 2005, 2, 184–199. [Google Scholar]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [Green Version]
- Warnke, P.H.; Seitz, H.; Warnke, F.; Becker, S.T.; Sivananthan, S.; Sherry, E.; Liu, Q.; Wiltfang, J.; Douglas, T. Ceramic scaffolds produced by computer-assisted 3D printing and sintering: Characterization and biocompatibility investigations. J. Biomed. Mater. Res. Part B 2010, 93, 212–217. [Google Scholar]
- Choi, S.-W.; Xie, J.; Xia, Y. Chitosan-based inverse opals: Three-dimensional scaffolds with uniform pore structures for cell culture. Adv. Mater. 2009, 21, 2997–3001. [Google Scholar]
- Khoda, A.K.M.B.; Ozbolat, I.T.; Koc, B. Engineered tissue scaffolds with variational porous architecture. J. Biomech. Eng. 2010, 133, 011001:1–011001:12. [Google Scholar]
- Wilson, C.E.; van Blitterswijk, C.A.; Verbout, A.J.; Dhert, W.J.A.; de Bruijn, J.D. Scaffolds with a standardized macro-architecture fabricated from several calcium phosphate ceramics using an indirect rapid prototyping technique. J. Mater. Sci. Mater. Med. 2011, 22, 97–105. [Google Scholar]
- Melchels, F.P.W.; Bertoldi, K.; Gabbrielli, R.; Velders, A.H.; Feijen, J.; Grijpma, D.W. Mathematically defined tissue engineering scaffold architectures prepared by stereolithography. Biomaterials 2010, 31, 6909–6916. [Google Scholar] [Green Version]
- Legeros, R.Z.; Lin, S.; Rohanizadeh, R.; Mijares, D.; Legeros, J.P. Biphasic calcium phosphate bioceramics: Preparation, properties and applications. J. Mater. Sci. Mater. Med. 2003, 14, 201–209. [Google Scholar]
- Engineering of Functional Skeletal Tissues; Bronner, F.; Farach-Carson, M.C.; Mikos, A.G. (Eds.) SpringerLink: Berlin, Germany, 2007; Volume 3.
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar]
- Silva, W.A., Jr.; Covas, D.T.; Panepucci, R.A.; Proto-Siqueira, R.; Siufi, J.L.C.; Zanette, D.L.; Santos, A.R.D.; Zago, M.A. The profile of gene expression of human marrow mesenchymal stem cells. Stem Cells 2003, 21, 661–669. [Google Scholar]
- Jaiswal, N.; Haynesworth, S.E.; Caplan, A.I.; Bruder, S.P. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J. Cell. Biochem. 1997, 64, 295–312. [Google Scholar]
- Yuan, H.; Yang, Z.; de Bruijn, J.D.; de Groot, K.; Zhang, X. Material-dependent bone induction by calcium phosphate ceramics: A 2.5-year study in dog. Biomaterials 2001, 22, 2617–2623. [Google Scholar]
- Lee, J.T.Y.; Tsang, W.H.; Chow, K.L. Simple modifications to standard TRIzol® protocol allow high-yield RNA extraction from cells on resorbable materials. J. Biomater. Nanobiotechnol. 2011, 2, 41–48. [Google Scholar]
- Lee, J.T.Y.; Chow, K.L. SEM sample preparation for cells on 3D scaffolds by freezing drying and HMDS. Scanning 2011. [Google Scholar] [CrossRef]
- Stein, G.S.; Lian, J.B. Molecular mechanisms mediating proliferation/differentiation interrelationships during progressive development of the osteoblast phenotype. Endocr. Rev. 1993, 14, 424–442. [Google Scholar]
- Dohi, Y.; Akahane, M.; Ohgushi, H. Molecular structure of osteocalcin and the role as an essential marker in the osteogenic differentiation cascade. J. Nara Med. Assoc. 2008, 59, 83–96. [Google Scholar]
- Lee, J.T.Y.; Wang, K.; Tsang, W.H.; Chow, K.L. Comparative in vitro osteoinductivity study of CaP ceramics (HA, α-TCP, β-TCP) using 10T1/2 cells with different controls and possible correlations with other systems. J. Biomater. Nanobiotechnol. 2011, 2, 162–171. [Google Scholar]
- Takahashi, Y.; Tabata, Y. Effect of the fiber diameter and porosity of non-woven PET fabrics on the osteogenic differentiation of mesenchymal stem cells. J. Biomater. Sci. Polym. Ed. 2004, 15, 41–57. [Google Scholar]
- Okamoto, M.; Dohi, Y.; Ohgushi, H.; Shimaoka, H.; Ikeuchi, M.; Matsushima, A.; Yonemasu, K.; Hosoi, H. Influence of the porosity of hydroxyapatite ceramics on in vitro and in vivo bone formation by cultured rat bone marrow stromal cells. J. Mater. Sci. Mater. Med. 2006, 17, 327–336. [Google Scholar]
- McCullough, K.C.; Spier, R.E. Monoclonal Antibodies in Biology and Biotechnology: Theoretical and Practical Aspects; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Crowther, J.R. ELISA: Theory and Practice; Humana Press: Totowa, NJ, USA, 1995. [Google Scholar]
- Suzuki, A.; Palmer, G.; Bonjour, J.-P.; Caverzasio, J. Catecholamines stimulate the proliferation and alkaline phosphatase activity of MC3T3-E1 osteoblast-like cells. Bone 1998, 23, 197–203. [Google Scholar]
- Lee, D.H.; Lim, B.-S.; Lee, Y.-K.; Yang, H.-C. Effects of hydrogen peroxide (H2O2) on alkaline phosphatase activity and matrix mineralization of odontoblast and osteoblast cell lines. Cell Biol. Toxicol. 2006, 22, 39–46. [Google Scholar]
- Igarashi, M.; Yogiashi, Y.; Mihara, M.; Takada, I.; Kitagawa, H.; Kato, S. Vitamin K induces osteoblast differentiation through pregnane X receptor-mediated transcriptional control of the Msx2 gene. Mol. Cell. Biol. 2007, 27, 7947–7954. [Google Scholar]
- Zhang, Z.; Lundeen, S.G.; Zhu, Y.; Carver, J.M.; Winneker, R.C. In vitro characterization of trimegestone: A new potent and selective progestin. Steroids 2000, 65, 637–643. [Google Scholar]
- Young, H.K.; Choi, E.M. Stimulation of osteoblastic differentiation and inhibition of interleukin-6 and nitric oxide in MC3T3-E1 cells by pomegranate ethanol extract. Phytother. Res. 2009, 23, 737–739. [Google Scholar]
- Sun, J.; Tsuang, Y.; Liao, C.; Liu, H.; Hang, Y.; Lin, F. The effects of calcium phosphate particles on the growth of osteoblasts. J. Biomed. Mater. Res. 1997, 37, 324–334. [Google Scholar]
- Jones, J.R.; Tsigkou, O.; Coates, E.E.; Stevens, M.M.; Polak, J.M.; Hench, L.L. Extracellular matrix formation and mineralization on a phosphate-free porous bioactive glass scaffold using primary human osteoblast (HOB) cells. Biomaterials 2007, 28, 1653–1663. [Google Scholar]
- Kasten, P.; Vogel, J.; Luginbühl, R.; Niemeyer, P.; Tonak, M.; Lorenz, H.; Helbig, L.; Weiss, S.; Fellenberg, J.; Leo, A.; et al. Ectopic bone formation associated with mesenchymal stem cells in a resorbable calcium deficient hydroxyapatite carrier. Biomaterials 2005, 26, 5879–5889. [Google Scholar]
- Kim, H.-W.; Lee, E.-J.; Kim, H.-E.; Salih, V.; Knowles, J.C. Effect of fluoridation of hydroxyapatite in hydroxyapatite-polycaprolactone composites on osteoblast activity. Biomaterials 2005, 26, 4395–4404. [Google Scholar]
- Kong, Y.-M.; Bae, C.-J.; Lee, S.-H.; Kim, H.-W.; Kim, H.-E. Improvement in biocompatibility of ZrO2-Al2O3 nano-composite by addition of HA. Biomaterials 2005, 26, 509–517. [Google Scholar]
- Lee, J.-Y.; Choo, J.-E.; Park, H.-J.; Park, J.-B.; Lee, S.-C.; Lee, S.-J.; Park, Y.-J.; Chung, C.-P. Synthetic peptide-coated bone mineral for enhanced osteoblastic activation in vitro and in vivo. J. Biomed. Mater. Res. Part A 2008, 87, 688–697. [Google Scholar]
- Le Guehennec, L.; Lopez-Heredia, M.-A.; Enkel, B.; Weiss, P.; Amouriq, Y.; Layrolle, P. Osteoblastic cell behaviour on different titanium implant surfaces. Acta Biomater. 2008, 4, 535–543. [Google Scholar]
- Zhao, G.; Schwartz, Z.; Wieland, M.; Rupp, F.; Geis-Gerstorfer, J.; Cochran, D.L.; Boyan, B.D. High surface energy enhances cell response to titanium substrate microstructure. J. Biomed. Mater. Res. Part A 2005, 74, 49–58. [Google Scholar]
- Hofmann, S.; Hagenmüller, H.; Koch, A.M.; Müller, R.; Vunjak-Novakovic, G.; Kaplan, D.L.; Merkle, H.P.; Meinel, L. Control of in vitro tissue-engineered bone-like structures using human mesenchymal stem cells and porous silk scaffolds. Biomaterials 2007, 28, 1152–1162. [Google Scholar]
- Karageorgiou, V.; Meinel, L.; Hofmann, S.; Malhotra, A.; Volloch, V.; Kaplan, D. Bone morphogenetic protein-2 decorated silk fibroin films induce osteogenic differentiation of human bone marrow stromal cells. J. Biomed. Mater. Res. Part A 2004, 71, 528–537. [Google Scholar]
- Karageorgiou, V.; Tomkins, M.; Fajardo, R.; Meinel, L.; Snyder, B.; Wade, K.; Chen, J.; Vunjak-Novakovic, G.; Kaplan, D.L. Porous silk fibroin 3-D scaffolds for delivery of bone morphogenetic protein-2 in vitro and in vivo. J. Biomed. Mater. Res. Part A 2006, 78, 324–334. [Google Scholar]
- Mygind, T.; Stiehler, M.; Baatrup, A.; Li, H.; Zou, X.; Flyvbjerg, A.; Kassem, M.; Bünger, C. Mesenchymal stem cell ingrowth and differentiation on coralline hydroxyapatite scaffolds. Biomaterials 2007, 28, 1036–1047. [Google Scholar]
- Jiang, T.; Abdel-Fattah, W.I.; Laurencin, C.T. In vitro evaluation of chitosan/poly (lactic acid-glycolic acid) sintered microsphere scaffolds for bone tissue engineering. Biomaterials 2006, 27, 4894–4903. [Google Scholar]
- Mauney, J.R.; Jaquiéry, C.; Volloch, V.; Heberer, M.; Martin, I.; Kaplan, D.L. In vitro and in vivo evaluation of differentially demineralized cancellous bone scaffolds combined with human bone marrow stromal cells for tissue engineering. Biomaterials 2005, 26, 3173–385. [Google Scholar]
- Takahashi, Y.; Yamamoto, M.; Tabata, Y. Osteogenic differentiation of mesenchymal stem cells in biodegradable sponges composed of gelatin and β-tricalcium phosphate. Biomaterials 2005, 26, 3587–3596. [Google Scholar]
- Shirosaki, Y.; Tsuru, K.; Hayakawa, S.; Osaka, A.; Lopes, M.A.; Santos, J.D.; Fernandes, M.H. In vitro cytocompatibility of MG63 cells on chitosan-organosiloxane hybrid membranes. Biomaterials 2005, 26, 485–493. [Google Scholar]
- Oliveira, J.M.; Rodrigues, M.T.; Silva, S.S.; Malafaya, P.B.; Gomes, M.E.; Viegas, C.A.; Dias, I.R.; Azevedo, J.T.; Mano, J.F.; Reis, R.L. Novel hydroxyapatite/chitosan bilayered scaffold for osteochondral tissue-engineering applications: Scaffold design and its performance when seeded with goat bone marrow stromal cells. Biomaterials 2006, 27, 6123–6137. [Google Scholar] [Green Version]
- Bigi, A.; Bracci, B.; Cuisinier, F.; Elkaim, R.; Fini, M.; Mayer, I.; Mihailescu, I.N.; Socol, G.; Sturba, L.; Torricelli, P. Human osteoblast response to pulsed laser deposited calcium phosphate coatings. Biomaterials 2005, 26, 2381–2389. [Google Scholar]
- Causa, F.; Netti, P.A.; Ambrosio, L.; Ciapetti, G.; Baldini, N.; Pagani, S.; Martini, D.; Giunti, A. Poly-ε-caprolactone/hydroxyapatite composites for bone regeneration: In vitro characterization and human osteoblast response. J. Biomed. Mater. Res. Part A 2006, 76, 151–162. [Google Scholar]
- Takahashi, Y.; Yamamoto, M.; Tabata, Y. Enhanced osteoinduction by controlled release of bone morphogenetic protein-2 from biodegradable sponge composed of gelatin and β-tricalcium phosphate. Biomaterials 2005, 26, 4856–4865. [Google Scholar]
- Gauthier, O.; Bouler, J.-M.; Aguado, E.; Pilet, P.; Daculsi, G. Macroporous biphasic calcium phosphate ceramics: Influence of macropore diameter and macroporosity percentage on bone ingrowth. Biomaterials 1998, 19, 133–139. [Google Scholar]
- Habibovic, P.; Sees, T.M.; van den Doel, M.A.; van Blitterswijk, C.A.; de Groot, K. Osteoinduction by biomaterials—Physicochemical and structural influences. J. Biomed. Mater. Res. Part A 2006, 77, 747–762. [Google Scholar]
- Habibovic, P.; Kruyt, M.C.; Juhl, M.V.; Clyens, S.; Martinetti, R.; Dolcini, L.; Theilgaard, N.; Van Blitterswijk, C.A. Comparative in vivo study of six hydroxyapatite-based bone graft substitutes. J. Orthop. Res. 2008, 26, 1363–1370. [Google Scholar]
- Von Doernberg, M.-C.; von Rechenberg, B.; Bohner, M.; Grünenfelder, S.; van Lenthe, G.H.; Müller, R.; Müller, R.; Gasser, B.; Mathys, R.; Baroud, G.; Auer, J. In vivo behavior of calcium phosphate scaffolds with four different pore sizes. Biomaterials 2006, 27, 5186–5198. [Google Scholar]
- Kasten, P.; Beyen, I.; Niemeyer, P.; Luginbühl, R.; Bohner, M.; Richter, W. Porosity and pore size of β-tricalcium phosphate scaffold can influence protein production and osteogenic differentiation of human mesenchymal stem cells: An in vitro and in vivo study. Acta Biomater. 2008, 4, 1904–1915. [Google Scholar]
- Kruyt, M.C.; de Bruijn, J.D.; Wilson, C.E.; Oner, F.C.; Van Blitterswijk, C.A.; Verbout, A.J.; Dhert, W.J.A. Viable osteogenic cells are obligatory for tissue-engineered ectopic bone formation in goats. Tissue Eng. 2003, 9, 327–336. [Google Scholar]
- Mastrogiacomo, M.; Scaglione, S.; Martinetti, R.; Dolcini, L.; Beltrame, F.; Cancedda, R.; Quarto, R. Role of scaffold internal structure on in vivo bone formation in macroporous calcium phosphate bioceramics. Biomaterials 2006, 27, 3230–3237. [Google Scholar]
- Tsuruga, E.; Takita, H.; Itoh, H.; Wakisaka, Y.; Kuboki, Y. Pore size of porous hydroxyapatite as the cell-substratum controls BMP-induced osteogenesis. J. Biochem. 1997, 121, 317–324. [Google Scholar]
- Kuboki, Y.; Jin, Q.; Kikuchi, M.; Mamood, J.; Takita, H. Geometry of artificial ECM: Sizes of pores controlling phenotype expression in BMP-induced osteogenesis and chondrogenesis. Connect. Tissue Res. 2002, 43, 529–534. [Google Scholar]
- Habibovic, P.; Yuan, H.; van der Valk, C.M.; Meijer, G.; van Blitterswijk, C.A.; de Groot, K. 3D microenvironment as essential element for osteoinduction by biomaterials. Biomaterials 2005, 26, 3565–3575. [Google Scholar]
- Eggli, P.S.; Muller, W.; Schenk, R.K. Porous hydroxyapatite and tricalcium phosphate cylinders with two different pore size ranges implanted in the cancellous bone of rabbits. A comparative histomorphometric and histologic study of bone ingrowth and implant substitution. Clin. Orthop. Relat. Res. 1988, 127–138. [Google Scholar]
- Kujala, S.; Ryhänen, J.; Danilov, A.; Tuukkanen, J. Effect of porosity on the osteointegration and bone ingrowth of a weight-bearing nickel-titanium bone graft substitute. Biomaterials 2003, 24, 4691–4697. [Google Scholar]
- Mantila Roosa, S.M.; Kemppainen, J.M.; Moffitt, E.N.; Krebsbach, P.H.; Hollister, S.J. The pore size of polycaprolactone scaffolds has limited influence on bone regeneration in an in vivo model. J. Biomed. Mater. Res. Part A 2010, 92, 359–368. [Google Scholar]
- Fisher, J.P.; Vehof, J.W.M.; Dean, D.; van der Waerden, J.P.C.M.; Holland, T.A.; Mikos, A.G.; Jansen, J.A. Soft and hard tissue response to photocrosslinked poly(propylene fumarate) scaffolds in a rabbit model. J. Biomed. Mater. Res. 2002, 59, 547–556. [Google Scholar]
- Götz, H.E.; Müller, M.; Emmel, A.; Holzwarth, U.; Erben, R.G.; Stangl, R. Effect of surface finish on the osseointegration of laser-treated titanium alloy implants. Biomaterials 2004, 25, 4057–4064. [Google Scholar]
- Bandyopadhyay, A.; Espana, F.; Balla, V.K.; Bose, S.; Ohgami, Y.; Davies, N.M. Influence of porosity on mechanical properties and in vivo response of Ti6Al4V implants. Acta Biomater. 2010, 6, 1640–168. [Google Scholar]
- Itoh, M.; Shimazu, A.; Hirata, I.; Yoshida, Y.; Shintani, H.; Okazaki, M. Characterization of CO3Ap-collagen sponges using X-ray high-resolution microtomography. Biomaterials 2004, 25, 2577–2583. [Google Scholar]
- Murphy, C.M.; Haugh, M.G.; O'Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar]
- Annaz, B.; Hing, K.A.; Kayser, M.; Buckland, T.; di Silvio, L. Porosity variation in hydroxyapatite and osteoblast morphology: A scanning electron microscopy study. J. Microsc. 2004, 215, 100–110. [Google Scholar]
- Rosa, A.L.; Beloti, M.M.; van Noort, R. Osteoblastic differentiation of cultured rat bone marrow cells on hydroxyapatite with different surface topography. Dental Mater. 2003, 19, 768–772. [Google Scholar]
- Hornez, J.-C.; Chai, F.; Monchau, F.; Blanchemain, N.; Descamps, M.; Hildebrand, H.F. Biological and physico-chemical assessment of hydroxyapatite (HA) with different porosity. Biomol. Eng. 2007, 24, 505–509. [Google Scholar]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospun poly (ε-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: Characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules 2006, 7, 2796–2805. [Google Scholar]
- Ahu Akin, F.; Zreiqat, H.; Jordan, S.; Wijesundara, M.B.J.; Hanley, L. Preparation and analysis of macroporous TiO2 films on Ti surfaces for bone-tissue implants. J. Biomed. Mater. Res. 2001, 57, 588–596. [Google Scholar]
- Hartman, O.; Zhang, C.; Adams, E.L.; Farach-Carson, M.C.; Petrelli, N.J.; Chase, B.D.; Rabolt, J.F. Microfabricated electrospun collagen membranes for 3-D cancer models and drug screening applications. Biomacromolecules 2009, 10, 2019–2032. [Google Scholar]
- Breyner, N.M.; Hell, R.C.R.; Carvalho, L.R.P.; MacHado, C.B.; Peixoto Filho, I.N.; Valério, P.; Pereira, M.M.; Goes, A.M. Effect of a three-dimensional chitosan porous scaffold on the differentiation of mesenchymal stem cells into chondrocytes. Cells Tissues Organs (Print) 2010, 191, 119–128. [Google Scholar]
- Francioli, S.E.; Candrian, C.; Martin, K.; Heberer, M.; Martin, I.; Barbero, A. Effect of three-dimensional expansion and cell seeding density on the cartilage-forming capacity of human articular chondrocytes in type II collagen sponges. J. Biomed. Mater. Res. Part A 2010, 95, 924–931. [Google Scholar]
- Drengk, A.; Zapf, A.; Stürmer, E.K.; Stürmer, K.M.; Frosch, K.-H. Influence of platelet-rich plasma on chondrogenic differentiation and proliferation of chondrocytes and mesenchymal stem cells. Cells Tissues Organs (Print) 2009, 189, 317–326. [Google Scholar]
- Anders, J.O.; Mollenhauer, J.; Beberhold, A.; Kinne, R.W.; Venbrocks, R.A. Gelatin-based haemostyptic Spongostan as a possible three-dimensional scaffold for a chondrocyte matrix? An experimental study with bovine chondrocytes. J. Bone Joint Surg. Ser. B 2009, 91, 409–416. [Google Scholar]
- Kelm, J.M.; Lorber, V.; Snedeker, J.G.; Schmidt, D.; Broggini-Tenzer, A.; Weisstanner, M.; Odermatt, B.; Mol, A.; Zünd, G.; Hoerstrup, S.P. A novel concept for scaffold-free vessel tissue engineering: Self-assembly of microtissue building blocks. J. Biotechnol. 2010, 148, 46–55. [Google Scholar]
- Lupanova, T.; Stefanova, N.; Petkova, D.; Staneva, G.; Jordanova, A.; Koumanov, K.; Pankov, R.; Momchilova, A. Alterations in the content and physiological role of sphingomyelin in plasma membranes of cells cultured in three-dimensional matrix. Mol. Cell. Biochem. 2010, 340, 215–222. [Google Scholar]
- Cukierman, E.; Pankov, R.; Stevens, D.R.; Yamada, K.M. Taking cell-matrix adhesions to the third dimension. Science 2001, 294, 1708–1712. [Google Scholar]
- Tan, G.-K.; Dinnes, D.L.M.; Myers, P.T.; Cooper-White, J.J. Effects of biomimetic surfaces and oxygen tension on redifferentiation of passaged human fibrochondrocytes in 2D and 3D cultures. Biomaterials 2011, 32, 5600–5614. [Google Scholar]
- Hsieh, T.M.; Benjamin Ng, C.W.; Narayanan, K.; Wan, A.C.A.; Ying, J.Y. Three-dimensional microstructured tissue scaffolds fabricated by two-photon laser scanning photolithography. Biomaterials 2010, 31, 7648–7652. [Google Scholar]
- Verma, P.; Verma, V.; Ray, P.; Ray, A.R. Agar-gelatin hybrid sponge-induced three-dimensional in vitro ‘liver-like’ HepG2 spheroids for the evaluation of drug cytotoxicity. J. Tissue Eng. Regen. Med. 2009, 3, 368–376. [Google Scholar]
- Fan, X.; Zou, R.; Zhao, Z.; Yang, P.; Li, Y.; Song, J. Tensile strain induces integrin β1 and ILK expression higher and faster in 3D cultured rat skeletal myoblasts than in 2D cultures. Tissue Cell 2009, 41, 266–270. [Google Scholar]
- Gurkan, U.A.; Kishore, V.; Condon, K.W.; Bellido, T.M.; Akkus, O. A scaffold-free multicellular three-dimensional in vitro model of osteogenesis. Calcif. Tissue Int. 2011, 88, 388–401. [Google Scholar]
- Verma, S.; Kumar, N. Effect of biomimetic 3D environment of an injectable polymeric scaffold on MG-63 osteoblastic-cell response. Mater. Sci. Eng. C 2010, 30, 1118–1128. [Google Scholar]
- Stoll, C.; John, T.; Endres, M.; Rosen, C.; Kaps, C.; Kohl, B.; Sittinger, M.; Ertel, W.; Schulze-Tanzil, G. Extracellular matrix expression of human tenocytes in three-dimensional air-liquid and PLGA cultures compared with tendon tissue: Implications for tendon tissue engineering. J. Orthop. Res. 2010, 28, 1170–1177. [Google Scholar]
- Brink, H.E.; Bernstein, J.; Nicoll, S.B. Fetal dermal fibroblasts exhibit enhanced growth and collagen production in two- and three-dimensional culture in comparison to adult fibroblasts. J. Tissue Eng. Regen. Med. 2009, 3, 623–633. [Google Scholar]
- Abdullah, M.; Khairurrijal. A simple method for determining surface porosity based on SEM images using OriginPro software. Indones. J. Phys. 2009, 20, 37–40. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lee, J.T.Y.; Chow, K.L.; Wang, K.; Tsang, W.-H. Is Macroporosity Absolutely Required for Preliminary in Vitro Bone Biomaterial Study? A Comparison Between Porous Materials and Flat Materials. J. Funct. Biomater. 2011, 2, 308-337. https://doi.org/10.3390/jfb2040308
Lee JTY, Chow KL, Wang K, Tsang W-H. Is Macroporosity Absolutely Required for Preliminary in Vitro Bone Biomaterial Study? A Comparison Between Porous Materials and Flat Materials. Journal of Functional Biomaterials. 2011; 2(4):308-337. https://doi.org/10.3390/jfb2040308
Chicago/Turabian StyleLee, Juliana T. Y., King L. Chow, Kefeng Wang, and Wai-Hung Tsang. 2011. "Is Macroporosity Absolutely Required for Preliminary in Vitro Bone Biomaterial Study? A Comparison Between Porous Materials and Flat Materials" Journal of Functional Biomaterials 2, no. 4: 308-337. https://doi.org/10.3390/jfb2040308