Development of Ti-Coated Ferromagnetic Needle, Adaptable for Ablation Cancer Therapy by High-Frequency Induction Heating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials

2.2. Experimental Procedure

3. Results and Discussion
3.1. Heating Properties of the Ferromagnetic Mild Steel Rod


3.2. Heating Properties of the Prototype Ti-Coated Ablation Needle


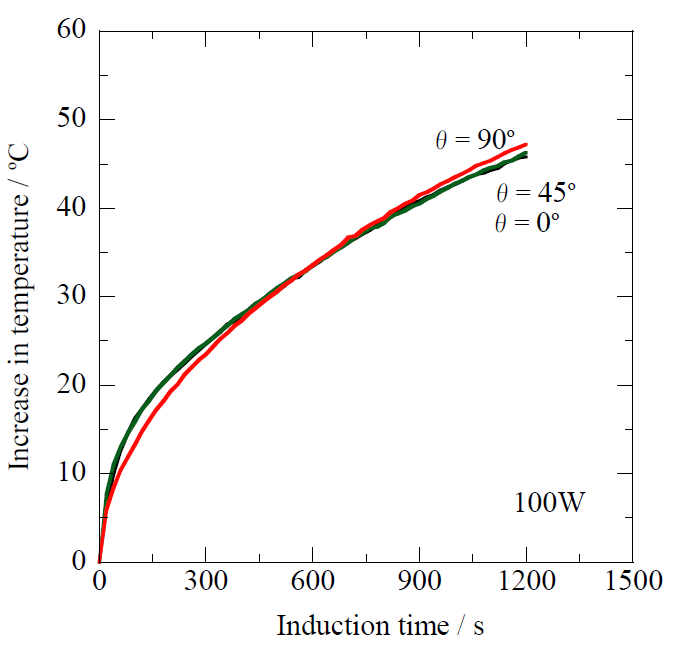
3.3. Applicability of the Prototype Ti-Coated Needle for Ablation Cancer Therapy
4. Conclusions
Acknowledgments
References
- Matsuura, M.; Nakajima, N.; Araki, K.; Ito, K. The usefulness of radiation therapy for hepatocellular carcinoma. Hepatogastroenterology 1998, 45, 791–796. [Google Scholar]
- Hawkins, M.A.; Dawson, L.A. Radiation therapy for hepatocellular carcinoma: From palliation to cure. Cancer 2006, 106, 1653–1663. [Google Scholar] [CrossRef]
- Dawson, L.A. The evolving role of radiation therapy in hepatocellular carcinoma. Cancer Radio ther. 2008, 12, 96–101. [Google Scholar] [CrossRef]
- Ganne-Carrié, N.; Trinhet, J.C. Systemic treatment of hepatocellular carcinoma. Eur. J. Gastro Enterol. Hepatol. 2004, 16, 275–281. [Google Scholar] [CrossRef]
- Okada, S. Chemotherapy in hepatocellular carcinoma. Hepatogastroenterology 1998, 45 (Suppl. 3), 1259–1263. [Google Scholar]
- Llovet, J.M. Updated treatment approach to hepatocellular carcinoma. J. Gastroenterol. 2005, 40, 225–235. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Gelber, R.D. Endocrine therapies of breast cancer. Semin. Oncol. 1996, 23, 494–505. [Google Scholar]
- Kimmick, G.G.; Muss, H.B. Endocrine therapy in metastatic breast cancer. Cancer Treat. Res. 1998, 94, 231–254. [Google Scholar] [CrossRef]
- Yamashita, H. Current research topics in endocrine therapy for breast cancer. Int. J. Cli. Oncol. 2008, 13, 380–383. [Google Scholar] [CrossRef]
- Buscarini, L.; Buscarini, E.; di Stasi, M.; Vallisa, D.; Quaretti, P.; Rocca, A. Percutaneous radiofrequency ablation of small hepatocelluler carcinoma: long-term results. Eur. Radiol. 2001, 11, 914–921. [Google Scholar] [CrossRef]
- Ni, Y.; Mulier, S.; Miao, Y.; Michel, L.; Marchal, G. A review of the general aspects of radio-frequency ablation. Abdom. Imaging 2005, 30, 381–400. [Google Scholar] [CrossRef]
- Lencioni, R.; Della Pina, C.; Barttolozzi, C. Percutaneous image-guided radiofrequency ablation in the therapeutic management of hepatocellular carcinoma. Abdom. Imaging 2005, 30, 401–408. [Google Scholar] [CrossRef]
- Crocetti, L.; Lencioni, R. Thermal ablation of hepatocellular carcinoma. Cancer Imaging 2008, 8, 19–26. [Google Scholar] [CrossRef]
- Ayav, A.; Germain, A.; Marchal, F.; Tierris, I.; Laurent, V.; Bazin, C.; Yuan, Y.; Robert, L.; Brunaud, L.; Bresler, L. Radiofrequency ablation of unresectable liver tumors: factors associated with incomplete ablation or local recurrence. Am. J. Surg. 2010, 200, 435–439. [Google Scholar] [CrossRef]
- Lam, V.W.; Ng, K.K.; Chok, K.S.; Cheung, T.T.; Yuen, J.; Tung, H.; Tso, W.K.; Fan, S.T.; Poon, R.T. Risk factors and prognostic factors of local reccurrence after radiofrequency ablation of hepatocellular carcinoma. J. Am. Coll. Surg. 2008, 207, 20–29. [Google Scholar] [CrossRef]
- Akahane, M.; Koga, H.; Kato, N.; Yamada, H.; Uozumi, K.; Tateishi, R.; Teratani, T.; Shiina, S.; Ohtomo, K. Complications of percutaneous radiofrequency ablation for hepato-cellular caicinoma: Imaging spectrum and management. Radiogr. 2005, 25 (Suppl. 1), 57–68. [Google Scholar]
- Maehara, T.; Konishi, K.; Kamimori, T.; Aono, H.; Naohara, T.; Kikkawa, H.; Watanabe, Y.; Kawachi, K. Heating of ferrite powder by an AC magnetic field for local hyperthermia. Jpn. J. Appl. Phys. 2002, 41, 1620–1621. [Google Scholar] [CrossRef]
- Naohara, T.; Aono, H.; Maehara, T.; Watanabe, Y.; Hirazawa, H.; Matsutomo, S. Computer simulation of heat generation ability in AC magnetic field. In Proceedings of International Symposium Electromagnetic Processing Materials, Dresden, Germany, 19–23 October 2009; pp. 193–196.
- Watanabe, Y.; Sato, K.; Yukumi, S.; Yoshida, M.; Yamamoto, Y.; Doi, T.; Sugishita, H.; Naohara, T.; Maehara, T.; Aono, H.; Kawachi, K. Development of a second radiofrequency ablation using sintered MgFe2O4 needles and alternating magnetic field for human cancer therapy. Bio-Med. Mater. Eng. 2009, 19, 101–110. [Google Scholar]
- Cullity, B.D.; Graham, C.D. Introduction to Magnetic Materials; Willey IEEE Press: Hoboken, NJ, USA, 2008; pp. 234–237. [Google Scholar]
- Naohara, T.; Aono, H.; Hirazawa, H.; Maehara, T.; Watanabe, Y.; Matsutomo, S. Heat generation ability in AC magnetic field of needle-type Ti-coated mild steel for ablation cancer therapy. Int. J. Comput. Math. Electr. Electron. Eng. 2011, 30, 1582–1588. [Google Scholar] [CrossRef]
- Brunette, D.M.; Tengvall, P.; Textor, M.; Thomsen, P. Titanium in Medicine: Materials Science, Surface Science, Engineering, Biological Responses and Medical Applications; Springer-Verlag: New York, NY, USA, 2001; pp. 14–19. [Google Scholar]
- Van Noort, R. Titanium: The implant material of today. J. Mate. Sci. 1987, 22, 3801–3811. [Google Scholar] [CrossRef]
- Skonski, R. Simple Model of Magnetism; Oxford University Press: New York, NY, USA, 2008; pp. 82–83. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Naohara, T.; Aono, H.; Maehara, T.; Hirazawa, H.; Matsutomo, S.; Watanabe, Y. Development of Ti-Coated Ferromagnetic Needle, Adaptable for Ablation Cancer Therapy by High-Frequency Induction Heating. J. Funct. Biomater. 2012, 3, 163-172. https://doi.org/10.3390/jfb3010163
Naohara T, Aono H, Maehara T, Hirazawa H, Matsutomo S, Watanabe Y. Development of Ti-Coated Ferromagnetic Needle, Adaptable for Ablation Cancer Therapy by High-Frequency Induction Heating. Journal of Functional Biomaterials. 2012; 3(1):163-172. https://doi.org/10.3390/jfb3010163
Chicago/Turabian StyleNaohara, Takashi, Hiromichi Aono, Tsunehiro Maehara, Hideyuki Hirazawa, Shinya Matsutomo, and Yuji Watanabe. 2012. "Development of Ti-Coated Ferromagnetic Needle, Adaptable for Ablation Cancer Therapy by High-Frequency Induction Heating" Journal of Functional Biomaterials 3, no. 1: 163-172. https://doi.org/10.3390/jfb3010163



